Abstract
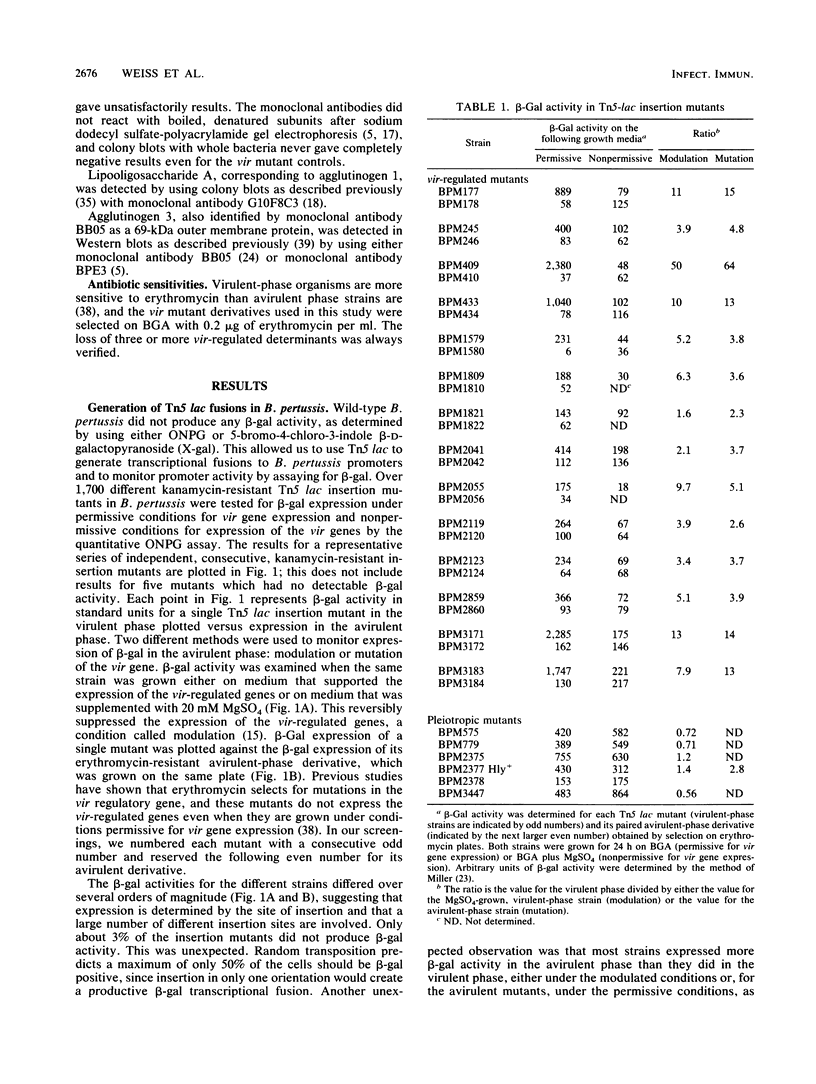
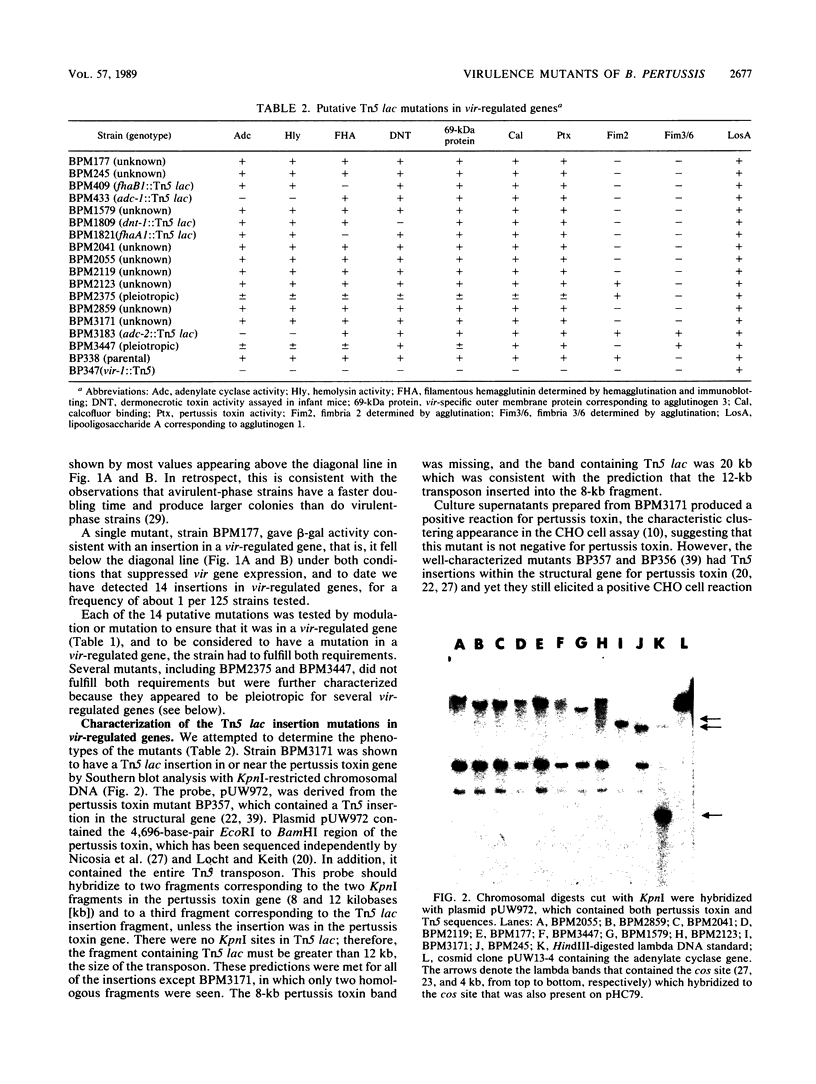
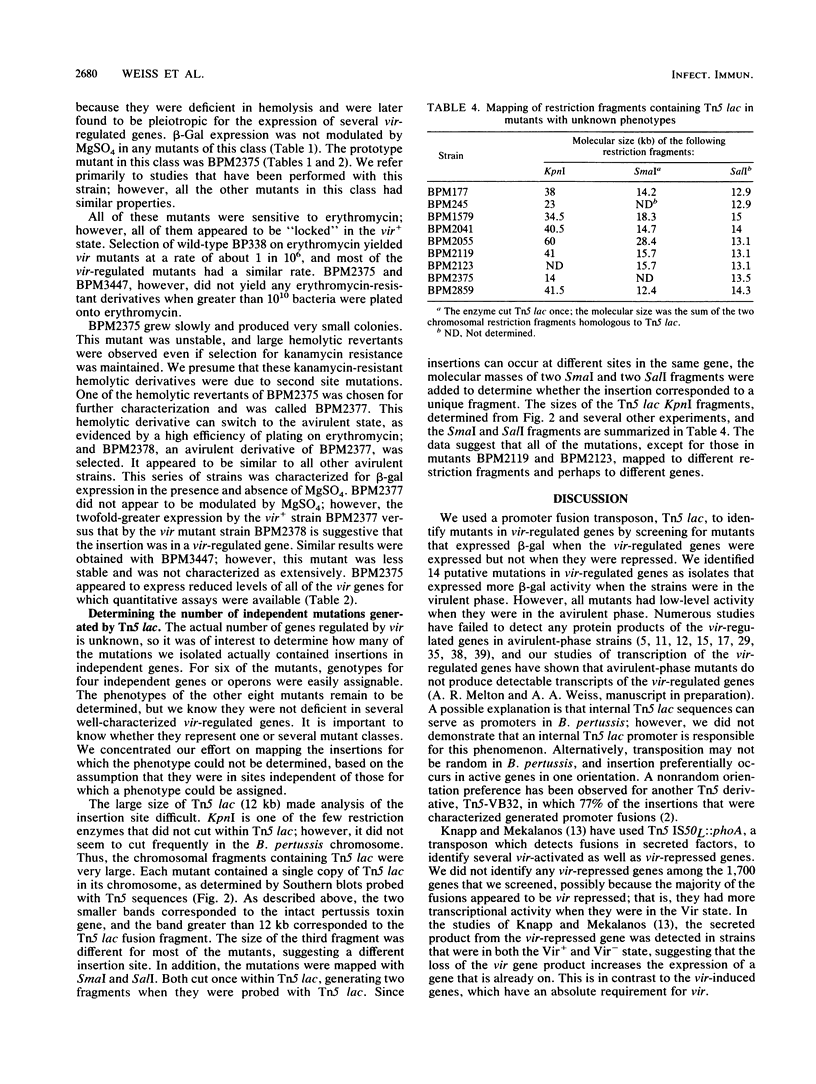
Mutants of Bordetella pertussis deficient in virulence-associated factors were identified by using the transposon Tn5 lac. Tn5 lac is a derivative of Tn5 which generates promoter fusions for beta-galactosidase. Tn5 lac insertions in the vir-regulated genes of B. pertussis were identified by selecting for kanamycin-resistant mutants that expressed beta-galactosidase when the vir-regulated genes were expressed but not when the vir-regulated genes were turned off. Fourteen different mutations in vir-regulated genes were identified. Two mutants were deficient in the production of the filamentous hemagglutinin, two mutants were deficient in the production of adenylate cyclase toxin and hemolysin, and one mutant was deficient in the production of dermonecrotic toxin. One insertion mapped adjacent to the pertussis toxin gene, but the mutant produced pertussis toxin. The phenotypes of the remaining eight mutants were not determined, but the mutants did not appear to be deficient in the production of the 69,000-dalton outer membrane protein (agglutinogen 3) or the capsule. Screening for mutations in either of the fimbrial genes proved to be problematic since the parental strain was found to switch from a fimbriated to a nonfimbriated state at a high frequency, which was suggestive of the metastable expression of pili in other bacteria. We used Southern blot analysis with a 30-mer specific for the fimbrial sequences. No bands with the predicted increase in size due to the 12 kilobases from Tn5 lac were observed, which suggests that none of these genes were mutated. Southern blot analysis also revealed that seven of the eight unidentified mutations mapped to different restriction fragments, which suggests that they could be deficient in as many as seven different genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellofatto V., Shapiro L., Hodgson D. A. Generation of a Tn5 promoter probe and its use in the study of gene expression in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1035–1039. doi: 10.1073/pnas.81.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Munoz J. J., Peacock M. G., Schad P. A., Cowell J. L., Burchall J. J., Lim M., Kent A., Steinman L., Falkow S. ADP-ribosyltransferase activity of pertussis toxin and immunomodulation by Bordetella pertussis. Science. 1988 Apr 29;240(4852):656–659. doi: 10.1126/science.2896387. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlie R. M., Coote J. G., Parton R. Complementation of mutations in Escherichia coli and Bordetella pertussis by B. pertussis DNA cloned in a broad-host-range cosmid vector. J Gen Microbiol. 1986 Nov;132(11):3221–3229. doi: 10.1099/00221287-132-11-3221. [DOI] [PubMed] [Google Scholar]
- ELDERING G., HORNBECK C., BAKER J. Serological study of Bordetella pertussis and related species. J Bacteriol. 1957 Aug;74(2):133–136. doi: 10.1128/jb.74.2.133-136.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E., Clarkson J. A. Reflections on the efficacy of pertussis vaccines. Rev Infect Dis. 1987 Sep-Oct;9(5):866–883. doi: 10.1093/clinids/9.5.866. [DOI] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Sauer K. T., Myers G. A., Cowell J. L., Guerrant R. L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect Immun. 1983 Jun;40(3):1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idigbe E. O., Parton R., Wardlaw A. C. Rapidity of antigenic modulation of Bordetella pertussis in modified Hornibrook medium. J Med Microbiol. 1981 Nov;14(4):409–418. doi: 10.1099/00222615-14-4-409. [DOI] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
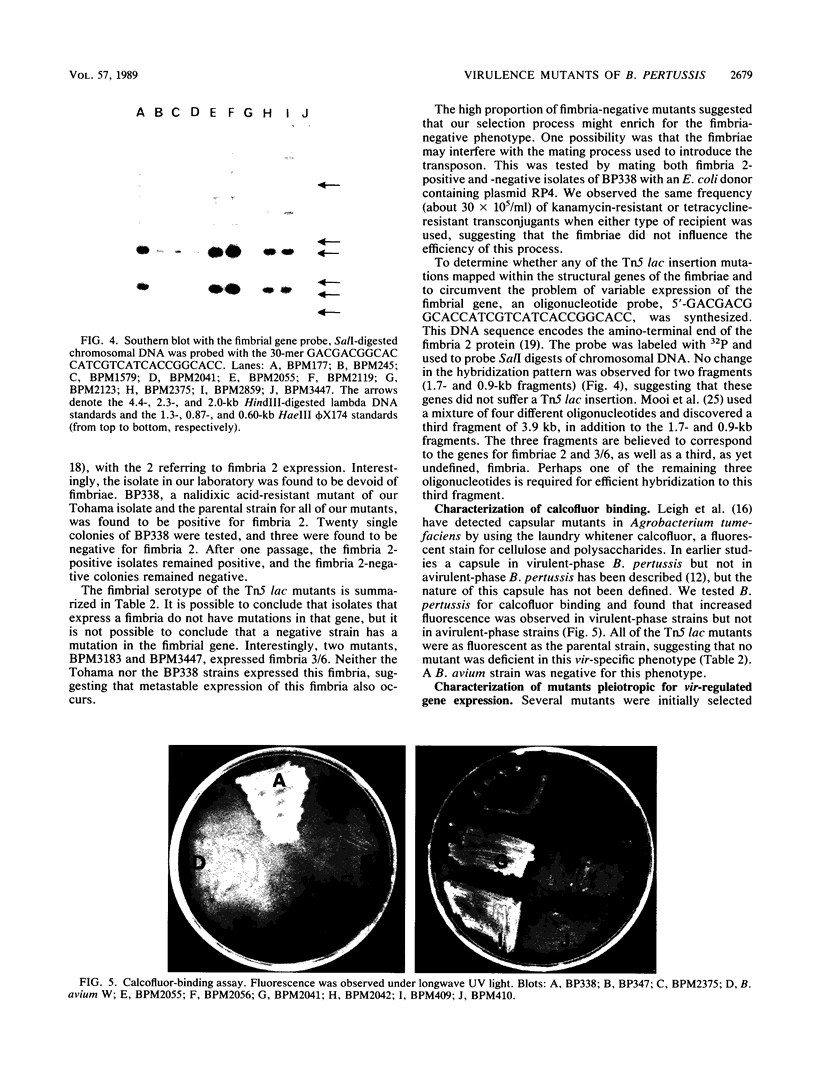
- Knapp S., Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988 Nov;170(11):5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Brennan M. J., David J. L., Carter P. H., Cowell J. L., Manclark C. R. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect Immun. 1988 Dec;56(12):3184–3188. doi: 10.1128/iai.56.12.3184-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Cowell J. L., Brennan M. J., Burns D. L., Manclark C. R. Agglutinating monoclonal antibodies that specifically recognize lipooligosaccharide A of Bordetella pertussis. Infect Immun. 1988 Mar;56(3):699–702. doi: 10.1128/iai.56.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livey I., Duggleby C. J., Robinson A. Cloning and nucleotide sequence analysis of the serotype 2 fimbrial subunit gene of Bordetella pertussis. Mol Microbiol. 1987 Sep;1(2):203–209. doi: 10.1111/j.1365-2958.1987.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Munoz J. J., Keith J. M. Detection of subunits of pertussis toxin in Tn5-induced Bordetella mutants deficient in toxin biological activity. Infect Immun. 1987 May;55(5):1309–1313. doi: 10.1128/iai.55.5.1309-1313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaraz J. A., Novotny P., Ivanyi J. Identification of a 68-kilodalton protective protein antigen from Bordetella bronchiseptica. Infect Immun. 1985 Mar;47(3):744–751. doi: 10.1128/iai.47.3.744-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van der Heide H. G., ter Avest A. R., Welinder K. G., Livey I., van der Zeijst B. A., Gaastra W. Characterization of fimbrial subunits from Bordetella species. Microb Pathog. 1987 Jun;2(6):473–484. doi: 10.1016/0882-4010(87)90054-4. [DOI] [PubMed] [Google Scholar]
- Muller A. S., Leeuwenburg J., Pratt D. S. Pertussis: epidemiology and control. Bull World Health Organ. 1986;64(2):321–331. [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect Immun. 1985 Oct;50(1):199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston N. W., Surapatana N., Carter E. J. A reappraisal of serotype factors 4, 5 and 6 of Bordetella pertussis. J Hyg (Lond) 1982 Feb;88(1):39–46. doi: 10.1017/s0022172400069874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANDFAST A. F. Some factors influencing the virulence for mice of Bordetella pertussis by the intracerebral route. Immunology. 1958 Apr;1(2):123–134. [PMC free article] [PubMed] [Google Scholar]
- Spears P. A., Schauer D., Orndorff P. E. Metastable regulation of type 1 piliation in Escherichia coli and isolation and characterization of a phenotypically stable mutant. J Bacteriol. 1986 Oct;168(1):179–185. doi: 10.1128/jb.168.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W., Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene. 1986;50(1-3):133–140. doi: 10.1016/0378-1119(86)90318-5. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Robbins K., Barrera O., Corwin D., Koomey J. M. Gene conversion involving the pilin structural gene correlates with pilus+ in equilibrium with pilus- changes in Neisseria gonorrhoeae. Cell. 1986 Oct 24;47(2):267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Genetic analysis of phase change in Bordetella pertussis. Infect Immun. 1984 Jan;43(1):263–269. doi: 10.1128/iai.43.1.263-269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Falkow S. Plasmid transfer to Bordetella pertussis: conjugation and transformation. J Bacteriol. 1982 Oct;152(1):549–552. doi: 10.1128/jb.152.1.549-552.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]