Abstract
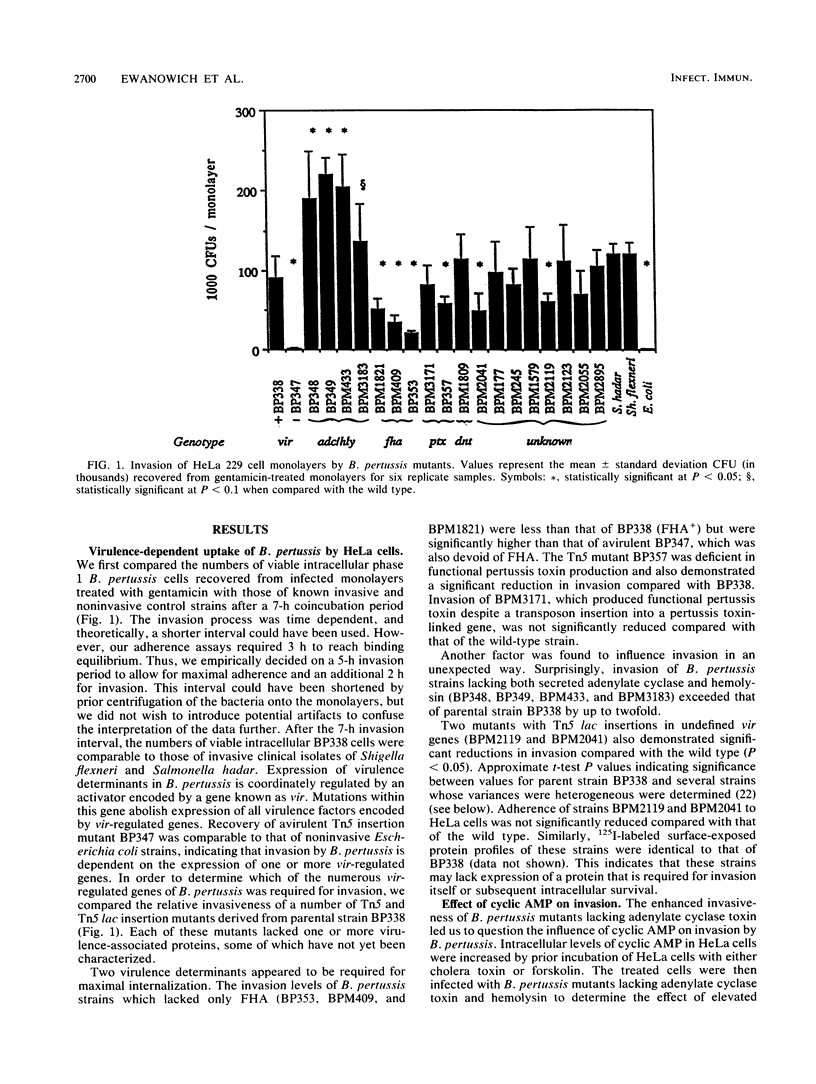
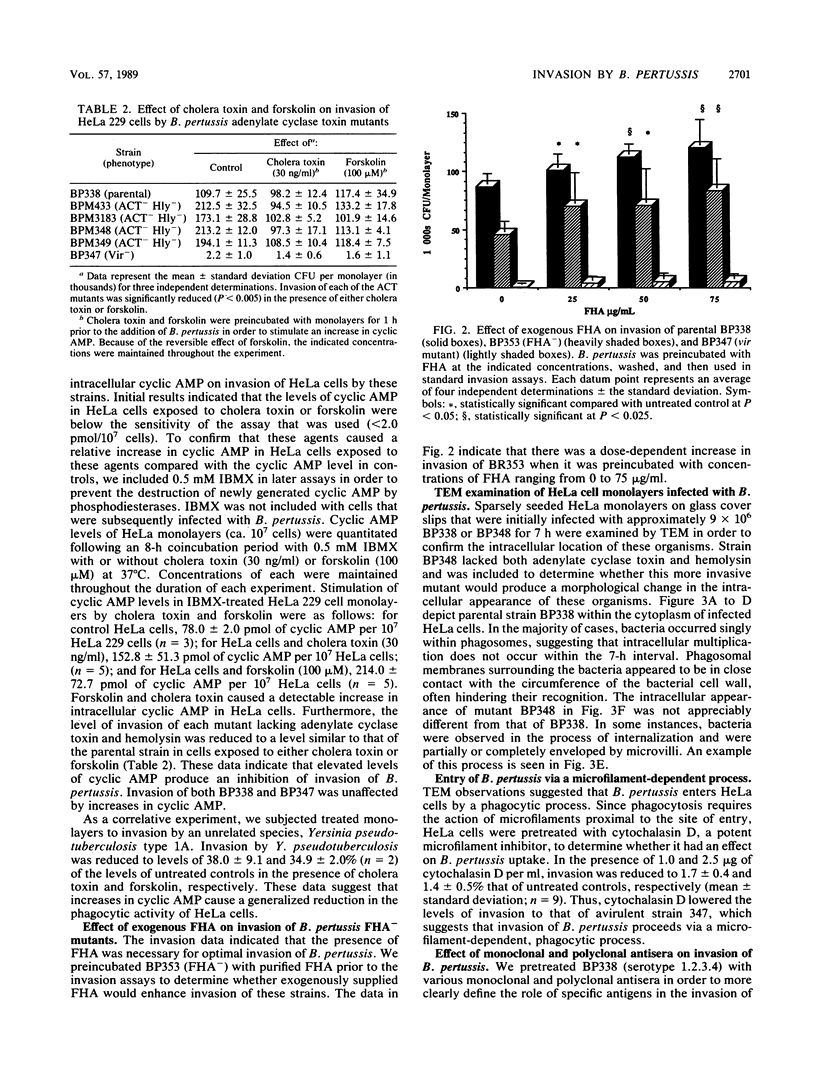
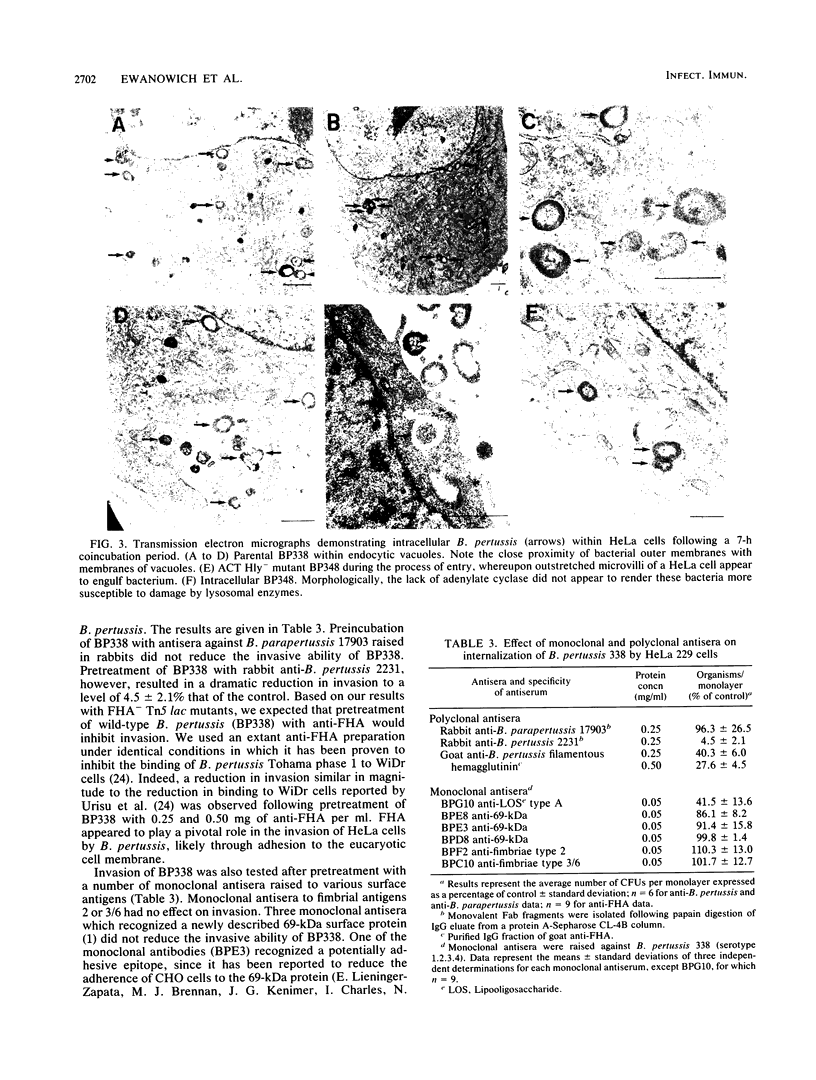
Phase-dependent invasive behavior of Bordetella pertussis was demonstrated by recovery of viable organisms from gentamicin-treated HeLa cell monolayers and by transmission electron microscopy. Several mutants of B. pertussis with Tn5 or Tn5 lac inserted into various vir-regulated genes were evaluated for differences in their invasive abilities. Mutants lacking filamentous hemagglutinin, pertussis toxin, and two as yet uncharacterized vir-regulated products had levels of invasion significantly lower than that of the parent strain BP338. In contrast, invasion by mutants lacking adenylate cyclase toxin was significantly increased compared with that of wild-type B. pertussis. This increase in invasion was eliminated when concentrations of intracellular cyclic 3'-5' AMP were stimulated by treating HeLa cells with cholera toxin or forskolin. Entry of B. pertussis occurred through a microfilament-dependent phagocytic process, as evidenced by the marked reduction in uptake following treatment of HeLa cells with cytochalasin D. Invasion was inhibited with polyclonal anti-B. pertussis and anti-filamentous hemagglutinin antisera. In addition, a monoclonal antibody against lipooligosaccharide A reduced uptake by 65.5%. The preservation of HeLa cell integrity and the limited replication of intracellular bacteria suggest that invasion may represent a means by which B. pertussis evades an active host immune response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD J. G., FISHEL C. W. Growth of Bordetella pertussis in tissue culture. J Bacteriol. 1959 Apr;77(4):465–474. doi: 10.1128/jb.77.4.465-474.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Gray D. F. Macrophage behaviour during the complaisant phase of murine pertussis. Immunology. 1969 Dec;17(6):875–887. [PMC free article] [PubMed] [Google Scholar]
- Confer D. L., Eaton J. W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982 Sep 3;217(4563):948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- Cox J. P., Karnovsky M. L. The depression of phagocytosis by exogenous cyclic nucleotides, prostaglandins, and theophylline. J Cell Biol. 1973 Nov;59(2 Pt 1):480–490. doi: 10.1083/jcb.59.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Sherburne R. K., Man S. F., Peppler M. S. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989 Apr;57(4):1240–1247. doi: 10.1128/iai.57.4.1240-1247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Fiederlein R. L., Glasser L., Galgiani J. N. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect Immun. 1987 Jan;55(1):135–140. doi: 10.1128/iai.55.1.135-140.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman B. L., Wardlaw A. C., Stevenson L. Q. Bordetella pertussis-induced hyperinsulinaemia without marked hypoglycaemia: a paradox explained. Br J Exp Pathol. 1981 Oct;62(5):504–511. [PMC free article] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. F., Cheers C. The steady state in cellular immunity. II. Immunological complaisance in murine pertussis. Aust J Exp Biol Med Sci. 1967 Aug;45(4):417–426. doi: 10.1038/icb.1967.40. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell J. W., Holt L. B., Desombre T. R. An electron-microscope study of intracerebral infection of mice with low-virulence Bordetella pertussis. J Med Microbiol. 1972 Feb;5(1):154–157. doi: 10.1099/00222615-5-1-154. [DOI] [PubMed] [Google Scholar]
- KENDRICK P. A., NADOLSKI E. B., ELDERING G., BAKER J. Antigenic relationships of Haemophilus pertussis, the parapertussis bacillus, and Brucella bronchiseptica as shown by cross protection tests in mice. J Bacteriol. 1953 Aug;66(2):166–169. doi: 10.1128/jb.66.2.166-169.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Cowell J. L., Brennan M. J., Burns D. L., Manclark C. R. Agglutinating monoclonal antibodies that specifically recognize lipooligosaccharide A of Bordetella pertussis. Infect Immun. 1988 Mar;56(3):699–702. doi: 10.1128/iai.56.3.699-702.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSE S. I. STUDIES ON THE LYMPHOCYTOSIS INDUCED IN MICE BY BORDETELLA PERTUSSIS. J Exp Med. 1965 Jan 1;121:49–68. doi: 10.1084/jem.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz J., Bergman R. K. Histamine-sensitizing factors from microbial agents, with special reference to Bordetella pertussis. Bacteriol Rev. 1968 Jun;32(2):103–126. doi: 10.1128/br.32.2.103-126.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. The concept of pertussis as a toxin-mediated disease. Pediatr Infect Dis. 1984 Sep-Oct;3(5):467–486. doi: 10.1097/00006454-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Redhead K. An assay of Bordetella pertussis adhesion to tissue-culture cells. J Med Microbiol. 1985 Feb;19(1):99–108. doi: 10.1099/00222615-19-1-99. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Zhang Y. L., Roberson R., Acton B., Trollfors B., Tolson N., Shiloach J., Bryla D., Muir-Nash J., Koeller D. Clinical, metabolic, and antibody responses of adult volunteers to an investigational vaccine composed of pertussis toxin inactivated by hydrogen peroxide. J Pediatr. 1988 Nov;113(5):806–813. doi: 10.1016/s0022-3476(88)80005-2. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Urisu A., Cowell J. L., Manclark C. R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986 Jun;52(3):695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Melton A. R., Walker K. E., Andraos-Selim C., Meidl J. J. Use of the promoter fusion transposon Tn5 lac to identify mutations in Bordetella pertussis vir-regulated genes. Infect Immun. 1989 Sep;57(9):2674–2682. doi: 10.1128/iai.57.9.2674-2682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



