Abstract
Despite ongoing eradication efforts, bovine tuberculosis (BTB) remains a challenge in Michigan livestock and wildlife. The objectives of this study were to (1) review the epidemiology of BTB in Michigan cattle, privately owned cervids, and wildlife between 1975 and 2010 and (2) identify important lessons learned from the review and eradication strategies. BTB information was accessed from the Michigan BTB Eradication Project agencies. Cattle herds (49), privately owned deer herds (4), and wild white-tailed deer (668) were found infected with BTB during the review period. BTB has occurred primarily in counties located at the northern portion of the state's Lower Peninsula. Currently used BTB eradication strategies have successfully controlled BTB spread. However additional changes in BTB surveillance, prevention, and eradication strategies could improve eradication efforts.
1. Introduction
After fifteen years of implementing the Michigan Bovine Tuberculosis Eradication Project, bovine tuberculosis (BTB) remains a challenge in Michigan livestock and wildlife. At least one BTB-infected cattle herd has been identified in Michigan annually since 1998. Because of this ongoing BTB challenge, regulatory requirements for cattle movements have affected cattle trade in Michigan. In addition, the state has spent approximately US$200 million on BTB eradication between 1994 and 2010 [1]. Annually, over US$7 million is spent on BTB surveillance in cattle alone. Additional resources are spent on indemnity payment, cleaning and disinfection of the premises of BTB infected cattle herds, wildlife surveillance, and implementation of other eradication strategies. Reviewing the epidemiology of the current BTB issues in Michigan could help advance BTB eradication strategies in Michigan, other regions of the country, or beyond.
BTB is a chronic bacterial disease caused by Mycobacterium bovis. M. bovis is primarily a pathogen of cattle but can also infect other mammals including humans. Among domestic animals, cattle are the primary reservoir. However, other animal species including deer, monkeys, European badgers, brush tailed opossums, and elephants have been shown to become endemically infected, thus serving as additional reservoirs for pathogen transmission [2].
BTB is mainly a respiratory disease and is transmitted primarily through aerosols [2, 3]. However, indirect transmission through ingestion of contaminated food items has been demonstrated in deer and cattle and is believed to be a major source of transmission in the current BTB outbreak in Michigan [4–7]. Although wildlife and domestic cattle commonly do not come in close physical contact with each other, transmission of M. bovis between domestic animals and wildlife has occurred over the years [8]. Domestic animals as well as wildlife are significant reservoir hosts for human tuberculosis, caused by M. bovis. The most common means humans acquire BTB is through the consumption of unpasteurized or insufficiently cooked animal products from BTB-infected animal [9].
At the end of the twentieth century, tuberculosis was the leading killer of humans in the United States (US). During this time period, M. bovis was found to be distinct from M. tuberculosis and there was evidence that M. bovis could be passed between animals and humans, and that in humans, M. bovis produces symptoms that were clinically indistinguishable from M. tuberculosis [10]. Due to the public health and economic relevance of BTB, the US BTB Eradication Program began in 1917. The program included comprehensive testing of imported and US bred cattle, improved animal tracking, destroying skin test positive animals (reactors), strengthening meat inspection for tuberculosis lesions, and commercialization of milk pasteurization. This program proved highly effective in controlling the disease, and by the 1960s, the number of BTB-reactor cattle detected in the US had markedly declined [10].
In 1974, the last known BTB-infected cattle herd in Michigan was depopulated; however a BTB positive wild white-tailed deer was harvested by a hunter in the following year [11]. It was widely believed that the deer acquired the M. bovis infection as a spill-over from livestock. With no further identified cases of BTB in cattle, the state acquired BTB “accredited-free” status in 1979. At that time the extent of BTB in Michigan wildlife was unknown. A second BTB positive wild white-tailed deer was identified in 1994, nine miles from the location of the index case [11]. With this occurrence, the Michigan Bovine TB Eradication Project began in 1995. The project was charged with increasing BTB surveillance in wildlife as well as in cattle and privately owned cervid (captive or farmed deer) herds surrounding any identified BTB wild white-tailed deer. The project involves a multiagency team of experts from the Michigan Department of Agriculture (MDA), the US Department of Agriculture Animal and Plant Health Inspection Services (USDA APHIS), Michigan Department of Natural Resources (MDNR), Michigan State University (MSU), and Michigan Department of Community Health (MDCH). Wildlife surveys conducted in the spring and fall of 1995 detected further cases among wild white-tailed deer. In 1998, cases of BTB infection began to reemerge among cattle herds in the state. As a consequence, Michigan lost its BTB “accredited-free” status in June 2000 and dropped to a “modified accredited” status [12]. Despite active eradication efforts, cases of BTB are still found in Michigan cattle, privately owned cervids, and wild white-tailed deer.
The objective of this study was to conduct a comprehensive descriptive epidemiological review of BTB in cattle, privately owned cervids, and wild white-tailed deer from 1975 to July 2010. Although previous epidemiological studies on BTB in Michigan have evaluated BTB challenge in cattle [13], privately owned cervid herds[14], wildlife [15–21], or both livestock and wildlife [22], none has provided extensive descriptive account of BTB challenge in both cattle and wildlife alongside with the lessons learned since over 15 years of eradication efforts. By reviewing and understanding the epidemiology of the current disease problem combined with a review of the strategies that have been implemented to eradicate the disease, important “lessons learned” can be identified, and new control strategies may emerge.
2. Materials and Methods
2.1. Sources of Data
Descriptive data on BTB in Michigan between 1995 and July 2010 were obtained from the partners of the Michigan BTB Eradication Project. Data on BTB infected cattle and privately owned cervid herds were obtained from MDA and USDA APHIS Veterinary Services (USDA APHIS VS). Data on BTB-infected wild white-tailed deer and other wildlife were obtained from Michigan Department of Natural Resources (MDNR) and USDA APHIS Wildlife Services (USDA APHIS WS).
2.2. Type of Data
The collected data comprised of host characteristics, geographical, and temporal distributions of the BTB-infected animals/herds. The host characteristics included type of herd operations (cattle), type of deer, origin of the infected animals into the herds, and herd size. The geographical distribution was limited to the county level. Annual records of BTB infection in animals/herds were used for temporal distribution. Additional information collected was the type of surveillance used to identify each BTB infected animal/herd, the type of BTB eradication used in each infected herd, the results of the epidemiological evaluation of the infected herds, and the various BTB eradication strategies/policies utilized by the Michigan BTB Eradication Project partners.
2.3. Data Analyses
The occurrence of BTB in cattle herds, privately owned cervids, and wild white-tailed deer was expressed either as incidence count, percentage proportion, incidence rate, herd prevalence, sample prevalence, or prevalence odds. Incidence count represented the total number of BTB-infected herds within the review period. Incidence rate was calculated as incidence count divided by the total population per year. In cattle herds, 12.5 years was used for the review time period (1998–July 2010). Captive cervid herd BTB prevalence was calculated as the number of BTB positive deer divided by the total number of deer in the herd. A BTB positive deer was classified as any deer bearing gross lesions consistent with BTB that tested positive for M. bovis on culture. Sample prevalence was calculated as the number of BTB positive wild white-tailed deer divided by the total number wild white-tailed deer tested. Prevalence odds of BTB infected wild white-tailed deer in an area were calculated as the probability that tested wild deer in the area were BTB positive (sample prevalence (p)) divided by the probability that the tested wild deer were not BTB positive (1-p).
3. Results
3.1. Area Description
The state of Michigan is located in the Upper Midwest region of the US. The state is made up of 83 counties and comprises two peninsulas: the Upper Peninsula (UP) and the Lower Peninsula (LP) (Figure 1). Michigan covers approximately 37 million acres. There are approximately 43,000 miles of rivers and streams, 11,000 inland lakes, and over 4,500 miles of shoreline along the Great Lakes. A variety of forest, wetland, and grasslands provide habitat to over 15,000 native species of insects, 1,815 native species of vascular plants, and 691 native species of animals. Among animal species, 68 different native wild mammals have been identified including white-tailed deer (Odocoileus Virginianus), elk (Cervus elaphus nelson), black bear (Ursus americanus), coyotes (Canis latrans), opossum (Didelphis virginiana), bobcats (Lynx rufus), and red fox (Vulpes vulpes fulva) [23].
Figure 1.
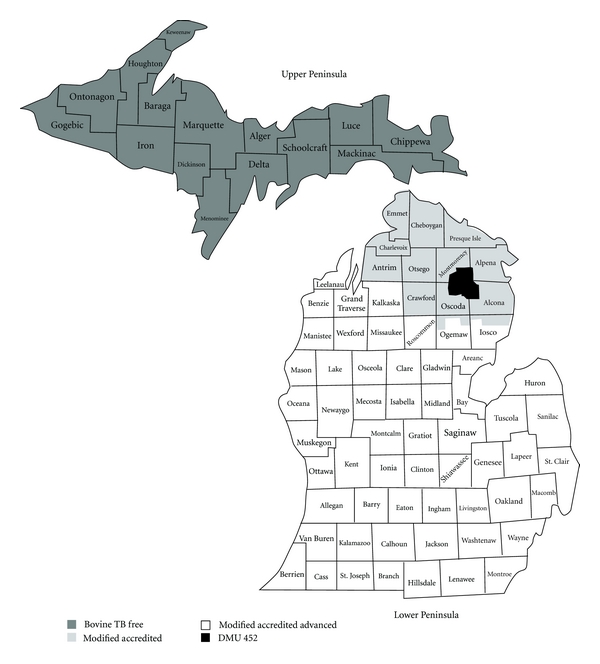
State of Michigan map showing the Upper and Lower Peninsula, counties, and BTB zones as of December 2009.
Livestock production is a significant part of the state's economy. Cattle are the most common livestock in the state and include dairy, cow-calf (beef), and feedlot operations. The 2007 agricultural census reported approximately 14,500 cattle herds in Michigan [24]. The cattle herd types (dairy, beef, mixed, and feedlot) and the total number of herds in BTB-affected counties are reported in Table 1. Other common domesticated livestock species include small ruminants such as sheep and goats, horses, swine, and poultry [24].
Table 1.
Descriptive epidemiology of BTB in MI cattle, privately owned and wild white-tailed deer (1975–July 2010).
| County | BTB (+) cattle herdsa | Total cattle herdsb | BTB Incidence rate/1000 cattle herd-yrsa | BTB (+) privately owned cervid herdsa | BTB (+) wild white-tailed deerc | Total wild white-tailed deer testedc | Prevalence odds of BTB (+) wild white-tailed deerc |
|---|---|---|---|---|---|---|---|
| Alcona | 13 | 119 | 8.7 | 0 | 240 | 18,451 | 0.0132 |
| Alpena | 21 | 231 | 7.3 | 0 | 186 | 18,776 | 0.0100 |
| Montmorency | 4 | 87 | 3.7 | 3 | 130 | 12,027 | 0.0109 |
| Oscoda | 3 | 80 | 3.0 | 0 | 74 | 9,624 | 0.0077 |
| Presque Isle | 2 | 140 | 1.1 | 1 | 13 | 9,404 | 0.0014 |
| Antrim | 3 | 98 | 2.4 | 0 | 1 | 5,133 | 0.0002 |
| Emmet | 3 | 135 | 1.8 | 0 | 2 | 3,413 | 0.0006 |
| Othersd | 0 | 13,564 | 0 | 0 | 22 | 107,441 | 0.0002 |
aData 1998–July 2010. bData from the 2007 Michigan Agricultural census [24]. cData 1975–July 2010. dOther counties in Michigan.
3.2. Cattle
3.2.1. BTB Surveillance in Cattle
Surveillance for BTB is primarily done through live animal skin testing and through tuberculosis lesion detection at slaughter facilities [25]. Common reasons for live animal testing include herd accreditation/reaccreditation, compliance with pasteurized milk ordinance (PMO) laws, herd surveillance in endemic areas of the state as required by Memorandums of Understanding with USDA ASPHIS VS, and tracing animals with lesions found on routine slaughter surveillance.
During herd surveillance, the caudal fold tuberculin (CFT) test is done on individual animals as a primary screening test [25]. All respondents (suspects) to the CFT and herd of origin are quarantined pending final classification as to whether any suspect animal is BTB infected or not. The suspects are subjected to a supplemental test, either the comparative cervical tuberculin (CCT) test or gamma interferon (γ-IFN) assay. An animal classified as CCT or γ-IFN positive responder either is designated as a “reactor” or remains a “suspect”. A “suspect” animal is retested with the γ-IFN assay within 30 days or the CCT after 60 days, and if the animal is not “negative”, it is automatically designated a “reactor”. Reactors are purchased for diagnostic purposes, humanely euthanized, and necropsied by a veterinary pathologist [25].
At necropsy, the animal is visually inspected for possible BTB lesions. Samples are taken from lymph nodes of the head, thorax, and abdomen, as well as from all visible BTB-like lesions and submitted for further testing. Histopathological screening, acid-fast staining of tissues, polymerase chain reaction (PCR), and bacterial culture are tests that are routinely conducted on these samples. Either PCR or bacterial culture is the confirmatory test in BTB screening. If BTB is confirmed in an animal, its herd of origin is declared BTB-infected and will remain under quarantine; otherwise the herd is released from quarantine [25].
3.2.2. BTB Eradication in Cattle
In BTB-infected herds, there are two disease eradication options for the herd. One is complete herd depopulation. The other is to develop a whole herd testing and removal plan with the cooperation of the owner and governmental agencies while the herd remains under quarantine (test and remove program). This plan includes the serial performance of BTB ante mortem screening tests over time and the subsequent removal of all test positive animals as outlined in the Bovine Tuberculosis Eradication: Uniform Methods and Rules [26, 27]. The herd is released from quarantine when testing reveals a BTB negative herd after a minimum of 8 whole herd tests over approximately a 4-year period as outlined in the 1999 Uniform Methods and Rules [26]. However, USDA APHIS VS typically did not utilize a test and remove program as outlined in the 1999 Uniform Methods and Rules until Michigan found an infected dairy herd in 2000. Most infected dairy herds prefer this program to depopulation as it allows for continuation of operations and cash flow. However, depopulation remains the disease eradication of choice in most infected beef herds.
3.2.3. BTB-Infected Cattle Herds
BTB in cattle herds reemerged in Michigan in 1998 after the last known case of BTB in the state was depopulated in 1974. Comprehensive details of this BTB incident and key policy changes are detailed in Table 2. Between 1998 and July 2010, the USDA APHIS VS recorded a total of 49 BTB-infected cattle herds in Michigan. At least one BTB cattle herd has been identified yearly since 1998 with higher numbers of herds found during the period from 2000 through 2003, and the highest peak in 2001 with 8 BTB-infected herds discovered (Figure 2). Since 2004, the number of infected herds has fluctuated between 1 and 4 herds/year. The average number of cattle per BTB-infected beef and dairy herd was 84 and 147, respectively. The average size of BTB-infected herds was larger than the average Michigan herd size of 14 and 130 cattle per beef and dairy herd respectively [24]. The number of cattle in BTB infected herds ranged from 6 to 495 (Figure 3). Included in these 49 infected herds are six premises which were removed from quarantine and then discovered to be reinfected at a later date. These reinfected herds either had completed the test and remove program successfully (n = 1) or were depopulated (n = 5).
Table 2.
Timeline of BTB in Michigan (1975–July 20010).
| Time | Event |
|---|---|
| 1974 | (i) Last known BTB-infected cattle herd in Michigan depopulated |
| 1975 | (i) BTB-infected wild white-tailed deer harvested by a hunter in Alcona County |
| 1979 | (i) State of Michigan designated as BTB-accredited free |
| 1994 | (i) BTB-infected wild white-tailed deer harvested by a hunter in Alpena County |
| 1995 | (i) BTB surveillance of hunter killed wild white-tailed deer, cattle, and privately owned cervid herds in 16 km radius around location of 1994 BTB infected wild white-tailed deer was initiated |
| (ii) MDNR conducted BTB surveillance in wild white-tailed deer within portions of Alcona, Alpena, Montmorency, and Oscoda counties (Deer Management Unit (DMU) 452) | |
| (iii) 18 of 403 (4.47%) wild white-tailed deer found infected with BTB | |
| (iv) Testing of all cattle and privately owned cervid herds located within 5 miles of any BTB positive wild white-tailed deer initiated | |
| 1996 | (i) Statewide BTB surveillance in wild white-tailed deer and other wildlife began |
| (ii) MDNR expanded BTB surveillance in wild white-tailed deer beyond DMU 452 to include all of Alcona, Alpena, Montmorency, and Oscoda counties | |
| (iii) Disease Control Permits issued | |
| (iv) 56 of 4,966 (1.13%) wild white-tailed deer found infected with BTB | |
| (v) 1 coyote found infected with BTB | |
| 1997 | (i) The 1st privately owned white-tailed deer herd found infected with BTB |
| (ii) 73 of 3,720 (1.96%) wild white-tailed deer found infected with BTB | |
| (iii) 2 coyotes found infected with BTB | |
| 1998 | (i) 3 beef cattle herds found infected with BTB |
| (ii) State of Michigan's BTB-free status suspended | |
| (iii) BTB testing of all cattle and cervid herds in 5-county area initiated | |
| (iv) Deer feeding banned, baiting restricted, and doe harvest increased in an Enforced Restricted Area (ERA) bordered by interstate road (I-75), state road (M-55), and shoreline of Lake Huron | |
| (v) DMU 452 expanded to encompass 5-county area (Alcona, Alpena, Montmorency, Oscoda, Presque Isle counties) | |
| (vi) Antlerless hunting permits issued liberally (1 per day) in the DMU 452 | |
| (vii) 78 of 9,057 (0.86%) wild white-tailed deer found infected with BTB | |
| (viii) 1 bear, 2 raccoons, and 2 coyotes found infected with BTB | |
| 1999 | (i) Baiting regulation initiated in the northeastern Lower Peninsula of the state |
| (ii) Unlimited antlerless hunting permits made available in the DMU 452 | |
| (iii) BTB testing for movement from any cattle herds East of I-75 and North of M-55 initiated | |
| (iv) 1 beef cattle herd found infected with BTB | |
| (v) 58 of 19,496 (0.3%) wild white-tailed deer found infected with BTB | |
| 2000 | (i) Baiting/feeding of Deer and Elk banned in counties with BTB positive wild white-tailed deer |
| (ii) Statewide official ear tag identification of cattle initiated | |
| (iii) Statewide BTB testing of all cattle herds by the end of 2003 initiated | |
| (iv) 2 dairy and 5 beef cattle herds found infected with BTB | |
| (v) State status dropped to Modified Accredited | |
| (vi) 53 of 25,858 (0.2%) wild white-tailed deer found infected with BTB | |
| 2001 | (i) DMU 452 redefined to what it was in 1996 but the area shifted slightly east from the original 1996 DMU 452 |
| (ii) USDA APHIS began fencing project on BTB high-risked cattle farms | |
| (iii) 8 beef cattle herds found infected with BTB | |
| (iv) 61 of 24,278 (0.25%) wild white-tailed deer found infected with BTB | |
| 2002 | (i) BTB program changed: |
| (a) Alcona, Alpena, Montmorency, Presque Isle counties—annual herd test of all cattle herds except feedlots was initiated. A negative BTB test required for movement of sexually active cattle if >6 months from whole herd test (WHT) | |
| (b) Cheboygan, Crawford, Iosco, Ogemaw, Oscoda, Otsego counties—Biennial WHT of all cattle herds except feedlots was initiated. A negative BTB test required for movement of sexually active cattle if >6 months from WHT | |
| (c) Antrim, Arenac, Charlevoix, Emmet, Gladwin, Kalkaska, Roscommon counties—2 WHT to be completed between 2000 and 2003. A negative BTB test required for movement of sexually active cattle if >6 months from WHT | |
| (i) Emmet County began annual WHT of all cattle herds except feedlots | |
| (ii) Antlerless hunting permits were increased for the northeast of the state | |
| (iii) 1 mixed, 2 dairy, and 4 beef cattle herds found infected with BTB | |
| (iv) 51 of 18,100 (0.28%) wild white-tailed deer found infected with BTB | |
| 2003 | (i) Statewide WHT completed |
| (ii) 2 dairy and 4 beef cattle herds found infected with BTB | |
| (iii) 32 of 17,302 (0.18%) wild white-tailed deer found infected with of BTB | |
| 2004 | (i) State of Michigan acquired split state status: Modified Accredited Zone (MAZ) and Modified Accredited Advanced Zone (MAAZ) |
| (ii) The gamma interferon assay approved for follow-up testing of caudal fold test suspects | |
| (iii) BTB program changed. | |
| (a) Annual WHT of all cattle herds in MAZ except feedlots initiated. Negative TB test for movement of sexually intact cattle if >60 days from WHT | |
| (b) Rest of Michigan-stratified random surveillance of 1500 herds every two years was initiated | |
| (iii) 2 dairy cattle herds found infected with BTB | |
| (iv) 28 of 15,131 (0.19%) wild white-tailed deer found infected with BTB | |
| 2005 | (i) Upper Peninsula part of the state elevated to BTB-Free status |
| (ii) 3 beef cattle herds found infected with BTB | |
| (iii) 16 of 7,364 (0.22%) wild white-tailed deer found infected with BTB | |
| 2006 | (i) The 2nd privately owned deer herd found infected with BTB |
| (ii) 2 dairy, 1 mixed, and 1 beef cattle herd found infected with BTB | |
| (iii) 41 of 7,914 (0.52%) wild white-tailed deer found infected with BTB | |
| 2007 | (i) Implementation of official electronic identification ear tags mandatory for all cattle within the state. |
| (ii) Annual WHT of feedlots within the MAZ implemented | |
| (iii) 1 dairy and 2 beef cattle herds found infected with BTB | |
| (iv) 27 of 8,316 (0.32%) wild white-tailed deer found infected with BTB | |
| 2008 | (i) One time WHT of all cattle herds located in Arenac, Clare, Gladwin, Grand Traverse, Iosco, Kalkaska, Missaukee, Ogemaw, Osceola, Roscommon, and Wexford counties within 3 years initiated |
| (ii) A beef and a mixed cattle herd infected with BTB | |
| (iii) The 3rd privately owned deer herd found infected with BTB | |
| (iv) 37 of 16,309 (0.23%) wild white-tailed deer found infected with BTB | |
| 2009 | (i) 1 beef cattle herd found infected with BTB |
| (ii) The 4th privately owned deer herd found infected with BTB | |
| (ii) 31 of 5,722 (0.54%) wild white-tailed deer found infected with BTB | |
| July 2010 | (i) 3 beef cattle herds found infected with BTB |
| (ii) 6 of 306 (1.96%) wild white-tailed deer found infected with BTB | |
Figure 2.
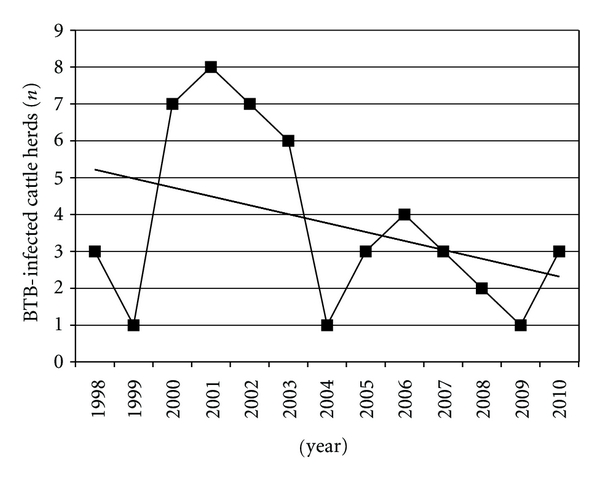
Annual incidence count of BTB cattle herds in Michigan (with linear trend line).
Figure 3.
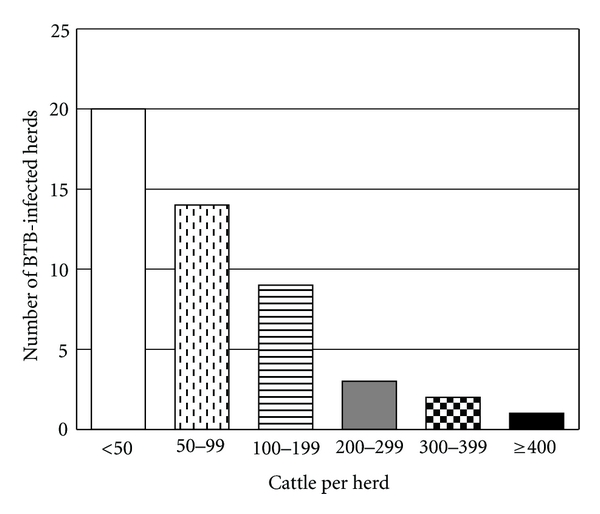
Herd sizes of BTB-infected cattle herds in Michigan, 1998–July 2010.
Geographically, BTB in cattle has only been found in 7 counties located in the Michigan's LP. The number of BTB-infected herds within the review period is represented in Figure 1. Within each county, the number of BTB-infected herds based on management operations is presented in Figure 4. The 6 reinfected herds comprised 1 dairy herd and 5 beef herds. The reinfected dairy herd was located in Montmorency County, while 3 beef herds were located in Alpena County, and 2 beef herds in Alcona County. Eleven herds had a previously BTB-infected herd located within a 3-mile radius: 6 were in Alcona county, 4 in Alpena, and 1 in Oscoda County. The BTB incidence rate per 1000 cattle herd-yrs ranged from 1.1 (Presque Isle) to 8.7 (Alcona) (Figure 5). The overall number of cattle herds in each county and those found infected with BTB is presented in Table 1.
Figure 4.
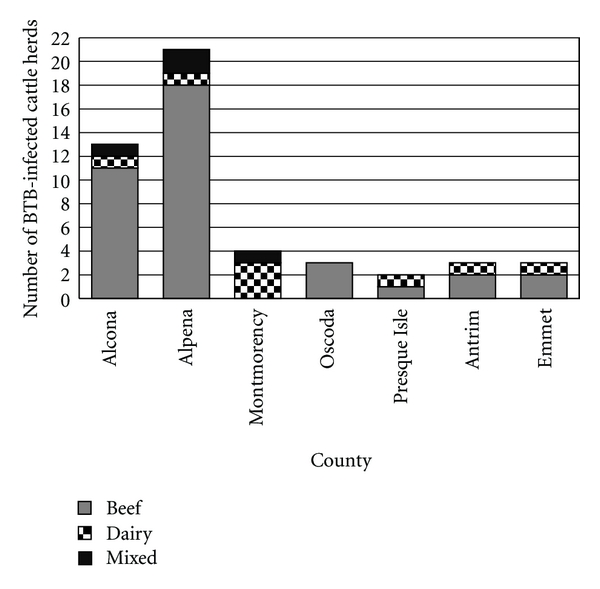
BTB incidence count in Michigan cattle herds by type, 1998–July 2010.
Figure 5.
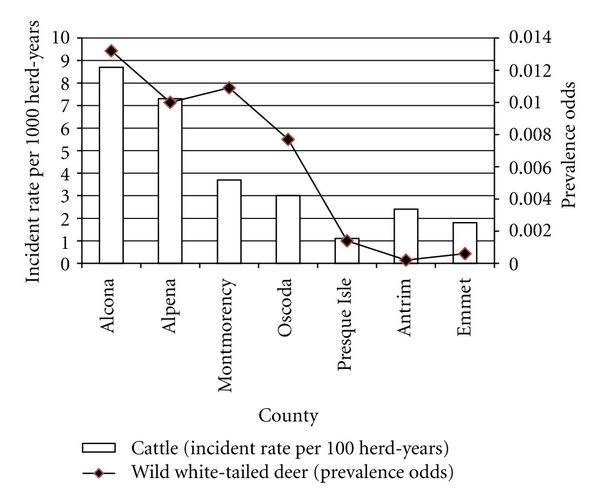
Prevalence odds and incident rate of BTB in Michigan wild white-tailed deer and cattle herds, respectively, 1975–July 2010.
Between 1995 and July 2010, the USDA and MDA have conducted approximately 35,000 whole herd tests implemented as a part of the Michigan Bovine TB Eradication Project. During these whole herd tests, 46 of the 49 BTB infected herds (94%) were identified. One herd was identified through each of the following: movement testing, slaughter surveillance, and trace testing (investigation of herds which moved cattle to and from BTB infected herds).
Of the 49 BTB infected herds, 47 herds had a history of purchasing or moving cattle into their herd prior to being found BTB infected (open herds). The other two herds had no history of introducing new animals into the herd prior to being found BTB infected (closed herds). In regards to BTB infected animals, 147 cattle presented at necropsy with gross lesions compatible with BTB (mode = 1, median = 1, and range = 0–32), 149 cattle had histopathological lesions compatible with M. bovis infections (mode = 1, median = 1, and range = 0–30), 104 cattle were confirmed BTB positive using PCR (mode = 1, median = 1, and range = 0–24), and 137 cattle were culture positive for M. bovis (mode = 1, median = 1, and range = 0–28). Out of 137 cattle confirmed through bacterial culture, 84% were raised on the farm, 7% were from unknown origin, and 9% were moved onto the farms. From the moved animals, 2 were purchased from other states, one from Ohio and the other from Texas; all others were moved through either intrastate purchases or leasing/borrowing of bulls.
The 5 most heavily BTB infected herds (greater numbers of M. bovis culture positive cattle) were located in the following counties: Alpena (28 cattle), Emmet (27 cattle), Alpena (9 cattle), Montmorency (9 cattle), and Presque Isle (6 cattle). These herds were each located in different counties and epidemiological investigations indicated that wildlife exposure was the most likely source of BTB transmission into the herd. The likely explanation for the higher disease prevalence in these herds is differences in individual herd management. Management practices that lead to closer confinement of cattle or sharing of focused feed and water sources are more likely to lead to intraherd transmission.
Cattle movement was believed to be the source BTB infection in the herd identified via trace testing. The source of BTB in the six infected herds located in Emmet (n = 3) and Antrim (n = 3) counties remains problematic. One herd brought no animals into the herd. Two herds brought in cattle from counties with a low prevalence of BTB in wild deer. The remaining three farms brought in cattle from counties with both high and low BTB prevalence in wild deer; however, on 1 farm the only BTB-affected animal originated from a BTB-free area. All cattle moving out of high prevalence areas were tested prior to movement for BTB with negative results. Regardless of location, all herds that were a source of livestock to BTB-affected farms had whole herd tests with no additional BTB found. In addition, all cattle farms adjoining BTB-affected farms were tested with no additional BTB-affected herds found. Finally, there is a low sample prevalence of BTB in wild white-tailed deer in these two counties (Figure 5). In one area with a high BTB prevalence in wild deer (Alcona County), five BTB infected herds had multiple possible routes of exposure. Each of the five herds shared fence-line contact with another BTB infected herd and moved animals between these herds, in addition to their location within an area with a high prevalence of BTB in wild white-tailed deer. The most probable source of BTB infection in each of these herds was not clear. Epidemiological reports on all other BTB-affected herds (n = 37) suspected white-tailed deer to be the source of infection as all source farms and adjoining farms were tested for BTB with negative results. Wild white-tailed deer had access to all farms with BTB infected herds. All but one BTB infected cattle farm had attributes attractive to deer. These attributes included apple trees, accessible stored feed, water sources, and woodlands providing cover.
As a control measure, 43 of the BTB cattle herds (88%) were depopulated, from which 5 premises became reinfected with BTB. All animals repopulating these farms had a negative test for BTB prior to entry onto the premises. Six (12%) herds entered a test-and-remove program, of which one dairy cattle herd became reinfected at two separate times. In this herd, the first reinfection occurred after the herd was removed from quarantine. This herd was again found infected on the final whole herd test of the test and remove program. The latest reinfection was not considered a separate infection because the herd was still quarantined at the time. Again, only BTB test negative animals were allowed onto these farms.
3.3. Privately Owned Cervids (Captive or Farmed Deer)
The USDA AHPIS VS and the MDA identified 4 privately owned white-tailed deer herds infected with BTB between 1975 and July 2010. The first incidence was reported in Presque Isle County in 1997; all others were in Montmorency County in 2006, 2008, and 2009. The first [28] and the second [29] infected herds were depopulated but due to inadequacy of indemnity funds, the disease control options were changed. Hence, the third and the fourth BTB infected privately owned white-tailed deer herds became hunting only operations, placed under long-term quarantine with no live animal movement off the operation.
The first infected herd had a herd prevalence of 5.3%. Out of the 262 deer in the herd, 9 had both gross and histopathological lesions compatible with BTB at depopulation while 14 deer were confirmed using bacterial culture. In the second infected herd, the herd prevalence was 1.2%. Out of the 330 deer in this herd, 9 and 5 deer had gross and histopatholgical BTB compatible lesions, respectively, 1 tested positive by PCR and 4 tested positive by bacterial culture. There were 140 deer in the third infected herd at the time it was found infected. Beyond the one deer that made the herd positive, no other infected deer has been reported in this hunt-only herd. In the fourth (last) infected deer herd, out of the original 280 deer in the herd, 2 deer were found with gross and histopathological BTB compatible lesions and later tested BTB positive by both PCR and bacterial culture. Subsequently, no further infected deer have been reported.
3.4. Wildlife
3.4.1. BTB Surveillance in Wild White-Tailed Deer
Starting in 1995, hunter-harvested, road-killed, and other dead wild white-tailed deer were tested for BTB infection. White-tailed deer have since been tested annually for BTB [25]. Most BTB examinations occur during the fall deer hunting season. Hunters are requested to voluntarily turn in the heads of harvested wild white-tailed deer for BTB examination; in addition, carcasses bearing lesions considered suspicious by either hunters or the MDNR are collected [3]. Hunter-harvested deer accounted for 91% of all deer tested between 1975 and 2006 [3] and remain a significant source of BTB surveillance in wild white-tailed deer. The principal tissues examined are the parotid, mandibular, and medial and lateral retropharyngeal lymph nodes found in the head [11]. Unlike in cattle, only lesioned tissue is subjected to mycobacterial culture.
3.4.2. BTB Eradication in Wild White-Tailed Deer
Reduction of both deer concentration and population has been the applied BTB eradication strategies in Michigan wild white-tailed deer. Restriction/ban of baiting and supplemental feeding in wild white-tailed deer was used to reduce deer concentration, while increased deer harvest was the approach aimed at reducing deer density (Table 2). These strategies were most intensively implemented in the area with the highest sample prevalence of BTB in the wild deer (Deer Management Unit (DMU) 452). This area contains portions of Alcona, Alpena, Montmorency, and Oscoda counties and has been the “core area” of BTB challenge in Michigan. Since 1995, there has been a 57% decline in BTB transmission rate among wild white-tailed deer located with DMU 452 [30]. The total number of statewide harvested wild white-tailed deer has increased annually from approximately 100,000 in 1975 to over 400,000 in 2009 [31]. Consequently, since 1995 deer population has decreased over the years; in DMU 452, deer population dropped by 60,000 (40%) in 2009 [30].
3.4.3. BTB-Infected Wild White-Tailed Deer
BTB in wild white-tailed deer was first reported in Michigan in 1975 with a second case in 1994 [11]. Since then, more BTB cases have been found in white-tailed deer as well as other wildlife including elk, black bear, bobcat, coyote, opossum, raccoon, and red fox [11, 12, 15, 16, 18, 20, 22]. The increased identification of BTB in the wildlife, especially white-tailed deer, has led to numerous policy changes by the MDNR aimed at BTB eradication. Details of Michigan DNR policy changes have been reported [12, 32] as they have extensive treatment of policy implications [20, 33, 34]. The key policy changes are presented in Table 2.
The total population of white-tailed deer tested between 1975 and the end of July 2010 and those infected with BTB is presented in Table 1. Out of 184,269 white-tailed deer tested, 668 were found infected with BTB. Among the BTB infected deer, 36% were from Alcona, 28% from Alpena, 20% from Montmorency, 2% from Presque Isle, 11% from Oscoda, and 3% came from all other counties. The county with the highest prevalence odds of finding BTB white-tailed deer was Alcona (0.0133); the prevalence odds in other counties were Alpena (0.01), Montmorency (0.0109), Oscoda (0.0077), Presque Isle (0.0014), Antrim (0.0002), Emmet (0.0006), and others (0.0002) (Figure 5). Schmitt [30] reported a higher annual sample prevalence of BTB in the DMU 452 than in the surrounding counties (Figure 6).
Figure 6.
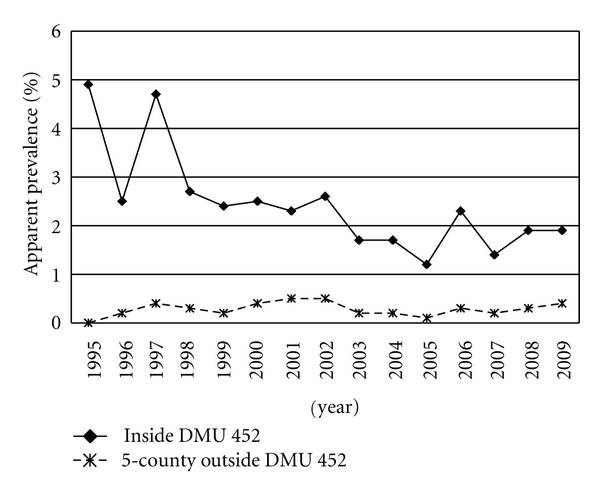
Sample prevalence of BTB in Michigan wild white-tailed deer.
4. Lessons Learned and Recommendations
4.1. BTB Remains an Ongoing Challenge
Despite ongoing control efforts, the continued identification of BTB in cattle and wildlife is strong evidence that BTB remains an ongoing challenge in Michigan. This is somewhat disheartening as significant resources have been expended to return Michigan to a BTB-free status. However, with the strategies that have been implemented during the past 15 years, the disease appears to have been confined to a geographical region of the state as was observed by other studies [12, 20]. The initiation of statewide BTB testing of all cattle herds in 2000 could be responsible for the spike in the BTB incidence between 2000 and 2003, as this was the first time most of these herds were ever BTB tested. Since then, there has been a declining trend in incidence and sample prevalence of BTB in cattle and wildlife, respectively, although recent evidence suggests that the downward trend has leveled off [30]. Earlier studies also observed a decline in sample BTB prevalence in Michigan's wild white-tailed deer [18, 20, 22]. These results suggest that progress has been made in the BTB eradication. However, it may be necessary to explore new and more aggressive control strategies in both cattle and wildlife that transcends political as well as social barriers, if complete eradication is to be accomplished.
4.2. Collaboration
Management of diseases that are transmissible between wildlife and domestic livestock can be a challenge and requires cooperation among their respective advocates in developing a reasonable and effective strategy that allows both to be maintained and prosper. Sharing of expertise is crucial in the eradication of a disease with many susceptible hosts as in BTB. All BTB cases in cattle and privately owned white-tailed deer herds as well as the majority of BTB infections in wildlife have been found in the northern portion of the Michigan's Lower Peninsula. The current containment of BTB to a geographical portion of Michigan is evidence of the successful collaborative efforts undertaken to eradicate BTB from Michigan. However, additional efforts and cooperation are needed to complete the eradication of BTB in Michigan. Increased cooperation between regulatory agencies, other stakeholders (e.g., hunters or local business owners), and livestock industry partners is needed. The development of a plan that is compatible with the long-term sustainability of both the livestock and wild life industries in Michigan should be targeted.
4.3. Surveillance
Whole herd testing of cattle farms has been crucial to identifying BTB infection, but this surveillance method is very expensive. Most BTB-affected cattle herds were found through annual whole herd surveillance. The state annually spends millions of dollars towards BTB control and eradication. Between 1994 and 2010, the State of Michigan has spent approximately US$200 million on BTB eradication [1]. Resources spent on whole herd testing of the livestock population contribute a significant part of the total expenses. Hidden costs rarely mentioned in the current BTB surveillance include such things such as injuries among the livestock owners, veterinarians, and technical staff that conduct BTB testing [35] and loss of production (e.g., temporary drop in milk production) that often occurs following restraint of cattle to administer and/or read ante mortem tests. Finally, there is an industry perception that the number of tests being done and the cost of surveillance in relation to the number of BTB herds found are excessive. This may lead to a decrease in long-term support of the current strategies from the cattle industry. Given these facts, there is a need for the exploration and subsequent adoption of less expensive, but just as reliable as surveillance methods such as targeted strategies using risk-based criteria. Examples where use of targeted risk-based criteria was successful for disease eradication/control include Bovine Spongiform Encephalopathy (BSE) program, Pseudorabies in Pigs, and Brucellosis in cattle and swine. A reliable assay that could be used for BTB surveillance at points of cattle concentration, such as slaughter houses or livestock markets, would also be beneficial and could be used in conjunction with risk-based targeted strategies. Development of sophisticated targeted screening strategies would most likely produce a significant reduction in resource expenditure while at the same time maintaining the necessary rapid identification of BTB infected herds to eradicate the disease.
4.4. BTB Transmission
The prevalence odds of BTB in wild white-tailed deer are highly correlated with the incidence rate of BTB in cattle herds (r = 0.8 and P value = .02). These prevalence odds estimates, calculated from sample prevalence, do not accurately represent the true odds of BTB infection in wild white-tailed deer. The impracticality of testing all wild deer and the imperfection of available screening tests makes the true prevalence of BTB in wild deer unknown in absolute numbers [18] but a good approximation of the extent to which the sample prevalence underestimates the true prevalence has been documented [19]. Since prevalence odds remain a measure of risk, this highly positive correlation result supports the theory of interspecies transmission. Furthermore, the majority of cattle herds infected with BTB shared common environmental and management features that are conducive to wild white-tailed deer-cattle interaction. These observations further support the claim that wildlife and specifically wild white-tailed deer are a reservoir of BTB infection for livestock in Michigan [22, 36, 37]. Therefore, successfully mitigating such wildlife-cattle space interaction would be a great stride towards BTB eradication in Michigan.
With the advent of mandatory radio frequency identification (RFID) in Michigan and movement permits in the Northeast LP, it has become much easier to track cattle movement and rule in/out the possibility of cattle movement as a source of BTB transmission. This is a clear example of the utility of unique individual animal identification system in a disease control program. Using this available information in epidemiological investigations has helped in the understanding that cattle movement is not the most likely source of transmission into most of the infected herds. However, it should be noted that interherd spread has been linked to cattle movement in at least one herd and fence-line contact between infected herds has been identified as a potential mode of transmission in some of the infected Michigan herds. Therefore, these transmission modes should not be ignored and efforts to mitigate the risk of BTB spread through these transmission modes should be continued.
4.5. Wild White-Tailed Deer-Cattle Space Interactions
Wild white-tailed deer-cattle space interactions include wild white-tailed deer's access to cattle's feed and water sources, where M. bovis could be transmitted to cattle via ingestion of contaminated food and water [7]. This example of space interaction would explain the lower number of BTB infected dairy herds, where the animals are primarily kept inside and usually have limited close contact with wild deer, or where livestock producers report no contact of their cattle with wild-white tailed deer. Livestock owners should sustain practices that reduce wild white-tailed deer-cattle space interactions such as storage of feed in enclosures that protect it from deer access, limiting cattle access to stagnant water sources and areas of cover that are also attractive to wild white-tailed deer, and removing feedstuffs from cattle areas that are attractive to deer (e.g., wild apple trees).
4.6. Deer Concentration and Density Reduction
Following the implementation of policies that reduced the population density and restricted practices which artificially increased wild white-tailed deer concentration, there has been a significant decreasing trend in the sample prevalence of BTB in wild white-tailed deer [20]. Since 2006, the sample prevalence has leveled-off. The cause of the slight increase in the sample prevalence of BTB in the wild deer in 2006 is not clearly known and could be associated with the epidemiology of BTB in the wild deer, which remains to be fully understood.
With policies that have led to an increased harvest [31], wild white-tailed deer population has decreased [30]. Similarly, changes in deer management practices, including restriction/ban of baiting and supplemental feeding in wild white-tailed deer, have likely helped reduce the transmission rate of BTB [30]. An increased harvest rate and a reduced transmission rate would cause a reduction in disease prevalence. Practices that have encouraged the reduction in deer concentration and deer density have likely contributed to the current containment of BTB. These practices will remain crucial for BTB eradication and should be supported.
4.7. Handling of BTB-Infected Herds
Among cattle herds infected with BTB in Michigan, depopulation has been the major strategy aimed at eradication. Depopulation is a BTB eradication strategy that is effective in areas with limited reservoirs of BTB infection and the disease challenge is not ongoing. Given the herd sizes of BTB infected cattle herds as it relates to the indemnity paid in the depopulation, this strategy is expensive and can be disrupting to the herd owner. It is even more frustrating when the depopulation strategy fails to achieve its purpose of eradicating the disease. Of the 6 herds with BTB reinfection, 5 were previously depopulated. There were no observable differences in the epidemiological data that would explain any vulnerability in those 6 cattle herds with BTB reinfection. However, detailed study of these herds and their management practices could provide insight into their vulnerability to BTB infection. Depopulation appears to not be a guaranteed BTB eradication strategy in an area with a wildlife reservoir of BTB infection and where some wildlife-livestock space interaction occurs, an observation that may have influenced a 2010 policy change in UDSA APHIS VS plans for herd-specific BTB eradication. In determining how best to handle a BTB infected cattle herd in an area with a wildlife reservoir of BTB, measures must be taken to understand the exposure/transmission risks for BTB and then strive to mitigate those risks. In previous studies, restricting deer's access to cattle feed and water was found to be associated with reduced odds of BTB infection in cattle herds while sharing of pastures, bulls, or fence-line contacts among cattle herds, especially those in close proximity to already infected herd, was associated with increased risk of acquiring BTB into the herd [13]. Therefore adopting herd-specific wildlife risk mitigation and other biosecurity practices needs to be implemented and strictly enforced as part of the BTB eradication project.
Long-term quarantine of BTB infected privately owned white-tailed deer herds with no live animal movement off the operation is a strategy that has been implemented due to inadequacy of indemnity funds. The result of this confinement remains undetermined. There are reports of deer escaping from privately owned white-tailed deer facilities in Michigan as a result of damaged fences [38]. Also, all of the BTB-affected deer farms have fencing that could allow nose-to-nose contact between wild and captive deer. Although the true prevalence of BTB in these quarantined BTB infected privately owned white-tailed deer herds is unknown, given that the herd prevalence of BTB in the depopulated privately owned white-tailed deer herds is as high as the sample prevalence of BTB in the wild white-tailed deer in the area, any escape deer from the quarantined herds could pose a BTB risk. Therefore, depopulation of infected privately owned white-tailed deer herds would be a recommended choice if BTB eradication is to be achieved sooner rather than later.
4.8. Research
The BTB outbreak in Michigan has highlighted many knowledge gaps in our understanding about BTB. For successful eradication to occur in Michigan and in other regions of the world, significant research aimed at enhancing current eradication strategies as well as developing new eradication tools needs to be carried out. Areas of research which should be supported include the following.
Vaccine Development. Extensive research on BTB vaccines for white-tailed deer is underway and the available results are promising [39–45]. The development of vaccines that could be successfully deployed in either livestock or wildlife and as part of a disease eradication program would be extremely beneficial globally. Vaccine may be even more important in other regions of the world without the infrastructure to implement a BTB control program using currently available strategies. Successful vaccine deployment in wildlife could transcend any social and political challenges of the current strategies which target more deer harvest. Therefore, current and future research efforts should be supported.
New Diagnostic Assays and Strategies. Currently available BTB diagnostic assays lack the desired sensitivity and specificity needed for effective BTB eradication in a timely manner. Development of inexpensive, accurate, and rapid diagnostic assays would be very valuable for the efficient identification of BTB infected animals/herds. Development and evaluation of new strategies to deploy diagnostic testing, such as at points of concentration, would be of further value. Newer ante mortem serological [46–48] and cell-mediated immune response [49] assays show potential improvements from the currently available assays and should be encouraged through research funding.
Disease Transmission Risk Factors. Studies that evaluate BTB transmission risk factors have been conducted [5–7, 13]. However, inadequate research has been done on quantifying how much each known risk factor contributes to disease transmission. Understanding and quantifying risk factors important for BTB transmission within and between species would be very valuable for the successful deployment of targeted surveillance strategies and for implementing herd control programs.
Ecology and Epidemiology of BTB in Noncattle Species. With the emergence of BTB reservoirs in wildlife, numerous studies that strive to understand BTB epidemiology have been conducted [17, 18, 34]. The results of these studies have advanced BTB eradication efforts. However, BTB epidemiology, especially in noncattle species, is still not well understood. Better understanding of how the disease is maintained and transmitted in these “new” hosts is necessary for successful control and eradication.
Sociological Aspects of Disease Eradication Programs. Effective disease control programs need commitment from all parties affected, whether directly or peripherally. As can be seen from a previous study [50], stakeholders attitudes have influenced the progress of BTB eradication in Michigan. Understanding societal concerns and developing strategies to mitigate these concerns is extremely important for successful deployment of a disease control program.
5. Conclusion
Despite ongoing eradication efforts, BTB remains a major challenge for Michigan. Policies and strategies implemented since 1994 have appeared to contain cases of BTB in cattle and privately owned deer herds as well as approximately 99.6% of the BTB infected wild white-tailed deer to the northern portions of the Lower Peninsula of Michigan, particularly counties in the North East portion. Active collaboration among the BTB Eradication Project partners, funding agencies, and the various stakeholders has contributed to the current progress and should be encouraged further for onward BTB eradication. Wild white-tailed deer remains the significant source of transmission of BTB to the livestock, most likely through indirect transmission. Mitigation strategies that decrease interactions and indirect transmission as well as supporting actions aimed at reducing the disease prevalence in wildlife should continue and be enhanced. These actions include decreasing wild white-tailed deer population density, decreasing opportunities for close congregation of wild deer, and developing novel strategies for increasing resistance to BTB such as vaccines. BTB surveillance strategies associated with the current eradication program have been effective but expensive. Therefore, development of effective but less expensive disease surveillance system would be beneficial. Finally, additional research is needed to improve our understanding about BTB epidemiology as well as disease eradication techniques that would transcend social and political issues. Supporting, conducting, and implementing the results of such research would greatly improve BTB eradication efforts.
Acknowledgments
The authors are grateful to the USDA for the research grant support and also indebted to the staff of the following agencies that made the data and information accessible: Michigan Department of Agriculture, Michigan Department of Natural Resources, USDA APHIS Veterinary Services, and USDA APHIS Wildlife Services.
References
- 1.Patrick B. Personal communications 2010. Animal Industry Division, Michigan Department of Agriculture, 2010.
- 2.O’Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle and Lung Disease. 1995;76(1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien DJ, Fitzgerald SD, Lyon TJ, et al. Tuberculous lesions in free-ranging white-tailed deer in Michigan. Journal of Wildlife Diseases. 2001;37(3):608–613. doi: 10.7589/0090-3558-37.3.608. [DOI] [PubMed] [Google Scholar]
- 4.Palmer MV, Whipple DL, Waters WR. Experimental deer-to-deer transmission of Mycobacterium bovis. American Journal of Veterinary Research. 2001;62(5):692–696. doi: 10.2460/ajvr.2001.62.692. [DOI] [PubMed] [Google Scholar]
- 5.Palmer MV, Waters WR, Whipple DL. Milk containing Mycobacterium bovis as a source of infection for white-tailed deer fawns (Odocoileus virginianus) Tuberculosis. 2002;82(4-5):161–165. doi: 10.1054/tube.2002.0334. [DOI] [PubMed] [Google Scholar]
- 6.Palmer MV, Waters WR, Whipple DL. Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. American Journal of Veterinary Research. 2004;65(11):1483–1489. doi: 10.2460/ajvr.2004.65.1483. [DOI] [PubMed] [Google Scholar]
- 7.Palmer MV, Whipple DL. Survival of Mycobacterium bovis on feedstuffs commonly used as supplemental feed for white-tailed deer (Odocoileus virginianus) Journal of Wildlife Diseases. 2006;42(4):853–858. doi: 10.7589/0090-3558-42.4.853. [DOI] [PubMed] [Google Scholar]
- 8.Palmer MV. Tuberculosis: a reemerging disease at the interface of domestic animals and wildlife. Current Topics in Microbiology and Immunology. 2007;315:195–215. doi: 10.1007/978-3-540-70962-6_9. [DOI] [PubMed] [Google Scholar]
- 9.Grange JM. Mycobacterium bovis infection in human beings. Tuberculosis. 2001;81(1-2):71–77. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- 10.Olmstead AL, Rhode PW. An impossible undertaking: the eradication of bovine tuberculosis in the United States. Journal of Economic History. 2004;64(3):734–772. [Google Scholar]
- 11.Schmitt SM, Fitzgerald SD, Cooley TM, et al. Bovine tuberculosis in free-ranging white-tailed deer from Michigan. Journal of Wildlife Diseases. 1997;33(4):749–758. doi: 10.7589/0090-3558-33.4.749. [DOI] [PubMed] [Google Scholar]
- 12.Hickling GJ. Dynamics of bovine tuberculosis in wild white-tailed deer in Michigan. MDNR Wildlife Division Report. 2002;(3363) http://ww2.dnr.state.mi.us/Publications/Pdfs/HuntingWildlifeHabitat/Reports/WLD-Library/3301-3399/3363.Pdf.
- 13.Kaneene JB, Bruning-Fann CS, Granger LM, Miller R, Porter-Spalding BA. Environmental and farm management factors associated with tuberculosis on cattle farms in northeastern Michigan. Journal of the American Veterinary Medical Association. 2002;221(6):837–842. doi: 10.2460/javma.2002.221.837. [DOI] [PubMed] [Google Scholar]
- 14.Kaneene JB, VanderKlok M, Bruning-Fann CS, et al. Prevalence of Mycobacterium bovis infection in cervids on privately owned ranches. Journal of the American Veterinary Medical Association. 2002;220(5):656–659. doi: 10.2460/javma.2002.220.656. [DOI] [PubMed] [Google Scholar]
- 15.Bruning-Fann CS, Schmitt SM, Fitzgerald SD, et al. Mycobacterium bovis in coyotes from Michigan. Journal of Wildlife Diseases. 1998;34(3):632–636. doi: 10.7589/0090-3558-47.632.636. [DOI] [PubMed] [Google Scholar]
- 16.Bruning-Fann CS, Schmitt SM, Fitzgerald SD, et al. Bovine tuberculosis in free-ranging carnivores from Michigan. Journal of Wildlife Diseases. 2001;37(1):58–64. doi: 10.7589/0090-3558-37.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Kaneene JB, Bruning-Fann CS, Dunn J, et al. Epidemiologic investigation of Mycobacterium bovis in a population of cats. American Journal of Veterinary Research. 2002;63(11):1507–1511. doi: 10.2460/ajvr.2002.63.1507. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien DJ, Schmitt SM, Fierke JS, et al. Epidemiology of Mycobacterium bovis in free-ranging white-tailed deer, Michigan, USA, 1995–2000. Preventive Veterinary Medicine. 2002;54(1):47–63. doi: 10.1016/s0167-5877(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien DJ, Schmitt SM, Berry DE, et al. Estimating the true prevalence of Mycobacterium bovis in hunter-harvested white-tailed deer in Michigan. Journal of Wildlife Diseases. 2004;40(1):42–52. doi: 10.7589/0090-3558-40.1.42. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien DJ, Schmitt SM, Fitzgerald SD, Berry DE, Hickling GJ. Managing the wildlife reservoir of Mycobacterium bovis: the Michigan, USA, experience. Veterinary Microbiology. 2006;112(2-4):313–323. doi: 10.1016/j.vetmic.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien DJ, Schmitt SM, Berry DE, et al. Estimating the true prevalence of Mycobacterium bovis in free-ranging elk in Michigan. Journal of Wildlife Diseases. 2008;44(4):802–810. doi: 10.7589/0090-3558-44.4.802. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt SM, O’Brien DJ, Bruning-Fann CS, Fitzgerald SD. Bovine tuberculosis in Michigan wildlife and livestock. Annals of the New York Academy of Sciences. 2002;969:262–268. doi: 10.1111/j.1749-6632.2002.tb04390.x. [DOI] [PubMed] [Google Scholar]
- 23.Paskus JJ, Derosier AL, Schools EH, et al. Biodiversity Assessment of Michigan Techinical Report. 2008 http://web4.msue.msu.edu/Mnfi/Reports/2007-11_Biodiversity_Assessment_of_Michigan_Technical_Report.Pdf, Tech. Rep.
- 24.USDA Census of Agriculture. 2007, http://www.agcensus.usda.gov/Publications/2007/Full_Report/Volume_1,_Chapter_2_US_State_Level/st99_2_011_011.pdf.
- 25.MDNR. Bovine Tuberculosis. 2010, http://www.michigan.gov/emergingdiseases.
- 26.USDA APHIS. Bovine Tuberculosis Eradication: Uniform Methods and Rules, Effective January, 1999.
- 27.USDA APHIS. Bovine Tuberculosis Eradication: Uniform Methods and Rules, Effective January, 2005.
- 28.Palmer MV, Whipple DL, Payeur JB, et al. Naturally occurring tuberculosis in white-tailed deer. Journal of the American Veterinary Medical Association. 2000;216(12):1921–1924. doi: 10.2460/javma.2000.216.1921. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien DJ, Schmitt SM, Lyashchenko KP, et al. Evaluation of blood assays for detection of Mycobacterium bovis in white-tailed deer (Odocoileus virginianus) in Michigan. Journal of Wildlife Diseases. 2009;45(1):153–164. doi: 10.7589/0090-3558-45.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt SM. Bovine tuberculosis in wildlife. MDA-DNRE TB Informational Meetings, 2010, http://www.michigan.gov/documents/emergingdiseases/DNRESchmittPublicMeeting062210_325662_7.pdf.
- 31.Frawley BJ. Michigan Deer Harvest Survey Report 2009 Seasons. MDNR Wildlife Report no. 3513, 2010, http://www.michigan.gov/documents/dnr/report3513_327318_7.pdf.
- 32.MDNR. History of Legislation and Regulations for Bovine Tuberculosis in Michigan Wildlife. 2005, http://www.michigan.gov/documents/Bait_Feed_History_138339_7.pdf.
- 33.Conner MM, Ebinger MR, Blanchong JA, Cross PC. Infectious disease in cervids of North America: data, models, and management challenges. Annals of the New York Academy of Sciences. 2008;1134:146–172. doi: 10.1196/annals.1439.005. [DOI] [PubMed] [Google Scholar]
- 34.De Lisle GW, Bengis RG, Schmitt SM, O'Brien DJ. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. OIE Revue Scientifique et Technique. 2002;21(2):317–334. doi: 10.20506/rst.21.2.1339. [DOI] [PubMed] [Google Scholar]
- 35.Wilkins MJ, Bartlett PC, Judge LJ, Erskine RJ, Boulton ML, Kaneene JB. Veterinarian injuries associated with bovine TB testing livestock in Michigan, 2001. Preventive Veterinary Medicine. 2009;89(3-4):185–190. doi: 10.1016/j.prevetmed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Miller R, Kaneene JB, Fitzgerald SD, Schmitt SM. Evaluation of the influence of supplemental feeding of white-tailed deer (Odocoileus virginianus) on the prevalence of bovine tuberculosis in the Michigan wild deer population. Journal of Wildlife Diseases. 2003;39(1):84–95. doi: 10.7589/0090-3558-39.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Wilkins MJ, Meyerson J, Bartlett PC, et al. Human Mycobacterium bovis infection and bovine tuberculosis outbreak, Michigan, 1994–2007. Emerging Infectious Diseases. 2008;14(4):657–660. doi: 10.3201/eid1404.070408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien DJ, Bernardi P, Dubay S, Mayhew S, Moritz WE, Purol D. A Risk-based Audit of the Captive/Privately owned Cervid Industry in Michigan. 2005. http://www.michigan.gov/documents/CPOCAuditReport_Final_118651_7.pdf.
- 39.Palmer MV, Thacker TC, Waters WR. Vaccination with Mycobacterium bovis BCG strains Danish and Pasteur in white-tailed deer (Odocoileus virginianus) experimentally challenged with Mycobacterium bovis. Zoonoses and Public Health. 2009;56(5):243–251. doi: 10.1111/j.1863-2378.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- 40.Nol P, Lyashchenko KP, Greenwald R, et al. Humoral immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination and experimental challenge with M. bovis. Clinical and Vaccine Immunology. 2009;16(3):323–329. doi: 10.1128/CVI.00392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer MV, Thacker TC, Waters WR. Vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacillus Calmette Guerín. Vaccine. 2007;25(36):6589–6597. doi: 10.1016/j.vaccine.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 42.Palmer MV, Thacker TC, Waters WR, Robbe-Austerman S, Lebepe-Mazur SM, Harris NB. Persistence of Mycobacterium bovis bacillus calmette-guérin in white-tailed deer (Odocoileus virginianus) after oral or parenteral vaccination. Zoonoses and Public Health. 2010;57(7-8):e206–e212. doi: 10.1111/j.1863-2378.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 43.Thacker TC, Palmer MV, Waters WR. T-cell mRNA expression in response to Mycobacterium bovis BCG vaccination and Mycobacterium bovis infection of white-tailed deer. Clinical and Vaccine Immunology. 2009;16(8):1139–1145. doi: 10.1128/CVI.00424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nol P, Palmer MV, Waters WR, et al. Efficacy of oral and parenteral routes of Mycobacterium bovis bacille Calmette-Guerin vaccination against experimental bovine tuberculosis in white-tailed deer (Odocoileus virginianus): a feasibility study. Journal of Wildlife Diseases. 2008;44(2):247–259. doi: 10.7589/0090-3558-44.2.247. [DOI] [PubMed] [Google Scholar]
- 45.Waters WR, Palmer MV, Whipple DL, Slaughter RE, Jones SL. Immune responses of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis BCG vaccination. Journal of Wildlife Diseases. 2004;40(1):66–78. doi: 10.7589/0090-3558-40.1.66. [DOI] [PubMed] [Google Scholar]
- 46.Waters WR, Palmer MV, Thacker TC, et al. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clinical and Vaccine Immunology. 2006;13(6):648–654. doi: 10.1128/CVI.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenton KA, Fitzgerald SD, Kaneene JB, Kruger JM, Greenwald R, Lyashchenko KP. Comparison of three immunodiagnostic assays for antemortem detection of Mycobacterium bovis stimulation in domestic cats. Journal of Veterinary Diagnostic Investigation. 2010;22(5):724–729. doi: 10.1177/104063871002200509. [DOI] [PubMed] [Google Scholar]
- 48.Lyashchenko KP, Greenwald R, Esfandiari J, et al. Animal-side serologic assay for rapid detection of Mycobacterium bovis infection in multiple species of free-ranging wildlife. Veterinary Microbiology. 2008;132(3-4):283–292. doi: 10.1016/j.vetmic.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 49.Okafor CC, Grooms DL, Bolin SR, Kaneene JB. Validity of the bovine TB gamma interferon assay on blood collected during exsanguination at slaughter. In: Proceedings of the Conference of Research Workers in Animal Diseases; 2010; Chicago, Ill, USA. [Google Scholar]
- 50.Dorn ML, Mertig AG. Bovine tuberculosis in Michigan: stakeholder attitudes and implications for eradication efforts. Wildlife Society Bulletin. 2005;33(2):539–552. [Google Scholar]


