Subimmunogenic vaccination with an agonist mimetope of insulin converts naive T cells into regulatory T cells and prevents type 1 diabetes in NOD mice.
Abstract
Type 1 diabetes (T1D) results from the destruction of insulin-secreting pancreatic β cells by autoreactive T cells. Insulin is an essential target of the autoimmune attack. Insulin epitopes recognized by diabetogenic T cell clones bind poorly to the class II I-Ag7 molecules of nonobese diabetic (NOD) mice, which results in weak agonistic activity of the peptide MHC complex. Here, we describe a strongly agonistic insulin mimetope that effectively converts naive T cells into Foxp3+ regulatory T cells in vivo, thereby completely preventing T1D in NOD mice. In contrast, natural insulin epitopes are ineffective. Subimmunogenic vaccination with strongly agonistic insulin mimetopes might represent a novel strategy to prevent T1D in humans at risk for the disease.
Type 1 diabetes (T1D) results from the destruction of pancreatic β cells by autoimmune T cells (Tisch and McDevitt, 1996). The etiology of the autoimmune process is unknown but it has been postulated that T cells with specificity for poorly agonistic self-peptides escape negative selection in the thymus and mount an autoimmune attack in peripheral tissue (Fairchild et al., 1993; Liu et al., 1995; Garcia et al., 1999; Hahn et al., 2005; Stadinski et al., 2010; Wucherpfennig and Sethi, 2011). Accordingly, T cell receptors of autoimmune T cell clones were found to bind to peptide–MHC complexes with low affinity and/or unusual topology (Sethi et al., 2011; Wucherpfennig and Sethi, 2011). Moreover, weak interactions of self-ligands with MHC molecules are caused by partially filling the MHC binding groove or by the use of unfavored binding registers (Stadinski et al., 2010). The result in both scenarios is a TCR ligand with relatively poor agonistic activity.
Here, we propose a different hypothesis concerning the development of autoimmune disease that also offers preventive measures. We argue that poor agonistic ligands not only permit escape from “recessive” tolerance, i.e., negative selection in the thymus, but that such ligands also fail to efficiently induce “dominant” tolerance i.e., Foxp3+ T reg cells that can control the disease in peripheral tissue. The hypothesis is based on previous observations that when applied under subimmunogenic conditions, TCR ligands of high agonist activity convert naive but not activated T cells into stable T reg cells expressing Foxp3 (Apostolou and von Boehmer, 2004; Kretschmer et al., 2005; Polansky et al., 2008). The notion is in line with experimental data showing an essential lifelong function of Foxp3+ T reg cells in preventing autoimmune disease (Fontenot et al., 2005; Lahl et al., 2007). In addition, it offers a straightforward strategy to prevent autoimmunity by converting T cells with specificity for poorly agonistic self-ligands into Foxp3+ T reg cells by delivering strongly agonistic mimetopes of the self-ligands. In models unrelated to autoimmunity, dominant tolerance has best been achieved with strong agonist ligands (Kretschmer et al., 2005; Daniel et al., 2010), even in WT mice where antigen-specific naive T cells are rather infrequent (Verginis et al., 2008). Here, we test whether in the case of autoimmune T1D, where insulin represents an essential autoantigen (Nakayama et al., 2005), subimmunogenic delivery of strongly agonistic insulin mimetopes can convert naive T cells into protective Foxp3+ T reg cells and thereby prevent T1D.
Our results show that dominant tolerance to essential insulin epitopes indeed fails in T1D and that its establishment by Foxp3+ T reg cell conversion using a strong agonistic insulin mimetope is capable of completely preventing the onset of T1D in the nonobese diabetic (NOD) mouse.
RESULTS
Agonist and T reg cell conversion activity of insulin epitopes and mimetopes
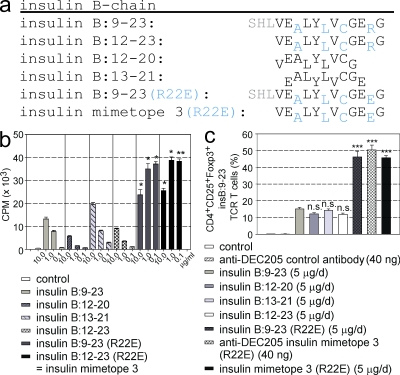
T cells expressing a transgenic TCR specific for the insulin B chain epitope 9–23 presented in the context of I-Ag7 MHC class II molecules (Jasinski et al., 2006) were used to determine the activities of several insulin epitopes and mimetopes (Fig. 1). These cells represent a subset of T cells that recognize the weakly agonistic natural epitope because high doses of the natural epitope are required to induce proliferation. The poor agonistic activity is likely to result from the weak binding of the natural insulin epitope to I-Ag7 in the third MHC-binding register (Stadinski et al., 2010; Fig. 1 a, MHC-binding residues for MHC pockets p1, p4, p6, and p9 are shown in subscript and are marked blue for the binding register 3) that is recognized by most T cell clones from diabetic mice, including the T cells used in this experiment (Stadinski et al., 2010). Accordingly, the arginine residue (R22) in the natural peptide that in this reading frame confronts another arginine in the positively charged p9 pocket of I-Ag7 was replaced with glutamic acid (Fig. 1 a, R22E). Both the full-length and the core peptide with the mutated residue induced much stronger proliferation (∼40-fold at 0.1 µg/ml), as well as conversion into Foxp3+ T reg cells (∼3-fold) when used under appropriate subimmunogenic conditions, i.e., either applied by subcutaneous peptide infusion using osmotic minipumps (5 µg/d for 14 d) or by a subcutaneous single shot injection using DEC205 fusion antibodies (40 ng/mouse; Fig. 1). The mutated core peptide was labeled mimetope 3 and used in all further experiments.
Figure 1.
Agonist activity of insulin B chain epitopes and mimetopes. (a) One-letter amino acid codes are shown for insulin B chain epitopes and mimetopes. MHC-binding residues are shown in subscript and in blue for binding register 3. Residues 9–11, which do not contribute to MHC binding, are shown in gray. (b) Analysis of proliferation of insulin B:9-23–specific TCR transgenic T cells stimulated with epitopes and mimetopes. Results are the averages ± SEM of triplicate wells from four independent experiments. (c) Agonist and T reg cell conversion activity. In vivo T reg cell conversion using naive CD4+ insulin B:9-23 TCR transgenic T cells transferred into congenic NOD Thy1.1+ mice at 4 wk of age. Error bars show percentages of Foxp3+ T reg cells (± SEM from three independent experiments; n = 4 per group) at 2 wk after injection of cells and infusion of peptide. Peptide was delivered either by a 2-wk infusion period using osmotic minipumps or by a single i.p. injection of DEC205 fusion antibody. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus insulin B:9-23.
T1D prevention and insulin autoantibodies
Insulin autoantibodies (IAAs) correlate with insulitis, the development of T1D, and T cell activation (Yu et al., 2000), the latter interfering with the effective conversion of T cells into Foxp3+ T reg cells (Fig. 2 a). We used the recently developed highly specific and sensitive method of a competitive IAA assay that has overcome previous problems in IAA detection (Babaya et al., 2009). In NOD mice, IAA can be detected as early as 15 d after birth (Reddy et al., 1988; Michel et al., 1989) with few mice exhibiting very high titers at the age of 4 wk, correlating with early onset of disease (Yu et al., 2000). To correlate IAA indices and possible effects of insulin epitopes and mimetopes on T1D prevention, IAA indices were determined in NOD mice at the age of 4 (before vaccination) and 6 wk (after vaccination). As shown in Fig. 2 there was indeed a correlation of IAA indices and development of diabetes, in that mice with higher indices developed diabetes earlier. Moreover, although the natural epitope afforded no protection, the mimetope 3 prevented the onset of T1D in the mice with moderate IAA (indices <1). Only in mice that already harbored high IAA with indices >1 at 4 wk of age was the mimetope 3 unable to completely prevent disease development. This is best explained by the assumption that mice with high indices of IAA already contain too many activated T cells that cannot be efficiently converted into Foxp3+ T reg cells (Fig. 2 a). Analysis of IAA before and after vaccination showed that subimmunogenic treatment with mimetope 3 substantially decreased IAA indices (Fig. 2 d), which is consistent with inducing immunosuppression. At 4 wk of age, ∼95% of mice exhibited IAA with indices <1 (moderate levels of IAA), and therefore were included in further experiments.
Figure 2.
Efficacy of Foxp3+ T reg cell vaccination as a function of IAA levels at 4 wk. (a) Naive CD4+CD25−CD62LhiCD44lowFoxp3GFP− cells or activated CD4+CD25−CD62LloCD44hiFoxp3GFP− cells were subjected to in vitro conversion assays (α-CD3 + α-CD28) in the presence or absence (= control) of TGFβ. After 3 d, Foxp3 expression was analyzed by FACS. Figure shows a representative dot plot from four independent experiments performed in triplicates per treatment group. (b and c) NOD mice were infused with insulin B:9-23 peptide (b) or insulin mimetope 3 (c) at 4–6 wk of age. IAA indices were measured at 4 wk of age, before vaccination. Vertical lines indicate the lower detection limit for IAAs. n = 12 for each group from three independent experiments. (d) IAA indices in NOD mice were measured at 4 wk of age (before vaccination) and at 6 wk of age (after vaccination). Nonvaccinated control mice were infused with PBS. Mice with IAA indices <1 were included. Bars show averages ± SEM of IAA indices. n = 8 from two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus insulin B:9-23.
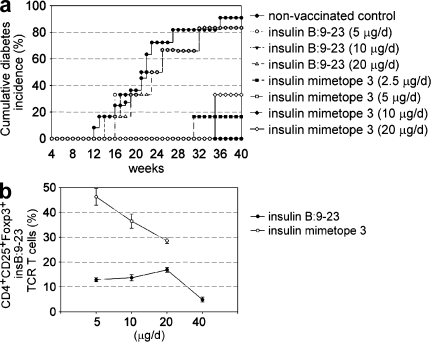
The protection by mimetope 3 was studied for longer time periods (Fig. 3 a), and with varying doses, in the aforementioned mice. A dose of 5 µg/d of mimetope 3 protected mice for 40 wk (which is when animals were sacrificed for analyses), whereas the same dose of the natural epitope had very little beneficial effect in preventing diabetes development. NOD mice were protected from developing T1D for up to 70 wk when they were followed for longer periods. Moreover, in mice with moderate levels of IAA, vaccination with mimetope 3 but not the natural insulin epitope was still effective in preventing the development of T1D, even when the peptide infusion was started in 12-wk-old mice (Fig. 3 b).
Figure 3.
Development of diabetes. (a) Cumulative diabetes incidence in NOD mice, nonvaccinated, vaccinated with insulin B:9-23 or insulin mimetope 3 at 4–6 wk of age. n = 12 from three independent experiments. (b) Cumulative diabetes incidence of NOD mice, nonvaccinated, vaccinated with insulin B:9-23 or insulin mimetope 3 at 12–14 wk of age. n = 6 from two independent experiments. **, P < 0.01; ***, P < 0.001 versus insulin B:9-23.
When the natural epitope and the mimetope were titrated (Fig. 4), it became clear that daily doses of mimetope 3 (2.5 or 20 µg/d) applied at 4–6 wk of age were somewhat less effective in achieving complete protection from diabetes development than 5 and 10 µg/d doses, whereas none of the doses of the natural epitope afforded significant protection from disease (Fig. 4 a). This is consistent with results that were obtained by testing various doses on Foxp3+ T reg cell conversion; none of the doses of the natural epitope (including 40 µg/d) resulted in Foxp3+ T reg cell conversion exceeding 15% of naive T cells. Mimetope 3 at a dose of 5 and 10 µg/d yielded between 40 and 50% of converted Foxp3+ T reg cells, whereas a dose of 20 µg/d resulted in <30% conversion (Fig. 4 b).
Figure 4.
Dose response of insulin epitope and mimetope. (a) Cumulative diabetes incidence in NOD mice that were nonvaccinated, vaccinated with insulin B:9-23, or vaccinated by insulin mimetope 3 at 4–6 wk of age (doses 2.5–20 µg/d). n = 6 from two independent experiments. (b) In vivo T reg cell conversion using naive CD4+ insulin B:9-23 TCR transgenic T cells transferred into congenic NOD Thy1.1+ mice. Percentages of Foxp3+ T reg cells (± SEM, n = 6 from two independent experiments) at 2 wk after vaccination with indicated doses of peptide. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus insulin B:9-23.
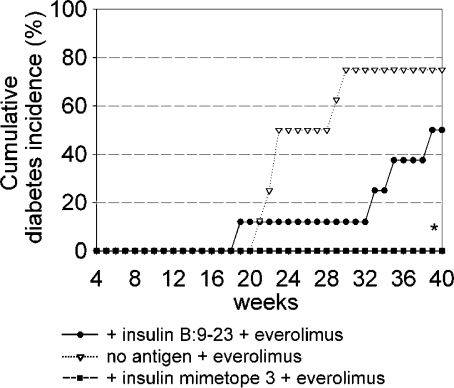
We then tested whether the rapamycin analogue everolimus had an effect on the development of T1D in normal NOD mice and in mice that had received the natural epitope or mimetope 3. As shown in Fig. 5, everolimus indeed delayed the onset of T1D in otherwise untreated mice and mice receiving the natural epitope, whereas no T1D developed in mice that also received mimetope 3. These data suggest that the simultaneous application of everolimus and mimetope represents a powerful tool for the prevention of T1D (see insulitis scores in Fig. 6) because everolimus prevents the immediate activation of effector cells as long as naive T cells are not yet converted while enhancing the conversion of naive T cells into T reg cells in vivo.
Figure 5.
Impact of the mTOR inhibitor everolimus on development of diabetes. Cumulative diabetes incidence of NOD mice treated with a combination of the natural insulin epitope or the mimetope plus everolimus (3 mg/kg i.p. for 14 d) or everolimus alone. n = 12 from three independent experiments. *, P < 0.05 versus insulin B:9-23 + everolimus.
Figure 6.
Histopathological evaluation of pancreas sections from either nonvaccinated or vaccinated NOD mice at the age of 20 or 40 wk. Representative hematoxylin and eosin–stained paraffin-embedded pancreas sections are shown in a and b. Bars, 100 µm. Grading of insulitis of the various treatment groups is shown in c and d. n = 12 from three independent experiments.
It has been established that the extent of infiltration by mononuclear cells in pancreatic islets of NOD mice corresponds to the extent of glycemia. Mice that have been treated with the natural epitope exhibited mostly strong (malignant) infiltration, whereas mimetope 3-treated mice showed mainly periinsulitis or partial insulitis with many morphologically intact β cells producing insulin, resulting in normoglycemia (Fig. 6 and data shown in Fig. 7). Quantitative assessment of a larger number of samples yielded insulitis scores as also shown in Fig. 6. Mimetope 3-vaccinated mice exhibited significantly lower insulitis scores in comparison to untreated mice or mice that had received the natural insulin epitope. The combined application of mimetope 3 and everolimus resulted in a further reduction of insulitis scores, underlining a superior effect in the prevention of T1D when used together (Fig. 6 and Fig. 7).
Figure 7.
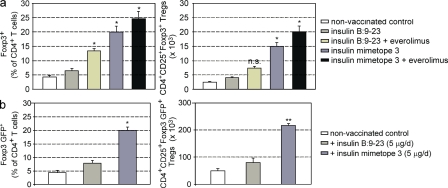
Analysis of Foxp3 expression in 30-wk-old NOD mice. Foxp3 immunohistochemistry in pancreatic cryosections. (a) Co-stainings of CD4 (green) and Foxp3 (red) expression in the respective treatment groups (b–d). Representative co-stainings of insulin (green) and Foxp3 (red) expression in pancreatic sections of mice that were nonvaccinated (b), vaccinated with insulin B:9-23 (c), and vaccinated with insulin mimetope 3 (d). n = 8 per group from two independent experiments.Bars, 100 µm. (e) Quantification of Foxp3+ cells in pancreatic sections as described in b–d. Bars show Foxp3+ cells per high-power field (± SEM from two independent experiments; n = 8 per group). *, P < 0.05; **, P < 0.01 versus insulin B:9-23.
Dominant tolerance in normoglycemic mice
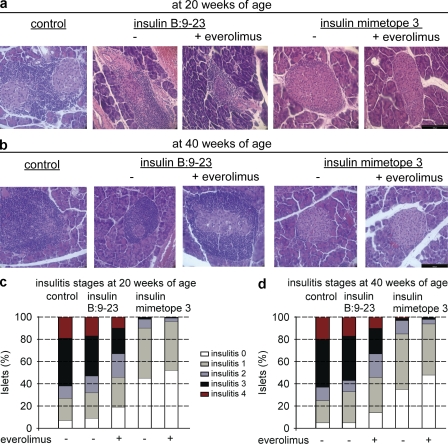
To test whether the observed protection was indeed caused by dominant tolerance executed by converted Foxp3+ T reg cells the number of Foxp3+ T reg cell was determined in several experimental mice. To this end, NOD or NOD Foxp3 GFP reporter mice that were untreated or injected with natural epitope or mimetope 3 were analyzed. As shown in Fig. 7 and Fig. 8 mice vaccinated with mimetope 3 always exhibited the highest number of Foxp3+ cells and insulin-producing β cells, which was further enhanced when everolimus was co-injected. The data of percentages of Foxp3+ T reg cells in pancreas-infiltrating CD4+ T cells confirm the superior effect of the mimetope in inducing Foxp3+ T reg cells as seen from absolute numbers of T reg cells (Fig. 8). It is clear that injection of the natural epitope also yielded a modest increase in Foxp3+ cells that was obviously insufficient to protect from T1D (Fig. 3 and Fig. 4). In addition, in NOD Foxp3 GFP reporter mice vaccination with mimetope 3 resulted in the highest percentages and most GFP+ cells in pancreatic lymph nodes (Fig. 8 b).
Figure 8.
Foxp3+ T reg cells in pancreata and pancreatic lymph nodes of NOD mice. (a) Percentages and absolute numbers of Foxp3+ T reg cells in pancreatic islet–infiltrating T cells of 30-wk-old NOD mice from the respective treatment groups, analyzed by flow cytometry. Bars represent means ± SEM from three independent experiments. n = 4 per group. (b) Percentages and absolute number of CD4 T cells expressing Foxp3 GFP in pancreatic lymph nodes from 20-wk-old NOD Foxp3-GFP reporter mice, analyzed by flow cytometry. Bars represent means ±SEM from two independent experiments, n = 4 per group. Mice were vaccinated at 4–6 wk of age.
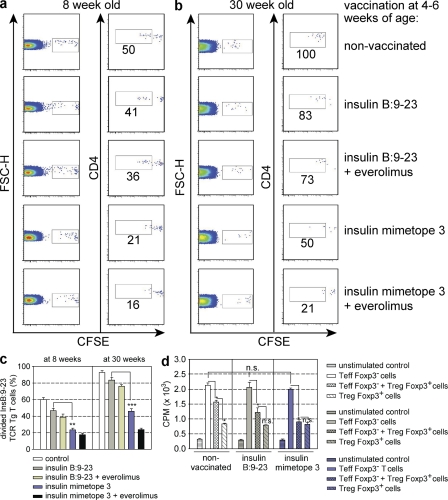
Because activated Foxp3+ T reg cells do not home properly to pancreatic lymph nodes (Tarbell et al., 2004, 2007), we did not attempt to transfer T reg cells from vaccinated mice to assess dominant tolerance. Instead, we used a previously described protocol (Sundstedt et al., 2003; Tarbell et al., 2004, 2007) of transferring CFSE-labeled, insulin-specific T cells into differently vaccinated mice and monitoring their proliferation. If dominant tolerance was established, proliferation should be suppressed and recessive forms of tolerance like deletion or anergy should not result in suppression of proliferation because recessive tolerance induced by strong agonist ligands is preceded by proliferation (Lanoue et al., 1997). Fig. 9 shows that mimetope 3–vaccinated mice displayed suppression of proliferation of insulin-specific T cells when the proportion of cells with diluted CFSE label was determined (Fig. 9a-c). Suppression of insulin-specific effector cells in mimetope 3–treated mice could be detected in 8- and 30-wk-old mice (Fig. 9, a–c). In additional experiments, we transferred CFSE-labeled, polyclonal-activated CD4 effector T cells purified from pancreatic lymph nodes of newly diabetic mice into mimetope-vaccinated NOD mice. It became clear that, in contrast to the natural epitope, vaccination with the mimetope resulted in suppression of polyclonal effector cells (Fig. S1).
Figure 9.
Analysis of dominant tolerance in vaccinated NOD mice. CFSE dilution profiles of insulin B:9-23 TCR transgenic T cells at day 4 after transfer into nonvaccinated or vaccinated 8- (a) or 30-wk-old (b) NOD mice. (c) Percentages of divided insulin B:9-23 TCR transgenic T cells after transfer into the vaccination groups as described in a and b. Bars represent means ± SEM from two independent experiments. n = 4 per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus insulin B:9-23. (d) Analysis of proliferation of T cells from pancreatic lymph nodes of NOD Foxp3-GFP reporter mice at 20 wk, vaccinated with either insulin B:9-23 or insulin mimetope 3 at 4–6 wk of age. Proliferation before or after depletion of Foxp3+ T cells in response to the insulin mimetope was analyzed. Results are the means ± SEM from two independent experiments. n = 4 per group.
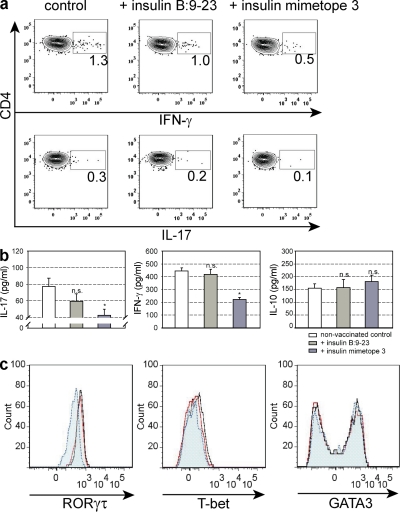
Finally, we determined the proliferative response of ex vivo cells from pancreatic lymph nodes from untreated, natural epitope–, or mimetope 3–vaccinated NOD Foxp3 GFP reporter mice in the presence or absence of Foxp3+ T reg cells. As shown in Fig. 9 d, Foxp3+ T reg cells from mimetope 3–vaccinated mice completely suppressed proliferation of effector cells, reflecting an enrichment of insulin-specific cells within the Foxp3+–T reg cell population, whereas T reg cells from nonvaccinated animals or mice treated with the natural epitope were less suppressive. Importantly, the proliferation of effector cells in the absence of Foxp3+ T reg cells was similar in all mice, indicating the absence of recessive tolerance. When the cytokine response of effector cells was analyzed, it became clear that there were no differences in IL-10 production, whereas vaccination with mimetope 3 resulted in reduced secretion of IFN-γ and IL-17 (Fig. 10).
Figure 10.
Characterization of effector T cells isolated from pancreatic lymph nodes of NOD Foxp3-GFP reporter mice at 20 wk. The mice were nonvaccinated, vaccinated with insulin B:9-23, or insulin mimetope 3 at 4–6 wk. Cytokine expression in T effector cells after depletion of Foxp3+ T cells in response to the insulin mimetope 3 was analyzed by intracellular staining (a) or by ELISA (b). *, P < 0.05 versus control. Representative dot plots are shown. Data are representative of two independent experiments. n = 4 per group. Expression of the lineage-specific transcription factors as assessed by intracellular staining is shown in c. Cells are gated on CD4+ T cells. Black solid line, nonvaccinated control; red solid line, insulin B:9-23 vaccinated; blue dotted line, insulin mimetope 3 vaccinated. Results are the means ± SEM from two independent experiments. n = 4 per group.
Collectively, these results indicate that in contrast to the natural insulin epitope, vaccination with mimetope 3 results in the highest number of stable Foxp3+ cells that suppress the proliferation of IFN-γ– and IL-17 cytokine-secreting, insulin-specific effector cells.
DISCUSSION
Vaccination with insulin or its mimetopes has been tested previously, to prevent or treat T1D using mucosal exposure or in the presence of adjuvant such as cholera toxin B subunit or IFA (Zhang et al., 1991; Daniel and Wegmann, 1996; Harrison et al., 1996; Bergerot et al., 1997; Chen et al., 2001; Alleva et al., 2002; Aspord and Thivolet, 2002; Martinez et al., 2003). The goal of these attempts was not to induce Foxp3+ T reg cells, and they afforded only incomplete protection, perhaps because of the fact that such maneuvers shifted the Th1 to Th2 ratio (Alleva et al., 2002). This would explain why with our tolerogenic vaccination protocol we see a drop in IAA indices, whereas in previous vaccination protocols an increase was noted (Orban et al., 2010). However, none of the previously established protocols reached the level of protection noted in the approach presented here and ensuing clinical trials using natural insulin epitopes were largely unsuccessful.
The findings demonstrated here support the hypothesis that weakly agonistic self-ligands recognized by autoreactive T cells fail to efficiently induce dominant tolerance. We therefore propose a novel concept of extrathymic Foxp3+ T reg cell conversion in autoimmune disease settings by applying minute amounts of strongly agonistic mimetopes of critical autoantigens, such as insulin in T1D. In contrast, natural insulin epitopes with poor agonistic activity that are recognized by diabetogenic T cells, irrespective of the applied dose, are ineffective in inducing stable Foxp3+ T reg cells. The ability to convert naive T cells through appropriate antigenic stimulation under subimmunogenic conditions into Foxp3+ T reg cells offers a convenient strategy to induce antigen-specific dominant tolerance for several reasons. Foxp3+ T reg cell vaccination requires only a single epitope because the induced T reg cells suppress immune responses of T cells to antigen from the same source through bystander suppression (Verginis et al., 2008), whereby the vicinity to antigen presenting cells is relevant. An additional advantage is that this mode of tolerance can be easily induced in the mature immune system by subimmunogenic delivery of antigen (Verginis et al., 2008; Daniel et al., 2010), i.e., in the absence of general immunosuppression that can have fatal consequences. The induced Foxp3+ T reg cells are stable (Polansky et al., 2008) and maintain their function even in an immunogenic context (Rubtsov et al., 2010) because reencounter of antigen causes neither loss of T reg cells nor loss of their activity (Klein et al., 2003).
Because the method is preventive, one should aim at a cohort of individuals with elevated risk of developing T1D. IAAs precede the development of T1D (Ziegler et al., 1989; Yu et al., 2000), i.e., most diabetes patients have IAA before T1D onset, therefore individuals with high-risk MHC alleles, such as HLA-DQ8 and DQ2, and moderate levels of IAA represent a well-defined population to vaccinate by this method.
The use of strongly agonistic ligands applied under subimmunogenic conditions results in complete protection from autoimmune disease development; this protection from autoimmune diabetes development has not previously been achieved with natural epitopes, irrespective of dose and mode of application, which is consistent with the finding that insulin-specific T cells in NOD mice exhibit specificity for poorly agonistic ligands (Stadinski et al., 2010). It is also consistent with the belief that high-affinity TCR ligands are the best inducers of T reg cells, although in these short-term experiments using models uunrelated to autoimmunity only TCR contact residues were modified (Gottschalk et al., 2010). The validity of the approach to create higher affinity TCR ligands appears to be limited to TCR transgenic systems (Gottschalk et al., 2010), as it is unlikely that a peptide that forms a higher affinity ligand for a given TCR will also form a higher affinity ligand for polyclonal T cell populations reacting with the same peptide.
Subimmunogenic doses of strongly agonistic mimetopes appear mandatory to achieve complete protection by immunosuppression. In contrast, high immunogenic doses of ligands lead to the activation of the PI3K–Akt–mTOR pathway (Merkenschlager and von Boehmer, 2010), which could interfere with extrathymic Foxp3 induction (Sauer et al., 2008). Recent studies show that Foxo proteins, which are inactivated by Akt, drive Foxp3 expression. These studies could explain the negative regulation of Foxp3 by the PI3K–Akt network (Merkenschlager and von Boehmer, 2010) after strong T cell activation. The best Foxp3+ T reg cell conversion is seen in T cells that undergo only limited proliferation, whereas higher doses of agonists result in more extensive proliferation and diminished Foxp3+ T reg cell conversion (Kretschmer et al., 2005). In support of these conclusions, robust T cell activation has been shown to negatively impact on the extrathymic T reg cell generation based on a cell-cycle dependent maintenance of a silenced state of the Foxp3 locus (Josefowicz et al., 2009). This mode of immunosuppression differs from methods that induced increased levels of IL-10 production by CD25− cells (Sundstedt et al., 2003).
The NOD mouse appears to represent a faithful model of human disease because it is controlled by the same genetic factors, such as MHC class II genes (Suri et al., 2005, 2008) and levels of intrathymic insulin expression (Pugliese et al., 1997; Jaeckel et al., 2004). Epitopes recognized by human CD4 T cells include the insulin B:9-23 (Alleva et al., 2001) peptide, which is identical to that of mouse insulin. Such cells were reported in cohorts of people at risk of developing T1D and with recent onset of T1D, but not in control subjects (Alleva et al., 2001). Human class II HLA-DQ8 and DQ2 alleles bear striking resemblance to the mouse class II I-Ag7 allele that confers susceptibility (Lee et al., 2001) with a positively charged p9 MHC-binding pocket that distinguishes the DQ8 and DQ2 alleles from other class II alleles that do not confer susceptibility. Thus, it seems reasonable to explore the possibility that the scenario as revealed in the NOD mouse also applies in human disease.
MATERIALS AND METHODS
Mice.
Insulin (B:9-23) TCR transgenic RAG−/− NOD mice were previously described (Jasinski et al., 2006) and were bred in the facility of the Dana Farber Cancer Institute. 4-wk-old female NOD mice were purchased from Taconic. Thy1.1+ congenic NOD mice and NOD Foxp3 GFP reporter mice were obtained from The Jackson Laboratory. Antigen-specific in vivo T reg cell conversion protocols, with or without everolimus treatment, were executed as previously described (Daniel et al., 2011). Before T reg cell conversion, mice were tested for IAA. Mice with high titers (indices ≥1) were excluded from analyses, as in these mice most insulin-specific T cells may already have been activated and hence resistant to T reg cell conversion. Diabetes development was monitored according to previously described protocols (Jaeckel et al., 2003, 2004). All animal care and procedures were executed according to National Institutes of Health guidelines and in accordance with the guidelines of the Animal Care and Use Committee of the Dana Farber Cancer Institute.
Histopathology of mouse NOD pancreata.
Pancreata were fixed in Bouin’s solution and embedded in paraffin, and serial sections were stained with hematoxylin and eosin for general morphology. Histology and scoring of insulitis in pancreatic sections were executed as previously described (Jaeckel et al., 2004; Krishnamurthy et al., 2006). Pancreatic islets were assigned scores as follows: 0, intact islets/no lesions; 1, periislet infiltrates; 2, <25% islet destruction; 3, >25% islet destruction; 4, complete islet destruction.
Peptides.
All peptides with amino acid sequences described in Fig. 1 a were synthesized at New England Peptide.
IAA assay.
Mouse high specificity/sensitivity competitive IAA assays in sera from NOD mice were performed in an ELISA format, as described previously (Babaya et al., 2009). In brief, high binding 96-well plates (Costar) were coated with human (recombinant) insulin (100 U/ml; Humulin; Lilly) overnight at 4°C. Unspecific-blocking was done using PBS containing 2% BSA for 2 h at room temperature. Preincubated serum (diluted 1:10) with or without insulin competition was added and incubated for 2 h at room temperature. After 4 wash steps, biotinylated anti–mouse IgG1 (Abcam), diluted 1:10,000 in PBS/BSA was added for 30 min at room temperature. After washing the plate, horseradish peroxidase–labeled streptavidin was added for 15 min. The plate was washed 5 times, and TMB substrate solution was added (OptEIA reagent set; BD). Each sample was run in duplicate with and without competition using human insulin. For each sample, an index was calculated based on the mean of the results.
Isolation of pancreatic islets and infiltrating T cells.
Cells were purified from mouse pancreata after in situ pancreas perfusion with collagenase, pancreas digestion, and islet purification using previously described protocols (Trembleau et al., 1995; Szot et al., 2007; Li et al., 2009). Purified islets were washed and subsequently selected by hand or separated by Ficoll gradient.
In vitro T cell proliferation assay.
Assays were performed in round-bottom 96-well tissue culture plates. CD11c+ DCs from NOD mice were used as antigen-presenting cells and were purified using anti-CD11c+ magnetic beads (Miltenyi Biotec; Daniel et al., 2011). Thymidine incorporation of stimulated CD4+TCRβ+-enriched insulin B:9-23 TCR transgenic T cells or of T cells purified from pancreatic lymph nodes of nonvaccinated or vaccinated NOD Foxp3 GFP reporter mice in response to the insulin mimetope was analyzed by scintillation counting after the addition of [3H]thymidine for the last 12 h of a 70-h culture period.
Analyses of dominant tolerance.
CD4+ insulin B:9-23 TCR transgenic T cells were labeled with CFSE at 2 µM and incubated for 4 min at 37°C in PBS plus 0.1% BSA. Labeled cells were adoptively transferred i.p. into T reg cell–vaccinated 8- or 30-wk-old NOD female mice, followed by i.p. co-injection with insulin B:9-23 peptide (15 µg/mouse). Analysis of CFSE dilution was done 4 d later in pancreatic lymph nodes using flow cytometry. Concentrations of IL-10, IFN-γ, and IL-17 were analyzed by mouse ELISA sets (OptEIA; BD or R&D Systems).
Immunohistochemistry.
Immunohistochemistry was performed essentially as previously described (Weigmann et al., 2008), with the following changes: 10 µm cryosections from pancreatic specimens of NOD mice were stained for Foxp3 with rat antibody (FJK-16s; eBioscience), rabbit anti-insulin antibody C27C9 (Abcam), and mouse anti-CD4 antibody (mAb51312; Abcam). Tissues were fixed in ice-cold acetone, followed by methanol blocking, biotin/avidin-blocking (Vector Laboratories), and protein-blocking reagent (Roth) for elimination of unspecific background staining. Sections were permeabilized with Perm-Medium B (ADG), followed by incubation with primary antibodies. Sections incubated with isotype-matched control antibodies served as negative control. Next, samples were incubated with biotinylated secondary IgG antibody (Dianova), incubated with streptavidin-HRP, and stained with tyramide-Cy3 or tyramide-FITC (Invitrogen) according to the manufacturer’s instructions.
Antibodies and flow cytometry.
mAbs specific for Thy1.1 (OX-7), Thy1.2 (53–2.1), CD4 (RM4-5), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), CD127 (A7R34), Foxp3 (FJK-16s), ROR-γτ (AFKJS-9), T-bet (eBio4B10), GATA3 (TWAJ), IL-17 (TC11-18H10) and IFN-γ (XMG1.2) were purchased from BD, eBioscience, or BioLegend and were used as biotin, FITC, PE, PE-Cy7, PerCP-Cy5.5, allophycocyanin, allophycocyanin-Cy7, Alexa Fluor 488 or Pacific blue conjugates. Enumeration of cells and acquisition are performed by using FACSAria and FACSDiva software (BD). Single-cell data analyses are done by the use of the FlowJo software (Tree Star, Inc.).
Statistical analysis.
Data were analyzed by one-way ANOVA with Tukey’s post hoc test or with the Holm-Sidak method for comparison of multiple columns. Wilcoxon signed rank test was used to analyze the impact of T reg cell vaccination on IAA levels before and after treatment. Cumulative incidence of diabetes was determined by Kaplan-Meier estimates, and statistical analysis of difference was determined by log-rank test. A value of P < 0.05 was considered significant. Analyses were done using SigmaStat 3.5 (Systat Software).
Online supplemental material.
Fig. S1 shows the analysis of dominant tolerance to polyclonal effector T cells in vaccinated NOD mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110574/DC1.
Acknowledgments
The authors thank Alexei Nikolaev for assistance in immunohistochemistry.
The studies were supported by National Institutes of Health grant AI-53102 (to H. von Boehmer). C. Daniel was supported by a Leopoldina research fellowship (BMBF-LPD 9901/8-184) and by the LOEWE (LipidSignaling Forschungszentrum Frankfurt) program of the Federal State of Hessen, Germany.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- IAA
- insulin autoantibody
- NOD
- nonobese diabetic
- T1D
- type 1 diabetes
References
- Alleva D.G., Crowe P.D., Jin L., Kwok W.W., Ling N., Gottschalk M., Conlon P.J., Gottlieb P.A., Putnam A.L., Gaur A. 2001. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J. Clin. Invest. 107:173–180 10.1172/JCI8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva D.G., Gaur A., Jin L., Wegmann D., Gottlieb P.A., Pahuja A., Johnson E.B., Motheral T., Putnam A., Crowe P.D., et al. 2002. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes. 51:2126–2134 10.2337/diabetes.51.7.2126 [DOI] [PubMed] [Google Scholar]
- Apostolou I., von Boehmer H. 2004. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199:1401–1408 10.1084/jem.20040249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C., Thivolet C. 2002. Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin. Exp. Immunol. 130:204–211 10.1046/j.1365-2249.2002.01988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaya N., Liu E., Miao D., Li M., Yu L., Eisenbarth G.S. 2009. Murine high specificity/sensitivity competitive europium insulin autoantibody assay. Diabetes Technol. Ther. 11:227–233 10.1089/dia.2008.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergerot I., Ploix C., Petersen J., Moulin V., Rask C., Fabien N., Lindblad M., Mayer A., Czerkinsky C., Holmgren J., Thivolet C. 1997. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 94:4610–4614 10.1073/pnas.94.9.4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Bergerot I., Elliott J.F., Harrison L.C., Abiru N., Eisenbarth G.S., Delovitch T.L. 2001. Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J. Immunol. 167:4926–4935 [DOI] [PubMed] [Google Scholar]
- Daniel D., Wegmann D.R. 1996. Intranasal administration of insulin peptide B: 9-23 protects NOD mice from diabetes. Ann. N. Y. Acad. Sci. 778:371–372 10.1111/j.1749-6632.1996.tb21146.x [DOI] [PubMed] [Google Scholar]
- Daniel C., Wennhold K., Kim H.J., von Boehmer H. 2010. Enhancement of antigen-specific Treg vaccination in vivo. Proc. Natl. Acad. Sci. USA. 107:16246–16251 10.1073/pnas.1007422107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Ploegh H., von Boehmer H. 2011. Antigen-specific induction of regulatory T cells in vivo and in vitro. Methods Mol. Biol. 707:173–185 10.1007/978-1-61737-979-6_11 [DOI] [PubMed] [Google Scholar]
- Fairchild P.J., Wildgoose R., Atherton E., Webb S., Wraith D.C. 1993. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int. Immunol. 5:1151–1158 10.1093/intimm/5.9.1151 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Garcia K.C., Teyton L., Wilson I.A. 1999. Structural basis of T cell recognition. Annu. Rev. Immunol. 17:369–397 10.1146/annurev.immunol.17.1.369 [DOI] [PubMed] [Google Scholar]
- Gottschalk R.A., Corse E., Allison J.P. 2010. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 207:1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Nicholson M.J., Pyrdol J., Wucherpfennig K.W. 2005. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat. Immunol. 6:490–496 10.1038/ni1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L.C., Dempsey-Collier M., Kramer D.R., Takahashi K. 1996. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J. Exp. Med. 184:2167–2174 10.1084/jem.184.6.2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel E., Klein L., Martin-Orozco N., von Boehmer H. 2003. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J. Exp. Med. 197:1635–1644 10.1084/jem.20030215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel E., Lipes M.A., von Boehmer H. 2004. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nat. Immunol. 5:1028–1035 10.1038/ni1120 [DOI] [PubMed] [Google Scholar]
- Jasinski J.M., Yu L., Nakayama M., Li M.M., Lipes M.A., Eisenbarth G.S., Liu E. 2006. Transgenic insulin (B:9-23) T-cell receptor mice develop autoimmune diabetes dependent on RAG genotype, H-2g7 homozygosity, and insulin 2 gene knockout. Diabetes. 55:1978–1984 10.2337/db06-0058 [DOI] [PubMed] [Google Scholar]
- Josefowicz S.Z., Wilson C.B., Rudensky A.Y. 2009. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol. 182:6648–6652 10.4049/jimmunol.0803320 [DOI] [PubMed] [Google Scholar]
- Klein L., Khazaie K., von Boehmer H. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891 10.1073/pnas.1533365100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M.C., von Boehmer H. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy B., Dudek N.L., McKenzie M.D., Purcell A.W., Brooks A.G., Gellert S., Colman P.G., Harrison L.C., Lew A.M., Thomas H.E., Kay T.W. 2006. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 116:3258–3265 10.1172/JCI29602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoue A., Bona C., von Boehmer H., Sarukhan A. 1997. Conditions that induce tolerance in mature CD4+ T cells. J. Exp. Med. 185:405–414 10.1084/jem.185.3.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Wucherpfennig K.W., Wiley D.C. 2001. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat. Immunol. 2:501–507 10.1038/88694 [DOI] [PubMed] [Google Scholar]
- Li D.S., Yuan Y.H., Tu H.J., Liang Q.L., Dai L.J. 2009. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4:1649–1652 10.1038/nprot.2009.150 [DOI] [PubMed] [Google Scholar]
- Liu G.Y., Fairchild P.J., Smith R.M., Prowle J.R., Kioussis D., Wraith D.C. 1995. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 3:407–415 10.1016/1074-7613(95)90170-1 [DOI] [PubMed] [Google Scholar]
- Martinez N.R., Augstein P., Moustakas A.K., Papadopoulos G.K., Gregori S., Adorini L., Jackson D.C., Harrison L.C. 2003. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J. Clin. Invest. 111:1365–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkenschlager M., von Boehmer H. 2010. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J. Exp. Med. 207:1347–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Boitard C., Bach J.F. 1989. Insulin autoantibodies in non-obese diabetic (NOD) mice. Clin. Exp. Immunol. 75:457–460 [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Abiru N., Moriyama H., Babaya N., Liu E., Miao D., Yu L., Wegmann D.R., Hutton J.C., Elliott J.F., Eisenbarth G.S. 2005. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 435:220–223 10.1038/nature03523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban T., Farkas K., Jalahej H., Kis J., Treszl A., Falk B., Reijonen H., Wolfsdorf J., Ricker A., Matthews J.B., et al. 2010. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J. Autoimmun. 34:408–415 10.1016/j.jaut.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky J.K., Kretschmer K., Freyer J., Floess S., Garbe A., Baron U., Olek S., Hamann A., von Boehmer H., Huehn J. 2008. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 38:1654–1663 10.1002/eji.200838105 [DOI] [PubMed] [Google Scholar]
- Pugliese A., Zeller M., Fernandez A., Jr, Zalcberg L.J., Bartlett R.J., Ricordi C., Pietropaolo M., Eisenbarth G.S., Bennett S.T., Patel D.D. 1997. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15:293–297 10.1038/ng0397-293 [DOI] [PubMed] [Google Scholar]
- Reddy S., Bibby N.J., Elliott R.B. 1988. Ontogeny of islet cell antibodies, insulin autoantibodies and insulitis in the non-obese diabetic mouse. Diabetologia. 31:322–328 [DOI] [PubMed] [Google Scholar]
- Rubtsov Y.P., Niec R.E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A.Y. 2010. Stability of the regulatory T cell lineage in vivo. Science. 329:1667–1671 10.1126/science.1191996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer S., Bruno L., Hertweck A., Finlay D., Leleu M., Spivakov M., Knight Z.A., Cobb B.S., Cantrell D., O’Connor E., et al. 2008. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA. 105:7797–7802 10.1073/pnas.0800928105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi D.K., Schubert D.A., Anders A.K., Heroux A., Bonsor D.A., Thomas C.P., Sundberg E.J., Pyrdol J., Wucherpfennig K.W. 2011. A highly tilted binding mode by a self-reactive T cell receptor results in altered engagement of peptide and MHC. J. Exp. Med. 208:91–102 10.1084/jem.20100725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadinski B.D., Zhang L., Crawford F., Marrack P., Eisenbarth G.S., Kappler J.W. 2010. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl. Acad. Sci. USA. 107:10978–10983 10.1073/pnas.1006545107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstedt A., O’Neill E.J., Nicolson K.S., Wraith D.C. 2003. Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo. J. Immunol. 170:1240–1248 [DOI] [PubMed] [Google Scholar]
- Suri A., Walters J.J., Gross M.L., Unanue E.R. 2005. Natural peptides selected by diabetogenic DQ8 and murine I-A(g7) molecules show common sequence specificity. J. Clin. Invest. 115:2268–2276 10.1172/JCI25350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri A., Levisetti M.G., Unanue E.R. 2008. Do the peptide-binding properties of diabetogenic class II molecules explain autoreactivity? Curr. Opin. Immunol. 20:105–110 10.1016/j.coi.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot G.L., Koudria P., Bluestone J.A. 2007. Murine pancreatic islet isolation. J. Vis. Exp. 7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V., Yamazaki S., Olson K., Toy P., Steinman R.M. 2004. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 199:1467–1477 10.1084/jem.20040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V., Petit L., Zuo X., Toy P., Luo X., Mqadmi A., Yang H., Suthanthiran M., Mojsov S., Steinman R.M. 2007. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204:191–201 10.1084/jem.20061631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R., McDevitt H. 1996. Insulin-dependent diabetes mellitus. Cell. 85:291–297 10.1016/S0092-8674(00)81106-X [DOI] [PubMed] [Google Scholar]
- Trembleau S., Penna G., Bosi E., Mortara A., Gately M.K., Adorini L. 1995. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 181:817–821 10.1084/jem.181.2.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verginis P., McLaughlin K.A., Wucherpfennig K.W., von Boehmer H., Apostolou I. 2008. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc. Natl. Acad. Sci. USA. 105:3479–3484 10.1073/pnas.0800149105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann B., Lehr H.A., Yancopoulos G., Valenzuela D., Murphy A., Stevens S., Schmidt J., Galle P.R., Rose-John S., Neurath M.F. 2008. The transcription factor NFATc2 controls IL-6–dependent T cell activation in experimental colitis. J. Exp. Med. 205:2099–2110 10.1084/jem.20072484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K.W., Sethi D. 2011. T cell receptor recognition of self and foreign antigens in the induction of autoimmunity. Semin. Immunol. 23:84–91 10.1016/j.smim.2011.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Robles D.T., Abiru N., Kaur P., Rewers M., Kelemen K., Eisenbarth G.S. 2000. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc. Natl. Acad. Sci. USA. 97:1701–1706 10.1073/pnas.040556697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.J., Davidson L., Eisenbarth G., Weiner H.L. 1991. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc. Natl. Acad. Sci. USA. 88:10252–10256 10.1073/pnas.88.22.10252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A.G., Ziegler R., Vardi P., Jackson R.A., Soeldner J.S., Eisenbarth G.S. 1989. Life-table analysis of progression to diabetes of anti-insulin autoantibody-positive relatives of individuals with type I diabetes. Diabetes. 38:1320–1325 10.2337/diabetes.38.10.1320 [DOI] [PubMed] [Google Scholar]