Abstract
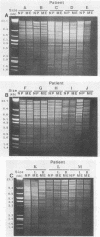
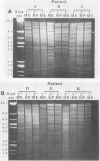
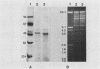
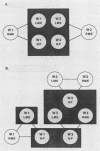
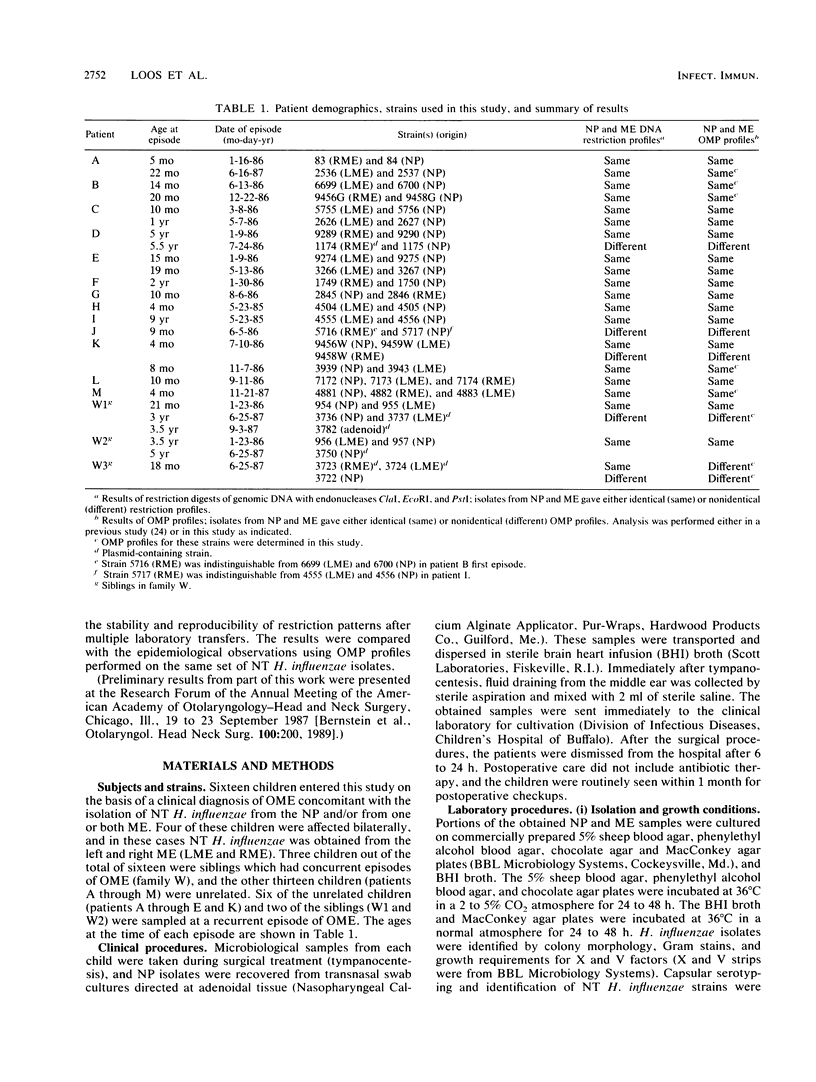
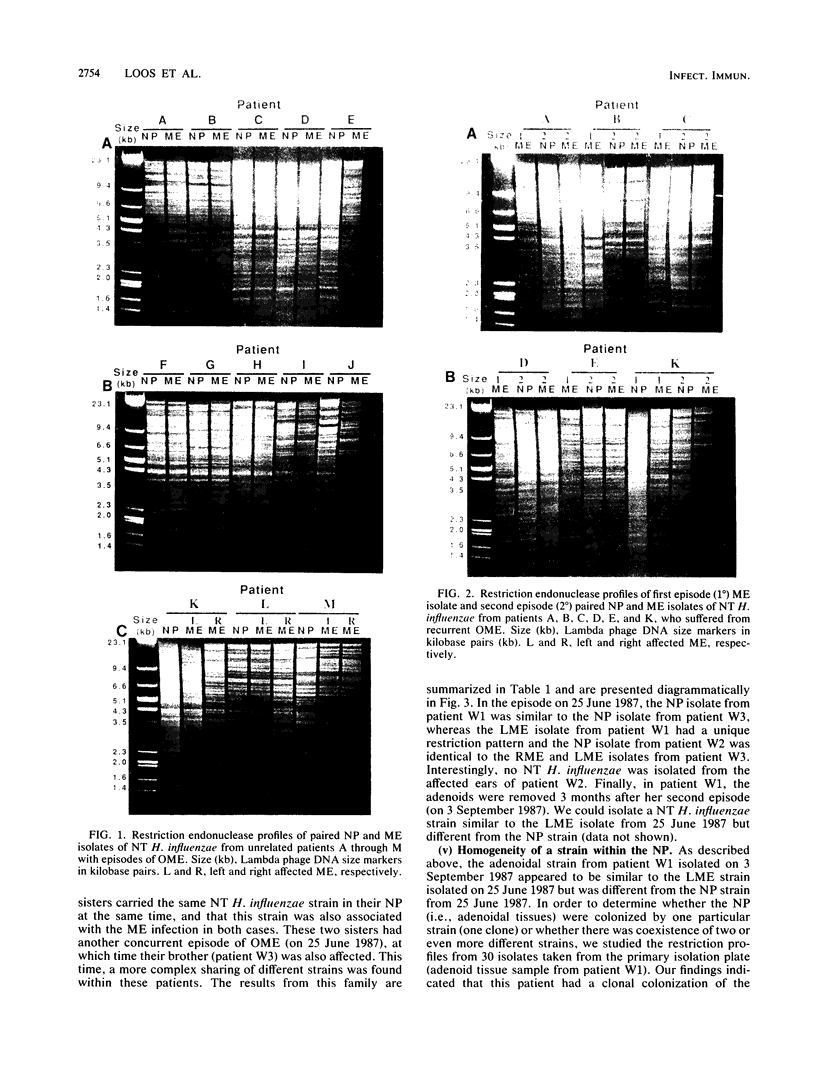
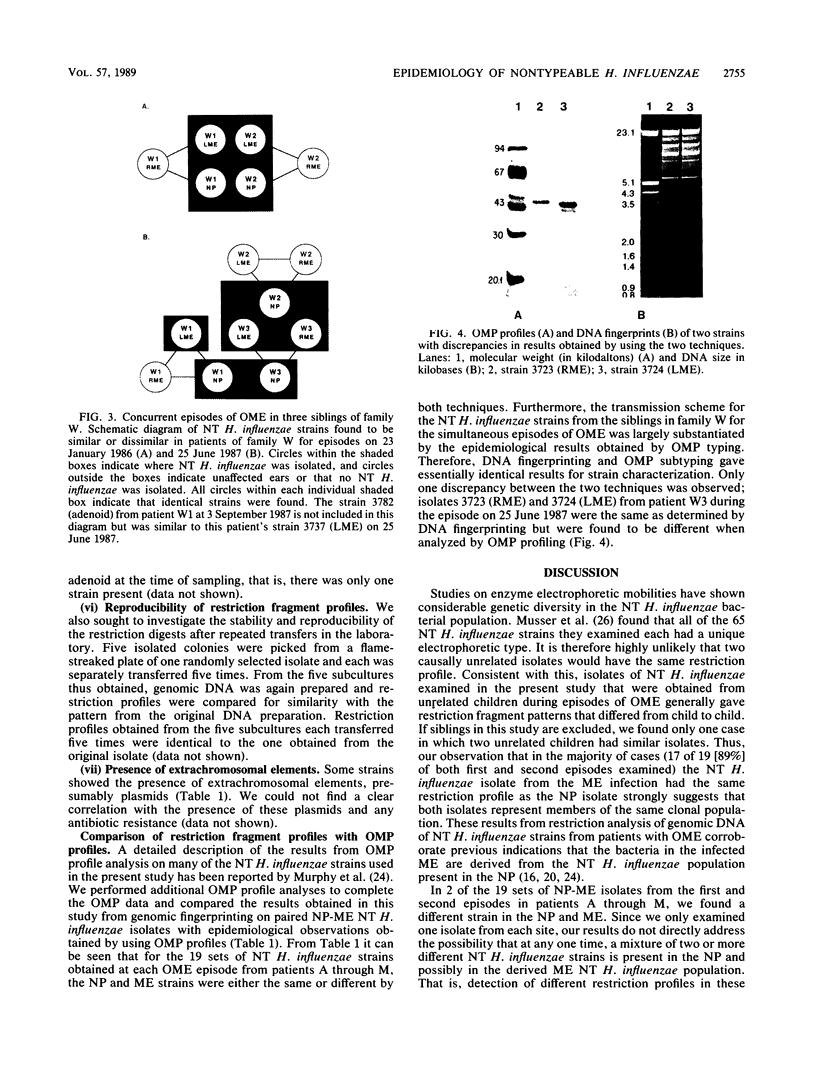
It is assumed that the causative bacteria in children suffering from otitis media reach the middle ear via the eustachian tube. The purpose of this investigation was to use endonuclease restriction of bacterial chromosomal DNA to compare isolates of nontypable (NT) Haemophilus influenzae obtained from the nasopharynx and from middle ear (ME) effusions of patients with otitis media. Strains of NT H. influenzae were isolated from the nasopharynx (NP) and affected ME from a group of 13 unrelated children with otitis media with effusion (OME). For 12 of these children, identical strains were isolated from the NP and ME in a first episode of OME. Each of these 12 sets differed from the other 11. Six of these children suffered from a second episode of OME with NT H. influenzae. Five of these children with recurrence again had identical NP and ME strains. These results suggest that at the time of an episode of OME, there is one predominant strain of NT H. influenzae that colonizes both the NP and ME. The strains of NT H. influenzae isolated from all six of the second episodes were different from strains from the first episode, indicating turnover of the predominant strain in the NT H. influenzae population between episodes. When we investigated three siblings with concurrent episodes of OME, we found that they shared several similar strains of NT H. influenzae, thereby demonstrating that within a family, transmission of NT H. influenzae from child to child is possible. These results from DNA fingerprinting were essentially identical when compared with results from outer membrane protein subtyping performed on the same set of strains. The analysis of endonuclease restriction patterns of total genomic DNA provides a sensitive measure of genetic dissimilarity between strains and represents an easily applicable method for epidemiological and transmission studies of bacterial infections associated with NT H. influenzae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austrian R., Howie V. M., Ploussard J. H. The bacteriology of pneumococcal otitis media. Johns Hopkins Med J. 1977 Sep;141(3):104–111. [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenkamp S. J., Shurin P. A., Marchant C. D., Karasic R. B., Pelton S. I., Howie V. M., Granoff D. M. Do children with recurrent Haemophilus influenzae otitis media become infected with a new organism or reacquire the original strain? J Pediatr. 1984 Oct;105(4):533–537. doi: 10.1016/s0022-3476(84)80415-1. [DOI] [PubMed] [Google Scholar]
- Campagnari A. A., Gupta M. R., Dudas K. C., Murphy T. F., Apicella M. A. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun. 1987 Apr;55(4):882–887. doi: 10.1128/iai.55.4.882-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J., Garcia-Tornel S., Musser J. M., Selander R. K., Smith A. L. Molecular Epidemiology of multiply resistant Haemophilus influenzae type b in day care centers. J Infect Dis. 1987 Sep;156(3):483–489. doi: 10.1093/infdis/156.3.483. [DOI] [PubMed] [Google Scholar]
- Coffey J. D., Jr Otitis media in the practice of pediatrics. Bacteriological and clinical observations. Pediatrics. 1966 Jul;38(1):25–32. [PubMed] [Google Scholar]
- Dickinson D. P., Loos B. G., Dryja D. M., Bernstein J. M. Restriction fragment mapping of Branhamella catarrhalis: a new tool for studying the epidemiology of this middle ear pathogen. J Infect Dis. 1988 Jul;158(1):205–208. doi: 10.1093/infdis/158.1.205. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Eijk P. P., Jansen H. M., Zanen H. C. Changes in outer membrane proteins of nontypable Haemophilus influenzae in patients with chronic obstructive pulmonary disease. J Infect Dis. 1988 Aug;158(2):360–365. doi: 10.1093/infdis/158.2.360. [DOI] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J., Kuklińska-Michalska D. Occurrence of Haemophilus influenzae strains in children with respiratory tract infections. Int J Pediatr Otorhinolaryngol. 1983 Dec;6(3):279–283. doi: 10.1016/s0165-5876(83)80129-3. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Collier A. M., Sanyal M. A., Watkins J. M., Fairclough D. L., Clyde W. A., Jr, Denny F. W. A longitudinal study of respiratory viruses and bacteria in the etiology of acute otitis media with effusion. N Engl J Med. 1982 Jun 10;306(23):1377–1383. doi: 10.1056/NEJM198206103062301. [DOI] [PubMed] [Google Scholar]
- Howie V. M., Ploussard J. H., Lester R. L., Jr Otitis media: a clinical and bacteriological correlation. Pediatrics. 1970 Jan;45(1):29–35. [PubMed] [Google Scholar]
- Jadavji T., Cheung R., Bannatyne R. M., Prober C. G. Rifampin alone or with trimethoprim for contacts of children with Haemophilus influenzae type b infections. CMAJ. 1986 Aug 15;135(4):328–331. [PMC free article] [PubMed] [Google Scholar]
- Kamme C., Nilsson N. I. Secretory otitis media: microbiology of the middle ear and the nasopharynx. Scand J Infect Dis. 1984;16(3):291–296. doi: 10.3109/00365548409070403. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Simonsen T., Spanne O., Lund V., Bjorvatn B. Isolates of Neisseria meningitidis from different sites in the same patient: phenotypic and genomic studies, with special reference to adherence, piliation, and DNA restriction endonuclease pattern. J Infect Dis. 1984 Sep;150(3):389–396. doi: 10.1093/infdis/150.3.389. [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., Oudbier J. H., Stuifbergen W. N., Jansz A., Zanen H. C. Application of whole-cell DNA restriction endonuclease profiles to the epidemiology of Clostridium difficile-induced diarrhea. J Clin Microbiol. 1987 Apr;25(4):751–753. doi: 10.1128/jcm.25.4.751-753.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. S., Henretig F. M., Teter M. J., McGowan K. L. Nasopharyngeal flora and acute otitis media. Infect Immun. 1983 Sep;41(3):987–991. doi: 10.1128/iai.41.3.987-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Antigenic heterogeneity of outer membrane proteins of nontypable Haemophilus influenzae is a basis for a serotyping system. Infect Immun. 1985 Oct;50(1):15–21. doi: 10.1128/iai.50.1.15-21.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Apicella M. A. Nontypable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev Infect Dis. 1987 Jan-Feb;9(1):1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Bernstein J. M., Dryja D. M., Campagnari A. A., Apicella M. A. Outer membrane protein and lipooligosaccharide analysis of paired nasopharyngeal and middle ear isolates in otitis media due to nontypable Haemophilus influenzae: pathogenetic and epidemiological observations. J Infect Dis. 1987 Nov;156(5):723–731. doi: 10.1093/infdis/156.5.723. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Dudas K. C., Mylotte J. M., Apicella M. A. A subtyping system for nontypable Haemophilus influenzae based on outer-membrane proteins. J Infect Dis. 1983 May;147(5):838–846. doi: 10.1093/infdis/147.5.838. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober C. G., Ipp M. M., Bannatyne R. M. Haemophilus influenzae type b in a nursery school: the value of biotyping. Pediatrics. 1982 Feb;69(2):215–218. [PubMed] [Google Scholar]
- Scherer S., Stevens D. A. Application of DNA typing methods to epidemiology and taxonomy of Candida species. J Clin Microbiol. 1987 Apr;25(4):675–679. doi: 10.1128/jcm.25.4.675-679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R., Rodriguez W. J., Khan W. N., Ross S. Acute purulent otitis media in children older than 5 years. Incidence of Haemophilus as a causative organism. JAMA. 1977 Sep 5;238(10):1032–1033. [PubMed] [Google Scholar]
- Shurin P. A., Howie V. M., Pelton S. I., Ploussard J. H., Klein J. O. Bacterial etiology of otitis media during the first six weeks of life. J Pediatr. 1978 Jun;92(6):893–896. doi: 10.1016/s0022-3476(78)80355-2. [DOI] [PubMed] [Google Scholar]
- Teele D. W., Klein J. O., Rosner B. A. Epidemiology of otitis media in children. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):5–6. doi: 10.1177/00034894800890s304. [DOI] [PubMed] [Google Scholar]
- Wald E. R., Rohn D. D., Chiponis D. M., Blatter M. M., Reisinger K. S., Wucher F. P. Quantitative cultures of middle-ear fluid in acute otitis media. J Pediatr. 1983 Feb;102(2):259–261. doi: 10.1016/s0022-3476(83)80536-8. [DOI] [PubMed] [Google Scholar]
- Wren B. W., Tabaqchali S. Restriction endonuclease DNA analysis of Clostridium difficile. J Clin Microbiol. 1987 Dec;25(12):2402–2404. doi: 10.1128/jcm.25.12.2402-2404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]