Abstract
Context:
Hypophysitis is a chronic inflammation of the pituitary gland that comprises an increasingly complex clinicopathological spectrum. Within this spectrum, lymphocytic and granulomatous hypophysitis are the most common forms, but newer variants have recently been reported.
Objective:
The aims of the study were to describe a new patient with IgG4-related hypophysitis, review the published literature, and provide diagnostic criteria.
Setting:
A 75-yr-old man presented with a 1-yr history of frontal headache. Initial studies revealed panhypopituitarism and a mass in both the sella turcica and the sphenoidal sinus. The patient underwent transphenoidal surgery, initiated high-dose prednisone followed by hormone replacement therapy, and was closely monitored for 3 yr.
Results:
Symptoms improved after prednisone, along with shrinkage of the pituitary and sphenoidal masses, but recurred when prednisone dose was lowered. Histopathology showed a marked mononuclear infiltrate in both the pituitary and sphenoidal specimens, mainly characterized by increased numbers of plasma cells. Many of the infiltrating plasma cells (>10 per high-power field) were IgG4-positive. Review of the literature identified 11 cases of IgG4-related hypophysitis (two diagnosed based on pituitary histopathology).
Conclusions:
We describe the first Caucasian patient with biopsy-proven IgG4-related hypophysitis and provide classification criteria for this disease.
Hypophysitis is a chronic inflammation of the pituitary gland of complex and still incompletely defined pathogenesis. It belongs to the group of nonhormone-secreting sellar masses, sharing with them comparable clinical presentation and radiographic appearance. These similarities often make it difficult to establish a diagnosis of certainty before pituitary surgery and pathological examination of the resected pituitary tissue (1). Nevertheless, more and more cases are diagnosed nowadays solely on clinical and imaging grounds.
Hypophysitis has been classified in a number of ways, none of them particularly useful to the clinician or the researcher. Classifications are based on anatomic location of the pituitary involvement, cause, and histopathological appearance (Table 1). Location distinguishes adenohypophysitis, infundibuloneurohypophysitis, or panhypophysitis depending on whether the clinical and radiological signs (and, more rarely available, the pathological findings) affect the anterior lobe, the posterior lobe and the stalk, or both.
Table 1.
Current classifications of hypophysitis
| Based on the anatomic location of pituitary involvement |
| Adenohypophysitis |
| Infundibuloneurohypophysitis |
| Panhypophysitis |
| Based on the histological appearance |
| Lymphocytic |
| Granulomatous |
| Xanthomatous |
| Necrotizing |
| IgG4 plasmacytic |
| Mixed forms (lymphogranulomatous, xanthogranulomatous) |
| Based on the cause |
| Primary (isolated or as part of a multiorgan systemic disease) |
| Secondary to |
| Sellar diseases (germinoma, Rathke cleft cyst, craniopharyngioma, pituitary adenoma) |
| Systemic diseases (Wegener's granulomatosis, tuberculosis, sarcoidosis, syphilis) |
| Injection of immunomodulatory drugs (CTLA-4 blocking antibody, interferon-α) |
The etiological classification identifies primary and secondary forms. Primary hypophysitis refers to the cases that do not currently have identifiable causes. Primary hypophysitis is the most common form of hypophysitis, has an autoimmune pathogenesis, and can occur in isolation or as part of a multiorgan disease (like polyglandular autoimmune syndromes and IgG-related systemic disease). Secondary hypophysitis includes the cases where a clear etiological agent can be identified [for example, the administration of immunomodulatory drugs like CTLA-4 blocking antibody (2) or interferon-α (3)], the cases where the inflammation of the pituitary is considered a reaction to sellar diseases (Rathke cleft cyst, craniopharyngioma, germinoma, and pituitary adenomas), and the cases where hypophysitis is part of a multiorgan systemic involvement (for example, Wegener's granulomatosis, tuberculosis, sarcoidosis, or syphilis).
When surgery of the pituitary gland is performed, the pituitary pathology reveals two more common forms (lymphocytic and granulomatous) and three rarer variants (xanthomatous, necrotizing, and plasma cell rich). Lymphocytic hypophysitis is the most common, with about 380 biopsy-proven patients published from 1962 to 2010 (4, 5). It is characterized by a marked infiltration of lymphocytes that populate the pituitary gland both in a diffuse fashion and occasionally with a focal formation. Lymphocytes are typically accompanied by scattered plasma cells, eosinophils, and fibroblasts, and in later disease stages by fibrosis. It is more common in women [female:male (F:M) ratio of 3:1], has a mean age at presentation of 38 (±15) yr, and uniquely presents in association with pregnancy and postpartum in about 40% of the women.
Granulomatous hypophysitis has been described in over 120 patients since 1908 (6). It shows a unique pathological appearance characterized by multinucleated giant cells that form true granulomas with palisading histiocytes, surrounded by numerous lymphocytes, mainly T cells, and some plasma cells. Like the lymphocytic form, it is more common in women (F:M ratio of 4:1), but presents at an older age (44 ± 16 yr) and is not associated with pregnancy.
Xanthomatous hypophysitis has been reported in 13 patients since 1998 (7). It is characterized by infiltration with foamy histiocytes and macrophages, accompanied by plasma cells, and lymphocytes. In chronic cases, fibrosis and acinar destruction are also seen (7). Xanthomatous hypophysitis is more common in women (F:M ratio of 3:1), has a mean age at presentation of 37 (± 16) yr, and lacks an association with pregnancy.
Necrotizing hypophysitis was reported in two patients in 1993 by the same medical center (8). The authors described two young men with central diabetes insipidus, partial hypopituitarism, and magnetic resonance imaging (MRI) features suggestive of hypophysitis (enlarged anterior pituitary and thickened stalk). Opening of the sellar floor released creamy, necrotic material and revealed involvement of pituitary lobes (both anterior and posterior) and the stalk up to the median eminence of the hypothalamus. Microscopic examination showed extensive necrosis surrounded by lymphoplasmacytic infiltration and fibrosis, with only scattered areas of glandular tissue remaining. No additional pathologically proven cases of necrotizing hypophysitis have been reported thus far. A third case appeared in the Japanese literature and described a 14-yr-old boy with a prolonged history of diabetes insipidus, but the diagnosis was established without pituitary biopsy, so it is less certain (9).1
A fifth pathological form of hypophysitis, characterized by an abundance of IgG4-producing plasma cells, has been reported during the last few years, first diagnosed on clinical grounds in 2004 (10) and then by pathology in 2007 (11). This form characterizes the disease called IgG4-related hypophysitis and is the topic of the present clinical case seminar.
Case Report
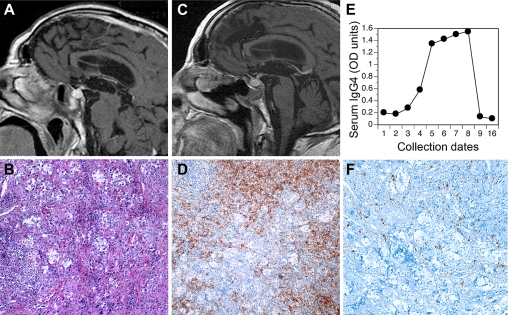
In January 2008, a 75-yr-old man was referred to our endocrine clinic for severe frontal headache of approximately 1-yr duration. Endocrine assessment revealed a complete hypopituitarism (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), characterized by central adrenal insufficiency (ACTH, 7 pg/ml; cortisol, 24 ng/ml), central hypothyroidism (TSH, 0.22 mU/liter; free T4, 4 pg/ml), central hypogonadism (LH, 0.3 mU/ml; testosterone, 0.1 ng/dl), decreased serum IGF-I (43 ng/ml), and borderline-low serum prolactin levels (2.3 ng/ml). Visual field perimetry was normal. Thyroglobulin, thyroperoxidase, adrenal, parietal cell, and transglutaminase antibodies were negative. The past medical history was unremarkable. Cranial MRI at that time showed a symmetrically enlarged pituitary gland that enhanced homogeneously and intensely after gadolinium injection (Fig. 1A). The pituitary stalk was thickened and also featured a small nodule near its proximal insertion. The physiological posterior pituitary bright spot could not be identified. In addition, mucosal thickening and a dense mass were noted within the sphenoidal sinus with thinning and erosion of the sellar floor. The sphenoidal mass enhanced avidly after gadolinium and showed small areas of necrosis (Fig. 1A). Based on the clinical and radiological findings, a diagnosis of autoimmune hypophysitis with associated sinusitis was suspected. Pituitary biopsy was scheduled after initiation of replacement therapy with cortisone acetate (30 mg/d) and L-thyroxine (75 μg/d).
Fig. 1.
Pituitary MRI and histopathology, highlighting a marked lymphoplasmacytic infiltration, rich in IgG4-producing cells, and serum IgG4 levels. A, Baseline (January 2008) T1-weighted magnetic resonance image showing enlargement of the pituitary gland, thickening of the stalk, with a depressed and eroded floor of the sella, and a mass in the sphenoidal sinus. B, Pituitary hematoxylin and eosin, 40×. C, MRI performed approximately 2.5 yr after the second surgery (July 2010) showing persistence of the pituitary inflammation. D, Pituitary CD138 (a marker for plasma cells), 40×. E, ELISA to detect serum IgG4 at multiple time points. The numbers on the x-axis correspond to the date IDs reported in Supplemental Table 1. F, Pituitary IgG4, 64×.
In February 2008, a transphenoidal sampling of the pituitary mass was attempted for diagnostic purposes. Upon entering the sphenoidal sinus, a very dark mass of about 1 cm in diameter was noted, mimicking the features of a fungal infection. The mass was biopsied, but the sellar floor was left intact for fear of spreading a possible infection. No clear evidence of communication between the sphenoidal sinus and the sella was seen during surgery. Pathology showed a marked mononuclear cell infiltrate, composed mainly of plasma cells and lymphocytes that diffusely populated edematous fragments of respiratory mucosa (Supplemental Fig. 1A). No infection or granulomas were seen. Cultures for bacteria and fungi were negative.
Immediately after surgery, cortisone acetate was replaced by high-dose glucocorticoids: prednisone 40 mg/d, tapered to 10 mg/d over a 4-wk period. The patient responded very well to the high-dose glucocorticoids. When the prednisone dose fell below 10 mg/d, however, headache recurred. Prednisone was increased to 15 mg/d, and a second transphenoidal surgery was recommended to clarify the nature of the pituitary mass.
In May 2008, the sellar floor was opened to obtain a pituitary biopsy, which showed a marked mononuclear infiltrate of the anterior pituitary with only a few islands of acinar cells remaining (Fig. 1B). Phenotypic characterization of the pituitary biopsy specimen revealed an abundance of CD138+ plasma cells (Fig. 1D), accompanied by CD20+ B lymphocytes (data not shown) and smaller populations of CD68+ macrophages (data not shown), and CD3+ T lymphocytes (data not shown). Similar findings were observed in the sphenoidal sinus (Supplemental Fig. 1). Plasma cells were polyclonal, some expressing κ and others λ light chains (data not shown). Considering the abundance and polyclonality of the infiltrating plasma cells, an IgG4-related disease was suspected and investigated by assessing the expression of IgG4 on formalin-fixed sphenoidal and pituitary sections pretreated with antigen-retrieval solution (Dako, Carpinteria, CA). The primary antibody was a mouse IgG1 monoclonal antihuman IgG4 (clone HP6025, diluted 1:1250; Invitrogen, Carlsbad, CA); the secondary antibody was a goat polyclonal antimouse IgG (diluted 1:1000; Jackson ImmunoResearch, West Grove, PA). The number of IgG4-positive plasma cells was derived by averaging the plasma cells present in five high-power fields, i.e. in fields analyzed with a 40× lens and a 10× eyepiece. Discrete IgG4-producing plasma cells were detected in both pituitary (Fig. 1F) and sphenoidal masses (Supplemental Fig. 1), representing about 15% of the total CD138+ plasma cells and accounting in some areas for more than 10 IgG4-positive cells per high-power field. These findings led us to establish a diagnosis of IgG4-related hypophysitis and paranasal sinusitis.
Shortly after the second surgery, the patient developed polydipsia and polyuria (4500 ml in 24 h) with decreased urine osmolality (200 mOsm/liter), corrected by desmopressin administration (0.15 mg/d). In the ensuing follow-up, headache recurred occasionally in the fall and winter of 2008, requiring reescalation of prednisone doses, but eventually prednisone could be tapered and suspended in April 2009. Since that time, the patient resumed replacement therapy with cortisone acetate and continued L-thyroxine and desmopressin therapy. At the last MRI study in September 2010, a moderate enlargement and inflammation of the pituitary gland persisted (Fig. 1C).
Pituitary antibodies were assessed by indirect immunofluorescence and found negative at all time points (Supplemental Table 1). Total serum IgG4 was measured with an in-house assay, developed because the volume of serum available was limited. ELISA plates were coated overnight with diluted (1:100) patient serum, then reacted with the same anti-IgG4 monoclonal antibody used for immunohistochemistry. Binding of the primary antibody was detected by the addition of a rabbit polyclonal antibody against mouse IgG1, conjugated to alkaline phosphatase (MP Biomedicals, Solon, OH). Results were expressed as absolute OD units, rather than as milligrams per deciliter, due to the lack of a standard curve. IgG4 levels were overall low, but showed a marked increase during a period of clinical recurrence (date IDs 5 through 8 in Fig. 1E and Supplemental Table 1). At the last follow-up visit, serum IgG4 was measured by nephelometry and found to be normal (40 mg/dl, date ID 16 in Fig. 1E).
Discussion
We report a well-characterized case of biopsy-proven IgG4-related hypophysitis. The disease is typically part of a multifocal systemic disease now called “IgG4-related autoimmune disease” (12) to emphasize the contribution of IgG4 in establishing the diagnosis.
IgG4, the least abundant of the four IgG antibodies, has long been associated with autoimmune and allergic diseases. For example, thyroperoxidase antibodies in Hashimoto thyroiditis (13) and BP180 antibodies in bullous pemphigoid (14) are enriched for the IgG4 subclass. Nevertheless, the antigens recognized by IgG4 antibodies in patients with IgG4-related diseases remain largely unknown. It is actually safe to say that IgG4 antibodies were ignored for diagnostic purposes until 2001 when Hamano et al. (15) linked them to autoimmune pancreatitis. This link has revolutionized our understanding of autoimmune pancreatitis, now called IgG4-related autoimmune (or sclerosing) pancreatitis (16), and made it possible to recognize that many diseases associated with autoimmune pancreatitis share similar pathological features, thus defining the existence of a multifocal systemic disease.
IgG4-related diseases are characterized by the infiltration of the target organ with numerous IgG4-producing plasma cells and lymphocytes (16). The number of IgG4-producing plasma cells and the associated pathological features vary depending upon the organ involved and, perhaps more significantly, on the type of specimen examined (biopsy vs. resection). For autoimmune pancreatitis, for example, the Mayo Clinic criteria (17) and the Japan-Korea criteria (18) require more than 10 IgG4-producing plasma cells per high-power field, whereas in China this number is now 50 (16). No guidelines are available for what this number should be in extrapancreatic lesions of the IgG4-related disease.
We have devised five criteria to establish a diagnosis of IgG4-related hypophysitis (Table 2). Criterion 1 is the presence of a mononuclear cell infiltrate rich in plasma cells within the pituitary gland and alone is sufficient to establish the diagnosis. Clearly this criterion is available only in a minority of patients, and properly so because pituitary surgery should be reserved to extreme cases given that the disease is highly responsive to steroids. As far as the number of IgG4-producing plasma cells, we suggest a cutoff of 10, in keeping with the Mayo Clinic and Japan-Korea criteria and with what has been reported for IgG4-related meningitis, another plasma cell-rich lesion of the central nervous system (19). When pituitary histopathology is not available, pituitary imaging (criterion 2) and the presence of IgG4 lesions in other organs (criterion 3) are sufficient to establish the diagnosis. When histopathology of other organs is not available, then imaging (criterion 2), increased serum IgG4 levels (criterion 4), and prompt response to glucocorticoids (criterion 5) can be used to establish a diagnosis of IgG4-related hypophysitis.
Table 2.
Diagnostic criteria for IgG4-related hypophysitis
| Criterion 1: Pituitary histopathology |
| Mononuclear infiltration of the pituitary gland, rich in lymphocytes and plasma cells, with more than 10 IgG4-positive cells per high-power field |
| Criterion 2: Pituitary MRI |
| Sellar mass and/or thickened pituitary stalk |
| Criterion 3: Biopsy-proven involvement in other organs |
| Association with IgG4-positive lesions in other organs |
| Criterion 4: Serology |
| Increased serum IgG4 (>140 mg/dl) |
| Criterion 5: Response to glucocorticoids |
| Shrinkage of the pituitary mass and symptom improvement with steroids |
| Diagnosis of IgG4-related hypophysitis is established when any of the following is fulfilled: |
| Criterion 1 |
| Criteria 2 and 3 |
| Criteria 2, 4, and 5 |
IgG4-related hypophysitis was first reported in 2004 in a 66-yr-old woman with multiple pseudotumors in the salivary glands, pancreas, and retroperitoneum (10) (ID 1 in Table 3), and then more extensively described in 2006 in a 70-yr-old man with swelling of the salivary glands caused by a marked infiltration with lymphocytes and IgG4-positive plasma cells (20). This patient (ID 2 in Table 3) had increased serum levels of IgG4, secondary hypogonadism, and enlargement and hyperintensity of the pituitary stalk, so that a diagnosis of IgG4-related hypophysitis was suspected, although not proven because the patient declined a biopsy of the pituitary gland. High-dose prednisone promptly corrected the submandibular swelling, reduced the stalk size, normalized the hypogonadism, and decreased serum IgG4 levels. It is very likely that previous cases of IgG4-related hypophysitis exist, published under different names. For example, pathologically proven cases of inflammatory pseudotumor of the pituitary (21, 22) resemble the pathology of IgG4-related hypophysitis, but a connection with IgG4 diseases was not established in those articles. In addition, a few other cases are reported in abstract form in Japan, where the disease appears to be more prevalent (23).
Table 3.
Summary of the key clinical features of 12 patients with IgG4-related autoimmune hypophysitis
| ID | First author, year (Ref.) | Patient's age (yr), gender, nationality | Clinical presentation | Laboratory and MRI | Diagnosis based on criterion | Involvement of other organs | Outcome | Follow-up time |
|---|---|---|---|---|---|---|---|---|
| 1 | Van der Vliet, 2004 (10) | 66, F, not reported | Nausea, headache, fever, swelling of salivary glands | Secondary hypothyroidism. Increased serum IgG4. Biopsy of submandibular mass showed lymphoid infiltration and fibrosis. Patient had a history of biopsy-proven autoimmune pancreatitis. CT scan showed a mass in the sella turcica, a mass in the upper lobe of the right lung, and a retroperitoneal mass. | 2 and 3 | Yes: sialadenitis, autoimmune pancreatitis, retroperitoneal fibrosis | Rapid clinical and radiological response to prednisone (60 mg/d) | 1 yr |
| 2 | Yamamoto, 2006 (20) | 70, M, Japanese | Swelling of salivary glands, sexual dysfunction | Secondary hypogonadism. Increased serum IgG4. Submandibular gland biopsy showed infiltration of lymphocytes and IgG4-positive plasma cells. Cranial MRI showed enlargement and hyperintensity of the pituitary stalk. | 2 and 3 | Yes: sialadenitis | Rapid response to prednisolone (40 mg/d) | Not noted |
| 3 | Tanabe, 2006 (25) | 71, M, Japanese | Fatigue, weight loss, polydipsia, swelling of salivary glands | Panhypopituitarism. Increased serum IgG4. Cranial MRI showed swelling of the pituitary gland. Abdominal CT revealed a retroperitoneal mass. Biopsy of salivary and abdominal masses showed fibrosis and chronic inflammation with infiltration of lymphocytes and IgG4-positive plasma cells. | 2 and 3 | Yes: sialadenitis and retroperitoneal fibrosis | Marked improvement with hydrocortisone (30 mg/d) and T4 replacement therapy | Not noted |
| 4 | Ralli, 2007 (24) | 67, M, not reported | Generalized weakness | Panhypopituitarism. Increased serum IgG4. Abdominal CT showed a pancreatic mass that upon biopsy was autoimmune pancreatitis. Cranial MRI showed enlargement and mass-like appearance of the infundibulum. Anterior pituitary was mildly enlarged. | 2, 4, and 5 | Yes: autoimmune pancreatitis | Dramatic improvement in symptoms after prednisone initiation (40 mg/d) | Not noted |
| 5 | Wong, 2007 (11) | 77, M, Chinese | Blurred vision | Secondary hypogonadism. Increased serum IgG4. Cranial MRI showed a pituitary mass extending above the sella and compressing the optic chiasm. Abdominal CT showed a mass in the pancreas and the gall bladder. Pathological exam of the pituitary, pancreatic, and gall bladder masses showed chronic inflammation with infiltration of lymphocytes and IgG4-positive plasma cells. | 1 | Yes: autoimmune pancreatitis, sclerosing cholangitis | Not noted | Not noted |
| 6 | Isaka, 2008 (26) | 55, M, Japanese | Fatigue, polyuria/polydipsia, nasal discharge | Central diabetes insipidus. Mild anterior pituitary dysfunction. Cranial MRI showed thickening of the infundibulum and loss of the posterior pituitary bright spot. Cranial CT showed a mass in the sphenoidal, maxillary, and frontal sinuses. Abdominal CT showed a mass around the abdominal aorta. Paranasal mass was biopsied and showed chronic inflammation with lymphocytes and IgG4-positive plasma cells. | 2 and 3 | Yes: retroperitoneal fibrosis, IgG4-related paranasal sinusitis | Prompt improvement after prednisone (50 mg/d) | Not noted |
| 7 | Tsuboi, 2008 (27) | 62, M, Japanese | Fatigue, weight loss, fever, joint stiffness | Central hypocortisolism, hypothyroidism, and diabetes insipidus. Cranial MRI showed enlarged and hyperintense pituitary stalk. CT scan showed bilateral pulmonary infiltrates, swollen pancreas, and retroperitoneal mass. Biopsy of the lung showed fibrosis and infiltration with lymphocytes and plasma cells, 23% of which were IgG4-positive. | 2 and 3 | Yes: interstitial lung disease, and likely retroperitoneal fibrosis | Glucocorticoid replacement corrected symptoms, and the pulmonary and retroperitoneal findings. Pituitary stalk and pancreas decreased in size | 8 months |
| 8 | Osawa, 2009 (28) | 74, F, Japanese | Fatigue, polydipsia | Panhypopituitarism, decreased visual acuity. Normal serum IgG4. Cranial MRI showed swelling of the infundibulum and the anterior pituitary, displacing the optic chiasm upward. Pituitary biopsy showed atrophic glandular tissue and massive infiltration of lymphocytes and plasma cells, some moderately positive for IgG4. | 1 | No | Marked improvement of symptoms after glucocorticoids | 15 months |
| 9 | Hori, 2010 (30) | 70, M, Japanese | Fatigue, thirst, nausea | Central diabetes insipidus and adrenal insufficiency. Increased serum IgG4. Cranial MRI showed enlarged infundibulum and posterior lobe with loss of the posterior pituitary bright spot. History of biopsy-proven sialadenitis, hepatitis, and interstitial lung disease. Specimens were stained for IgG4 and found to contain numerous IgG4-positive plasma cells. | 2 and 3 | Yes: sialadenitis, hepatitis, interstitial lung disease | Prompt response to hydrocortisone replacement therapy (15 mg/d). Then sialadenitis and interstitial pneumonia recurred and later improved spontaneously | 5 yr |
| 10 | Haraguchi, 2010, case 1 (29) | 74, F, Japanese | Fatigue, anorexia, polyuria, polydipsia | Panhypopituitarism, central diabetes insipidus. Increased serum IgG4. Cranial MRI showed swelling of the pituitary gland and stalk. | 2, 4, and 5 | No | Dramatic response to prednisone (30 mg/d) | 4 months |
| 11 | Haraguchi, 2010, case 2 (29) | 68, M, Japanese | Polyuria, polydipsia | Cranial MRI showed mass-like enlargement of the stalk, normal anterior pituitary. Three years later he developed leg edema caused by biopsy-proven retroperitoneal fibrosis. Four years later he developed headache and expansion of the retroperitoneal mass. | 2 and 3 | Yes: retroperitoneal fibrosis | Good response to prednisolone (30 mg/d) | 7 yr |
| 12 | Leporati, 2011, present case | 75, M, Caucasian | Headache | Panhypopituitarism. Cranial MRI showed enlarged anterior pituitary, thickened stalk, and a mass in the sphenoidal sinus. | 1 | Yes: IgG4-related paranasal sinusitis | Good response to prednisolone (40 mg/d) | 3 yr |
M, Male; F, female; CT, computed tomography.2
After two additional reports diagnosed on clinical and radiological grounds (24, 25), the first pathologically proven case of IgG4-related hypophysitis appeared in 2007 (11) (ID 5 in Table 3). It involved a senior male who presented nondescriptively with symptoms of pituitary expansion (blurred vision) and secondary hypogonadism, and a past medical history of autoimmune pancreatitis and sclerosing cholangitis. Due to the optic nerve compression, the patient underwent transphenoidal resection. Histopathology of the pituitary mass showed a dense lymphoplasmacytic infiltrate among residual nests of adenohypophyseal cells and fibrosis. Immunohistochemistry for IgG4 and κ/λ light chains highlighted numerous polyclonal plasma cells in the pituitary, pancreas, and gall bladder specimens. The key features of the remaining six published cases (26–30) of IgG4-related hypophysitis are summarized in Table 3.
Our patient (ID 12 in Table 3) showed a concomitant involvement of both pituitary and sphenoidal tissues, similar to the case described by Isaka et al. (26) (ID 6 in Table 3), to a case of inflammatory pseudotumor of the pituitary (22), and to a case of biopsy-proven lymphocytic/granulomatous hypophysitis reported by Amagasa and colleagues in 2001 (31). These authors postulated the existence of a defect in the dura mater and bony sellar floor, so that an inflammatory process could extend directly from the sella to the sphenoidal sinus or vice versa. In our patient, no macroscopic connections were noted during the first transphenoidal surgery, suggesting that the involvement of the sphenoidal sinus is yet another manifestation of the multifocal IgG4-related autoimmune disease.
Serum IgG4 levels are typically elevated in IgG4-related hypophysitis, but decrease upon initiation of glucocorticoid therapy and in later disease stages. One patient with biopsy-proven IgG4-related hypophysitis (ID 6 in Table 3) had normal serum IgG4 levels at diagnosis, as seen in our patient. These findings reflect variations in the autoimmune activity and emphasize the need for repeated IgG4 measurements. It remains difficult at this time to establish whether IgG4-related hypophysitis is distinct from lymphocytic hypophysitis or whether instead it represents a different pathological outcome of the same disease. The clinical and pathological features suggest to us a common autoimmune origin that later diverges into a lymphocytic-dominant or a plasmacytic-dominant phenotype depending upon the antigenic repertoire.
In conclusion, we report the first Caucasian patient with biopsy-proven IgG4-related hypophysitis and provide criteria that can be used to diagnose this condition before surgery. A correct preoperative diagnosis is critical because it can spare the patient from a major surgery for a disease that responds well to glucocorticoids.
Supplementary Material
Acknowledgments
The authors are grateful to Drs. Cristina Cupini, Rodolfo Fonte, and Enio Martino for the initial care of the patient.
This work was supported by National Institutes of Health Grant DK080351 (to P.C.).
Disclosure Summary: The authors have nothing to disclose.
A fourth patient with necrotizing hypophysitis was published while this article was being revised (32).
An additional patient with IgG4-related hypophysitis was published while this article was being revised (33).
- F:M
- Female:male
- MRI
- magnetic resonance imaging.
References
- 1. Howlett TA, Levy MJ, Robertson IJ. 2010. How reliably can autoimmune hypophysitis be diagnosed without pituitary biopsy. Clin Endocrinol (Oxf) 73:18–21 [DOI] [PubMed] [Google Scholar]
- 2. Blansfield JA, Beck KE, Tran K, Yang JC, Hughes MS, Kammula US, Royal RE, Topalian SL, Haworth LR, Levy C, Rosenberg SA, Sherry RM. 2005. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother 28:593–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tebben PJ, Atkinson JL, Scheithauer BW, Erickson D. 2007. Granulomatous adenohypophysitis after interferon and ribavirin therapy. Endocr Pract 13:169–175 [DOI] [PubMed] [Google Scholar]
- 4. Caturegli P, Lupi I, Landek-Salgado M, Kimura H, Rose NR. 2008. Pituitary autoimmunity: 30 years later. Autoimmun Rev 7:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. 2005. Autoimmune hypophysitis. Endocr Rev 26:599–614 [DOI] [PubMed] [Google Scholar]
- 6. Shi J, Zhang JM, Wu Q, Chen G, Zhang H, Bo WL. 2009. Granulomatous hypophysitis: two case reports and literature review. J Zhejiang Univ Sci B 10:552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burt MG, Morey AL, Turner JJ, Pell M, Sheehy JP, Ho KK. 2003. Xanthomatous pituitary lesions: a report of two cases and review of the literature. Pituitary 6:161–168 [DOI] [PubMed] [Google Scholar]
- 8. Ahmed SR, Aiello DP, Page R, Hopper K, Towfighi J, Santen RJ. 1993. Necrotizing infundibulo-hypophysitis: a unique syndrome of diabetes insipidus and hypopituitarism. J Clin Endocrinol Metab 76:1499–1504 [DOI] [PubMed] [Google Scholar]
- 9. Ogawa R. 1995. A child with necrotizing infundibulo-neurohypophysitis. Hormone to Rinsho 43:33–36 [Google Scholar]
- 10. van der Vliet HJ, Perenboom RM. 2004. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Ann Intern Med 141:896–897 [DOI] [PubMed] [Google Scholar]
- 11. Wong S, Lam WY, Wong WK, Lee KC. 2007. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol 38:1720–1723 [DOI] [PubMed] [Google Scholar]
- 12. Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. 2003. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 38:982–984 [DOI] [PubMed] [Google Scholar]
- 13. Kotani T, Kato E, Hirai K, Kuma K, Ohtaki S. 1986. Immunoglobulin G subclasses of anti-thyroid peroxidase autoantibodies in human autoimmune thyroid diseases. Endocrinol Jpn 33:505–510 [DOI] [PubMed] [Google Scholar]
- 14. Liu Z. 2002. Are anti-BP180 IgG1 or IgG4 autoantibodies pathogenic? J Invest Dermatol 119:989–990 [DOI] [PubMed] [Google Scholar]
- 15. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. 2001. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 344:732–738 [DOI] [PubMed] [Google Scholar]
- 16. Cheuk W, Chan JK. 2010. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol 17:303–332 [DOI] [PubMed] [Google Scholar]
- 17. Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB. 2006. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol 4:1010–1016; quiz 934 [DOI] [PubMed] [Google Scholar]
- 18. Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T, Nishimori I, Notohara K, Naruse S, Ko SB, Kihara Y. 2008. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol 43:403–408 [DOI] [PubMed] [Google Scholar]
- 19. Lindstrom KM, Cousar JB, Lopes MB. 2010. IgG4-related meningeal disease: clinico-pathological features and proposal for diagnostic criteria. Acta Neuropathol 120:765–776 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. 2006. A case of Mikulicz's disease (IgG4-related plasmacytic disease) complicated by autoimmune hypophysitis. Scand J Rheumatol 35:410–411 [DOI] [PubMed] [Google Scholar]
- 21. Al-Shraim M, Syro LV, Kovacs K, Estrada H, Uribe H, Al-Gahtany M. 2004. Inflammatory pseudotumor of the pituitary: case report. Surg Neurol 62:264–267; discussion 267 [DOI] [PubMed] [Google Scholar]
- 22. Hansen I, Petrossians P, Thiry A, Flandroy P, Gaillard RC, Kovacs K, Claes F, Stevenaert A, Piguet P, Beckers A. 2001. Extensive inflammatory pseudotumor of the pituitary. J Clin Endocrinol Metab 86:4603–4610 [DOI] [PubMed] [Google Scholar]
- 23. Shimatsu A, Oki Y, Fujisawa I, Sano T. 2009. Pituitary and stalk lesions (infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: an emerging clinical entity. Endocr J 56:1033–1041 [DOI] [PubMed] [Google Scholar]
- 24. Ralli S, Lin J, Farrell J. 2007. Autoimmune pancreatitis. N Engl J Med 356:1586. [DOI] [PubMed] [Google Scholar]
- 25. Tanabe T, Tsushima K, Yasuo M, Urushihata K, Hanaoka M, Koizumi T, Fujimoto K, Kubo K, Uehara T, Shigematsu S, Hamano H, Kawa S. 2006. IgG4-associated multifocal systemic fibrosis complicating sclerosing sialadenitis, hypophysitis, and retroperitoneal fibrosis, but lacking pancreatic involvement. Intern Med 45:1243–1247 [DOI] [PubMed] [Google Scholar]
- 26. Isaka Y, Yoshioka K, Nishio M, Yamagami K, Konishi Y, Inoue T, Hirano A, Hosoi M, Imanishi M. 2008. A case of IgG4-related multifocal fibrosclerosis complicated by central diabetes insipidus. Endocr J 55:723–728 [DOI] [PubMed] [Google Scholar]
- 27. Tsuboi H, Inokuma S, Setoguchi K, Shuji S, Hagino N, Tanaka Y, Yoshida N, Hishima T, Kamisawa T. 2008. Inflammatory pseudotumors in multiple organs associated with elevated serum IgG4 level: recovery by only a small replacement dose of steroid. Intern Med 47:1139–1142 [DOI] [PubMed] [Google Scholar]
- 28. Osawa S, Ogawa Y, Watanabe M, Tominaga T. 2009. Hypophysitis presenting with atypical rapid deterioration: with special reference to immunoglobulin G4-related disease-case report. Neurol Med Chir (Tokyo) 49:622–625 [DOI] [PubMed] [Google Scholar]
- 29. Haraguchi A, Era A, Yasui J, Ando T, Ueki I, Horie I, Imaizumi M, Usa T, Abe K, Origuchi T, Eguchi K. 2010. Putative IgG4-related pituitary disease with hypopituitarism and/or diabetes insipidus accompanied with elevated serum levels of IgG4. Endocr J 57:719–725 [DOI] [PubMed] [Google Scholar]
- 30. Hori M, Makita N, Andoh T, Takiyama H, Yajima Y, Sakatani T, Fukumoto S, Iiri T, Fujita T. 2010. Long-term clinical course of IgG4-related systemic disease accompanied by hypophysitis. Endocr J 57:485–492 [DOI] [PubMed] [Google Scholar]
- 31. Amagasa M, Yuda F, Kojima H, Noshita N, Sato S. 2001. Natural course of lymphocytic infundibuloneurohypophysitis. Clin Neuropathol 20:229–232 [PubMed] [Google Scholar]
- 32.Gutenberg A, Caturegli P, Metz I, Martinez R, Mohr A, Brück W, Rohde V. Necrotizing infundibulo-hypophysitis: an entity too rare to be true? Pituitary. 2011 Apr 10; doi: 10.1007/s11102-011-0307-2. 10.1007/s11102-011-0307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel SM, Szostek JH. 2011. IgG4-related systemic disease in a Native American man. Intern Med 50:931–934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.