Transforming growth factor β and its regulation of epithelial mesenchymal transition contribute to the initiation of pulmonary metastatic outgrowth specifically through the down-regulation of epithelial cadherin.
Abstract
Reduced epithelial cadherin (E-cad) is a hallmark of invasive carcinomas that have acquired epithelial-mesenchymal transition (EMT) phenotypes. Here we show that down-regulated E-cad expression induced by transforming growth factor-β (TGF-β) and EMT preceded breast cancer outgrowth in three-dimensional (3D) organotypic assays and in the lungs of mice. Pharmacological inhibitors against focal adhesion kinase prevented metastatic outgrowth of newly seeded organoids, but not that of their fully established counterparts. Interrogating the D2-HAN (hyperplastic alveolar nodule) model of breast cancer dormancy and metastasis showed that dormant D2.OR cells produced branched organoid morphologies in 3D-cultures, and expressed robust quantities of E-cad that was uncoupled from regulation by TGF-β. In contrast, metastatic D2.A1 organoids were spherical and wholly lacked E-cad expression. Interestingly, D2.A1 cells engineered to re-express E-cad formed branched organoids, down-regulated β1 integrin expression, and failed to undergo metastatic outgrowth. The tumor-suppressing function of E-cad was inactivated by increased microenvironmental rigidity, and was not recapitulated by expression of an E-cad mutant lacking its extracellular domain. Twist expression, but not that of Snail, reinitiated metastatic outgrowth in dormant D2.OR cells. Our findings show that EMT and its down-regulated expression of E-cad circumvent breast cancer dormancy in part by facilitating β1 integrin expression necessary for metastatic outgrowth.
INTRODUCTION
Dissemination of tumor cells from the primary lesion is the most common event in the metastatic process and leads to the shedding of millions of carcinoma cells into the circulation each day (Yoshida et al., 2000; Cowin and Welch, 2007). Fortunately, metastasis is a highly inefficient and sequential cascade that requires carcinoma cells that have escaped the primary tumor to survive in the circulation, invade target organs, and reinitiate secondary tumor outgrowth. Indeed, initiation of metastatic outgrowth is the final critical event required to produce lethal metastatic disease. It therefore stands to reason that elucidating and targeting the molecular mechanisms that initiate the outgrowth of disseminated cancer cells could significantly improve the clinical course of patients with metastatic breast cancer. Expression of epithelial cadherin (E-cad) is a hallmark of a fully differentiated epithelium where it functions to maintain cell-cell junctions, thereby inhibiting aberrant cell proliferation and migration. Indeed, loss of E-cad function via genetic inactivation or epigenetic silencing is a common characteristic of systemically invasive carcinomas (Graff et al., 1998, 2000; Nass et al., 2000; Lombaerts et al., 2006). Epithelial-mesenchymal transition (EMT) is a physiological process that is hijacked by breast cancer cells, which enables them to initiate systemic dissemination by 1) down-regulating E-cad expression or activity; 2) separating cell-cell junctions; 3) invading the surrounding tissues; and 4) intravasating the vasculature or lymphatic system (Thiery, 2002; Wendt et al., 2009a). Recently, EMT and its accompanying reduction in E-cad expression were shown to be essential for the extravasation of cancer cells into secondary organs (Drake et al., 2009). Unfortunately, the specific impact of EMT and its inactivation of E-cad function in facilitating the metastatic outgrowth of disseminated breast cancer cells remains unknown.
The ability of breast cancer cells to initiate metastatic outgrowth has recently been linked to the expression and activity of β1 integrin and its downstream effector, focal adhesion kinase (FAK) (Barkan et al., 2008; Shibue and Weinberg, 2009). Moreover, alterations within the cytoskeletal architecture also appear necessary to enable dormant breast cancer metastases to reinitiate proliferative programs coupled to metastatic outgrowth (Barkan et al., 2008). EMT is classically associated with reorganization of the actin cytoskeleton (Miettinen et al., 1994), and recent studies by our group and others have established integrins and FAK as essential mediators of EMT induced by transforming growth factor-β (TGF-β) in normal and malignant mammary epithelial cells (MECs) (Bhowmick et al., 2001; Galliher and Schiemann, 2006; Wendt and Schiemann, 2009; Wendt et al., 2010). In addition, TGF-β1 signaling has been associated with the selection and expansion of cancer stem cells, a phenomenon mimicked by the constitutive expression of EMT-associated transcription factors (e.g., Twist, Snail, and ZEB1) and by the targeted deletion of E-cad in MECs (Mani et al., 2008; Battula et al., 2010; Taube et al., 2010). In light of these findings, we hypothesized that EMT endows disseminated cancer cells with the ability to overcome systemic dormancy and initiate metastatic outgrowth, leading to the subsequent formation of secondary macroscopic metastases.
Herein we engineered several breast cancer cell lines that possessed differing degrees of metastatic competency to stably express firefly luciferase, which was used to longitudinally track their growth in compliant and rigid three-dimensional (3D) cultures and in the lungs of mice. In doing so, we show that down-regulated E-cad expression induced by TGF-β and EMT was sufficient to prevent MEC differentiation and organoid branching, and instead produced dense, more spherical cultures that underwent metastatic outgrowth. We also characterized the EMT status of the D2-HAN (hyperplastic alveolar nodule) derivatives, D2.A1 and D2.OR, which are established models of the success and failure of pulmonary outgrowth, respectively. Indeed, recent studies have shown that these D2 cell derivatives differ not in their ability to extravasate into the lung, but in their ability to initiate metastatic outgrowth within the pulmonary microenvironment (Rak et al., 1992; Morris et al., 1994). Interestingly, the lung shares an elastic modulus reminiscent of that of the normal mammary gland (Butcher et al., 2009), indicating that disseminated breast cancer cells endure dramatic changes in tissue compliance as a part of the metastatic cascade (i.e., compliant mammary gland → rigid primary tumor → compliant disseminated sites). Along these lines, the differential metastatic outgrowth activities exhibited by these D2 cell derivatives can be recapitulated in vitro using compliant 3D organotypic cultures (Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). We show that systemically dormant D2.OR cells express robust quantities of E-cad and readily differentiate into branching organoid structures in 3D cultures, whereas their outgrowth-proficient D2.A1 cell counterparts are devoid of E-cad expression and fail to undergo MEC differentiation programs. Importantly, heterologous E-cad expression in metastatic D2.A1 cells induced their formation of branched organoid structures, as well as ablated their outgrowth in 3D cultures. Interestingly, the ability of E-cad to prevent the outgrowth of D2.A1 cells in 3D cultrues could be circumvented by inclusion of collagen within recombinant basement membrane cushions, suggesting that microenvironmental rigidity negates the tumor-suppressing functions of E-cad. Mechanistically, the down-regulated expression of E-cad induced by TGF-β and Twist, but not by Snail, was both necessary and sufficient to stabilize β1 integrin expression needed for efficient outgrowth of metastatic breast cancer cells. Collectively our findings establish E-cad and its response to EMT induced by TGF-β as a critical determinant for whether disseminated breast cancer cells acquire dormant or proliferative metastatic programs.
RESULTS
Down-regulated E-cad expression is required for 3D outgrowth of breast cancer cells
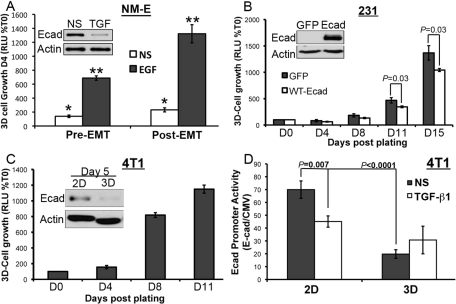
We recently demonstrated that inducing EMT before the intravenous inoculation of epidermal growth factor receptor (EGFR)-transformed breast cancer cells dramatically increased their ability to colonize the lungs and initiate secondary tumor outgrowth (Wendt et al., 2010). To address whether EMT induced by TGF-β could specifically increase the initiation of outgrowth, we treated these same EGFR-transformed NMuMG (NM-E) cells with TGF-β1 for 48 h to induce an EMT response that included decreased E-cad expression (Figure 1A, inset). Afterward, these pre- and post-EMT NM-E cell populations were sparsely seeded (5000 cells/cm2) into compliant 3D cultures to mimic metastatic outgrowth in the pulmonary microenvironment (Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). Bioluminescence quantification showed that the ability of TGF-β to induce EMT readily enhanced the 3D outgrowth of NM-E cells in the absence or presence of exogenous EGF (Figure 1A). We also reexpressed E-cad in the mesenchymal-like and E-caddeficient human MDA-MB-231 breast cancer cells, an experimental manipulation previously shown to decrease their metastatic potential and normalize their acinar morphology in 3D cultures (Mbalaviele et al., 1996; Zantek et al., 1999; Wang et al., 2002). Accordingly, bioluminescence growth assays demonstrated that heterologous E-cad expression in MDA-MB-231 cells significantly inhibited their growth in 3D cultures (Figure 1B). We extended these analyses to murine 4T1 breast cancer cells, which are highly metastatic despite their robust expression of E-cad (Figure 1C; Galliher-Beckley and Schiemann, 2008; Wendt and Schiemann, 2009). Interestingly, 4T1 cells exhibited a biphasic growth pattern in 3D cultures that was characterized by an initial latency phase, followed by a dramatic proliferative phase (Figure 1C). Examination of 4T1 cells about to undertake the outgrowth process (i.e., 5 d postplating in 3D culture) revealed a dramatic down-regulation of E-cad protein as compared with cells grown for the same period of time on standard tissue culture plastic (Figure 1C).
FIGURE 1:
Down-regulated E-cad expression is required for 3D outgrowth of breast cancer cells. (A) NM-E cells were treated with TGF-β1 (5 ng/ml) for 48 h (Post-EMT) before their 3D culture for an additional 4 d in the absence (NS) or presence of EGF (50 ng/ml) as indicated; 3D outgrowth was monitored by a bioluminescence growth assay. Inset, Immunoblot verifying down-regulation of E-cad in NM-E cells upon TGF-β1induced EMT. Data are the mean (±SE) of three independent experiments completed in triplicate. *, **, p < 0.05. (B) 3D outgrowth of human MDA-MB-231 breast cancer cells (231) expressing E-cad or GFP as a control was monitored longitudinally by bioluminescence. Data are the mean (±SE) of three independent experiments completed in triplicate. Inset, Immunoblot analysis verifying recombinant E-cad expression. (C) 3D outgrowth of murine 4T1 breast cancer cells was monitored by bioluminescence. Data are the mean (±SE) of three independent experiments completed in triplicate. Inset, E-cad protein levels were monitored by immunoblot analyses 5 d after propagating the cells in 2D or 3D cultures. (D) 4T1 cells engineered to stably express an E-cadluciferase reporter construct were grown in the absence or presence of TGF-β1 (5 ng/ml) under 2D or 3D conditions as in (C). Data are the mean (±SE) E-cadfirefly luciferase/CMVrenilla luciferase ratios obtained from three independent experiments completed in triplicate.
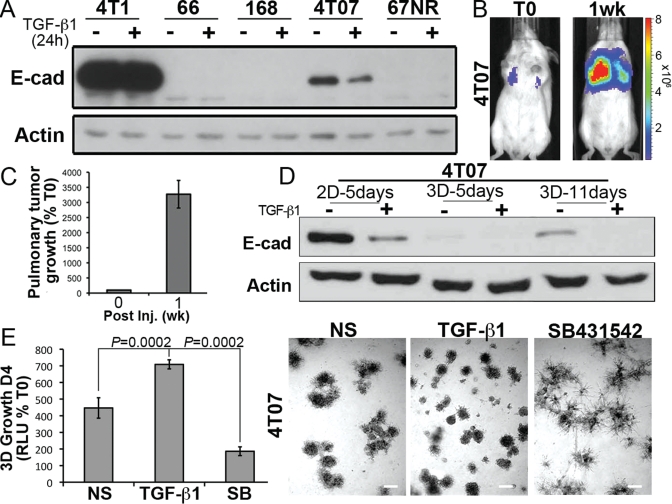
To further explore the connections between E-cad expression and 4T1 cell proliferation, we engineered 4T1 cells that stably expressed 1) firefly luciferase under the control of the human E-cad promoter, and 2) renilla luciferase under the control of the constitutively active cytomegalovirus (CMV) promoter. Using this dual bioluminescence reporter system, we show that the initiation of 3D outgrowth (Day 5) was concomitant with dramatically diminished E-cad promoter activity, even in the absence of exogenous TGF-β (Figure 1D). Finally, we extended these analyses to 4T07 cells, which are isogenic to 4T1 cells and are 1) systemically invasive and unable to metastasize from orthotopic primary tumors (Aslakson and Miller, 1992; Wendt et al., 2010); 2) competent to form lung tumors when injected into the lateral tail vein of syngeneic BALB/c mice (Figure 2, B and C); and 3) adept at down-regulating E-cad expression in response to TGF-β (Figure 2D). Similar to our findings in the 4T1 line, administration of TGF-β to 4T07 cells dramatically down-regulated their expression of E-cad, as did their propagation in 3D cultures (Figure 2D). Interestingly, E-cad expression returned upon prolonged (11 d) culture under 3D conditions (Figure 2D). These data suggest that diminution of E-cad expression is required to initiate organoid outgrowth, whereas macroscopic metastasis formation requires a mesenchymal-epithelial transition (MET) that includes the reexpression of E-cad (Hugo et al., 2007; Chao et al., 2010). Moreover, the 3D outgrowth latency exhibited by 4T1 organoids was not observed in 4T07 organoids, presumably due to their lower levels of E-cad expression relative to their 4T1 counterparts (Figures 1C and 2, A and E). Importantly, treatment of 4T07 cells in 3D culture with TGF-β increased the initiation of 3D outgrowth, whereas inclusion of a small molecule antagonist to TβR-I (TGF-b receptor type I) dramatically inhibited the initiation of 3D outgrowth (Figure 2E). The diminished 3D outgrowth of 4T07 organoids brought about by the inhibition of autocrine TGF-β signaling resulted in MEC differentiation and the acquisition of branched organoid morphologies, which contrasted sharply with the increased appearance of dense and independent organoids elicited by administration of TGF-β (Figure 2F; Wendt et al., 2010). Taken together, these findings are consistent with the notion that E-cad expression is down-regulated to allow breast cancer cells to abandon their inherent mammary branching phenotypes in favor of proliferative spheroids capable of initiating metastatic outgrowth.
FIGURE 2:
4T07 cells down-regulate E-cad to initiate 3D outgrowth. (A) Members of the murine 4T1 progression series were incubated in the absence or presence of TGF-β1 (5 ng/ml) for 24 h before monitoring their expression of E-cad and actin, which served as a loading control. (B) 4T07 cells (1 × 106) were injected into the lateral tail vein of 4-wk-old female BALB/c mice and imaged 30 min later (T0), and again 1 wk later, as indicated. Shown are bioluminescent images of representative mice from each time point. (C) Bioluminescence quantification of mice described in (B) (n = 4 mice). All mice succumbed to pulmonary tumor burden 12 d postinoculation. (D) 4T07 cells were grown in 2D or compliant 3D cultures for varying times in the absence or presence of TGF-β1 (5 ng/ml) as indicated. Afterward, E-cad expression was monitored by immunoblotting; actin immunoreactivity is provided as a loading control. Data are representative of three independent experiments. (E) Compliant 3D outgrowth of 4T07 cells propagated in the absence (NS) or presence of TGF-β1 (5 ng/ml) or the TβR-I inhibitor, SB431452 (SB, 10 μM), was quantified by bioluminescence. D4, Day 4 postplating. Afterward, the resulting organoids were imaged under phase-contrast microscopy (50×). Data are the mean (±SE) of two independent experiments completed in triplicate.
TGF-β is sufficient to decrease mammary branching of D2.OR cells
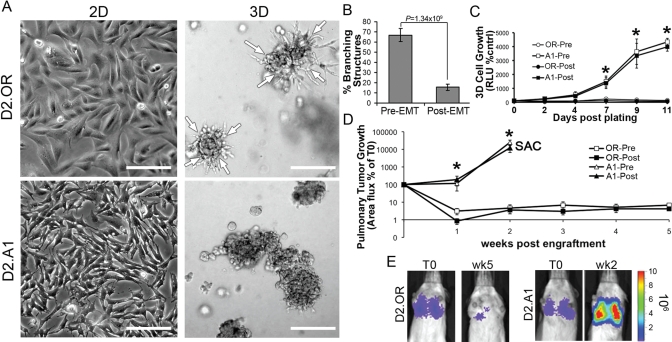
We next sought to use the isogenic D2-HAN cell system, which represents an established model to recapitulate the success and failure of metastatic pulmonary outgrowth (Rak et al., 1992; Morris et al., 1994; Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). Morphologically, nonmetastatic D2.OR cells are less mesenchymal-like than their metastatic D2.A1 counterparts when grown on tissue-culture plastic (Figure 3A). More importantly, D2.OR cells displayed a branched morphology when propagated in 3D cultures, whereas D2.A1 cells grew as independent metastatic clusters (Figure 3A, Supplemental Figure S1, Supplemental Movie S1). Furthermore, propagating D2.OR cells at higher densities in 3D cultures resulted in a dramatic aggregation and formation of branched structures within 18 h of plating (Supplemental Figure S1). Similar manipulations to D2.A1 cells, however, showed these metastatic MECs to be immotile and immediately proficient to undergo proliferative programs (Supplemental Figure S1). Indeed, although traditional microscopy intuitively depicts branched D2.OR organoids as being outwardly invasive, our time-lapse microscopy clearly shows that these branched organoid structures formed in response to cellular aggregation and inward migration (Supplemental Movie S2). Thus the 3D culture morphology of D2.OR cells manifests independent of their ability to proliferate. We recently demonstrated the role of EMT induced by TGF-β to prevent organoid branching in 3D cultures, an event that correlated with enhanced pulmonary outgrowth of breast cancer cells in mice (Wendt et al., 2010). Although identical experimental manipulations directed at D2.OR cells did reduce their ability to form branched organoid structures (Figure 3B), we were unable to rescue their 3D outgrowth in an EMT-dependent manner (Figure 3C). Moreover and irrespective of TGF-β signaling, we observed ∼510% of D2.OR cell inoculated into the lateral tail veins of mice to remain dormant in the lungs for a span of up to 5 wk (Figure 3, D and E). Collectively these findings suggest that the inherent program of nonmetastatic breast cancer cells to communicate and migrate toward one another during the formation of branched, multicellular organoids may underlie their inability to initiate proliferative programs within compliant pulmonary microenvironments.
FIGURE 3:
TGF-βmediated EMT decreases mammary branching of D2.OR cells. (A) D2.OR or D2.A1 cells were plated on either tissue-culture plastic (2D) or reconstituted basement membrane (3D) for 5 d, and subsequently were imaged under phase-contrast microscopy (200×). Arrows indicate the inward directional migration of the D2.OR branching structures. (B) D2.OR cells were left untreated (Pre-EMT) or treated with TGF-β1 (Post-EMT) for 48 h on plastic. Afterward, the cells were subcultured and grown in compliant 3D cultures for 5 d, at which point the mean percentage of branching structures was quantified from nine random fields of view over three independent experiments. (C) 3D outgrowth of Pre- and Post-EMT D2.OR and D2.A1 cells was quantified by bioluminescence. Data are the mean (±SE) of three independent experiments completed in triplicate. (D) Pre- and Post-EMT D2.OR and D2.A1 cells were injected into the lateral tail vein of BALB/c mice (1 × 106 cells/mouse). Data are the mean area flux values (±SE, n = 5 per group) normalized to injected values. Mice that received injections of D2.A1 cells succumbed to pulmonary tumor burden 2 wk following tumor cell inoculation (SAC). (E) Bioluminescent images of representative mice described in (C), imaged at the time of injection (T0) and 2 (wk2) or 5 (wk5) wk later. In (C) and (D), * p < 0.01 between the D2.OR and D2.A1 cells.
Outgrowth-proficient cells lack E-cad expression
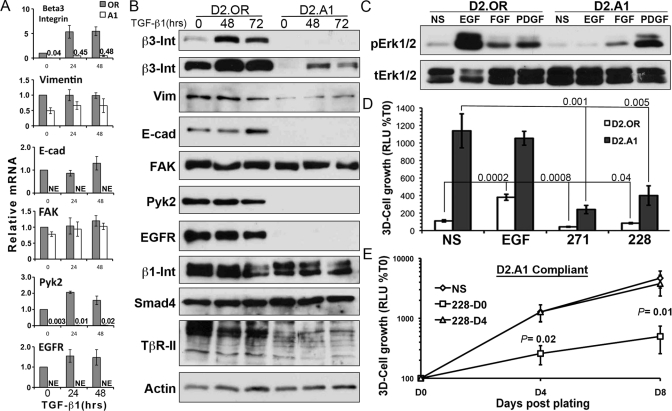
Given the differential requirement of EMT to enhance the pulmonary outgrowth of NM-E cells (Figure 1A; Wendt et al., 2010) and not that of D2.OR cells (Figure 3, CE), we next sought to verify the response of D2-HAN derivatives to TGF-β by characterizing a repertoire of target genes known to be regulated by this multifunctional cytokine. Both D2.OR and D2.A1 cells readily up-regulated the expression of β3 integrin in response to TGF-β (Figure 4, A and B), a molecule we established as one of the most sensitive and robust markers of TGF-β signaling (Galliher and Schiemann, 2006; Wendt and Schiemann, 2009; Wendt et al., 2010). Moreover, both D2-HAN derivatives displayed enhanced actin stress fiber formation in response to TGF-β1 stimulation (Supplemental Figure S2). Examination of other EMT markers identified several absolute gene expression differences between these D2-HAN derivatives. For instance, systemically dormant D2.OR cells expressed abundant quantities of EGFR, Pyk2, and E-cad (Figure 4, A and B), all of which were conspicuously absent in their metastatic D2.A1 counterparts. Surprisingly, administration of TGF-β to D2.OR cells failed to down-regulate their expression of E-cad (Figure 4, A and B). Moreover, chronic and continued culture of D2.OR cells with TGF-β actually increased the levels of E-cad mRNA (unpublished data) and protein (Supplemental Figure S3A). Inclusion of a TβR-I antagonist, SB431542, to these cultures resulted in a dramatic diminution of E-cad expression (Supplemental Figure S3A). In stark contrast, chronic TGF-β treatment of the NM-E cells led to a robust EMT that included down-regulated E-cad expression that readily reversed upon removal of exogenous TGF-β (Supplemental Figure S3, B and C). These findings identify a clear defect in the ability of the D2.OR cells to down-regulate E-cad expression as part of their EMT program, a deficit that may underlie their preferential acquisition of dormant phenotypes during pulmonary metastasis.
FIGURE 4:
Outgrowth-proficient cells lack E-cad expression. (A) D2.OR and D2.A1 cells were stimulated with TGF-β1 (5 ng/ml) for 048 h and subsequently were analyzed for alterations in the indicated transcripts by RT-PCR. Data are the mean (±SE) of three independent experiments. NE, no expression. (B) D2.OR and D2.A1 cells were stimulated with TGF-β1 for the indicated times and analyzed by immunoblot for the indicated proteins. Data are representative of at least two independent analyses. Int, Integrin; Vim, Vimentin. (C) Quiescent D2.OR and D2.A1 cells were stimulated with the indicated growth factors for 30 min and analyzed for the presence of phospho-ERK1/2 (p-Erk1/2) and total Erk1/2 (t-Erk1/2) as a loading control. Data are representative of three independent experiments. (D) 3D-outgrowth of luminescent D2.OR and D2.A1 cells in the presence of EGF (50 ng/ml) or inhibitors. Data are the mean Day 5 values (±SE) of two independent experiments completed in triplicate. NS, no stimulation; 271, FAK/Pyk2 inhibitor; 228, FAK inhibitor. (E) 3D outgrowth of D2.A1 cells was monitored longitudinally in the absence or presence of the FAK inhibitor, PF228, that was added either at the initiation of the experiment (228-D0) or 4 d after cell plating (228-D4). Data are the mean (±SE) of three independent experiments completed in triplicate.
We next examined the functional significance that results from the differential expression of EGFR observed between these D2-HAN derivatives. For instance, stimulating D2.OR cells with EGF resulted in a robust activation of extracellular signal-regulated kinase 1/2 (ERK1/2), a response that was undetectable in D2.A1 cells (Figure 4C). D2.A1 cells, however, readily active ERK1/2 in response to either fibroblast growth factor and platelet-derived growth factor (Figure 4C), demonstrating the competency of the Ras/mitogen-activated protein kinase pathway to be activated in these metastatic MECs. Along these lines, the 3D outgrowth of D2.OR organoids was strongly induced by EGF, which failed to enhance the growth of D2.A1 organoids presumably due to their deficiency in EGFR expression (Figure 4D). We also conducted 3D-outgrowth assays in the presence of either the dual FAK and Pyk2 inhibitor, PF-562,271 (PF271), or the FAK-specific inhibitor, PF-573,228 (PF228; Roberts et al., 2008), both of which dramatically decreased the 3D outgrowth of D2.A1 organoids (Figure 4D). Because D2.A1 cells fail to express detectable levels of Pyk2 (Figure 4, A and B), these findings implicate FAK as a critical effector operant in mediating the 3D outgrowth of D2.A1 cells. This conclusion is supported by a recent study that observed genetic depletion of FAK to prevent pulmonary outgrowth of D2.A1 cells (Shibue and Weinberg, 2009). It remains unclear, however, as to whether FAK regulates the initiation, the maintenance, or both phases of metastatic outgrowth. To address this question, we performed a longitudinal D2.A1 outgrowth study in which the cells were immediately incubated with either diluent or PF228, or in which the cells were allowed to grow for 4 d before the addition of PF228. Figure 4E shows that FAK antagonism significantly impeded the initiation of D2.A1 outgrowth but provided no therapeutic benefit in preventing the outgrowth of established D2.A1 organoids. Thus FAK protein tyrosine kinase activity appears essential solely for the initiation of proliferative programs by metastatic cell clusters, not for their continued outgrowth. These findings are consistent with those of several recent reports showing that therapeutic targeting of FAK was effective in decreasing the establishment of pulmonary metastases, but not the later stages of their eventual outgrowth in the lung (van Nimwegen et al., 2005; Wendt and Schiemann, 2009). Furthermore, given the established role of FAK in facilitating TGF-βinduced EMT (Cicchini et al., 2008; Wendt and Schiemann, 2009), including the inactivation of E-cad function, our findings also demonstrate that the diminution of E-cad expression facilitates the initiation of metastatic outgrowth.
3D culture is required to manifest the TGF-β paradox
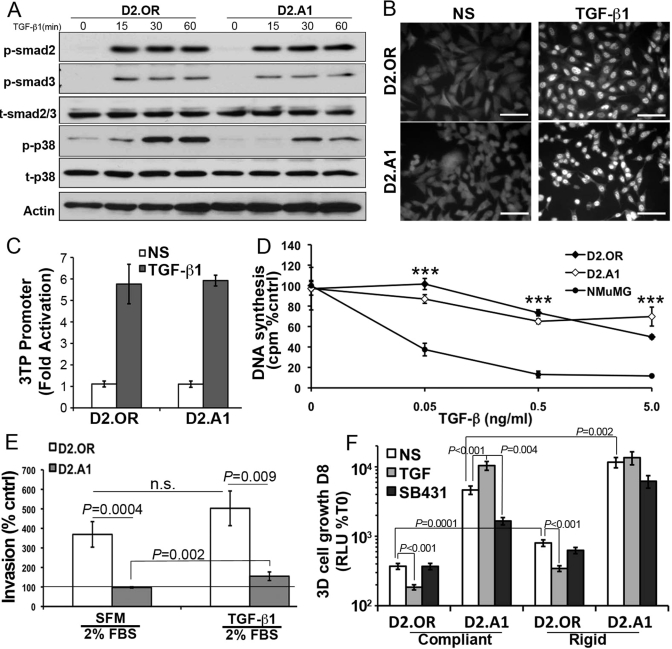
The switch in TGF-β function from a tumor suppressor to a tumor promoter is referred to as the “TGF-β Paradox” (Tian et al., 2010). In breast cancer, this phenomenon is characterized by a decrease in Smad2/3 activity and acquired resistance to the cytostatic activities of TGF-β (Wendt et al., 2009b), both of which are brought about by TGF-β stimulation of EMT (Supplemental Figure S4; Gal et al., 2008; Neil et al., 2008). Interestingly, the phosphorylation (Figure 5A), nuclear translocation (Figure 5B), and transcriptional activation (Figure 5C) mediated by Smad2/3 were similarly induced by TGF-β in both D2.OR and D2.A1 cells. Moreover, in traditional two-dimensional (2D) culture, both D2-HAN derivatives were similarly resistant to TGF-βmediated growth arrest as compared with NM-E cells (Figure 5D). These findings, together with those in Figure 4, argue that the derivation of D2.A1 cells likely did not transpire via a TGF-βdriven EMT event. Along these lines, we observed dormant D2.OR cells to be more invasive than their D2.A1 counterparts in Matrigel-coated transwell assays (Figure 5E). Administration of TGF-β, however, significantly enhanced D2.A1 cell invasion, whereas identical TGF-β treatments of D2.OR cells failed to significantly enhance their invasion through reconstituted basement membranes (Figure 5E). Intriguingly, Figure 5F shows that propagating these D2.HAN derivatives in 3D cultures readily manifested and recapitulated the “TGF-β Paradox” in vitro. For instance, TGF-β dramatically inhibited the outgrowth of D2.OR organoids in compliant 3D cultures, but significantly stimulated the proliferation of D2.A1 organoids under identical culture conditions (Figure 5F). Additionally, D2.A1 cell outgrowth was critically dependent on autocrine TGF-β signaling as evidenced by the ability of TβR-I antagonism to significantly inhibit D2.A1 outgrowth (Figure 5F). We also incorporated type I collagen to these 3D cultures to make them mechanically rigid, which significantly enhanced the basal growth of D2-HAN derivatives (Figure 5F; Barkan et al., 2010). Despite their enhanced growth in rigid 3D cultures, D2.OR cells remained sensitive to the cytostatic activities of TGF-β, whereas their D2.A1 counterparts remained insensitive to the antiproliferative activities of this cytokine (Figure 5F). Collectively these findings clearly demonstrate the necessity of 3D culture systems to manifest the “TGF-β Paradox.” Moreover, our results also indicate that the function of TGF-β to either suppress or promote pulmonary outgrowth largely reflects its ability to govern the expression levels of E-cad in metastatic breast cancer cells.
FIGURE 5:
3D culture is required to manifest the TGF-β paradox. (A) Quiescent D2.OR or D2.A1 cells were stimulated with TGF-β1 (5 ng/ml) for the indicated times and analyzed for the presence of phospho-Smad2 (p-smad2), phospho-Smad3 (p-smad3), and phospho-p38 mitogen-activated protein kinase (p-p38). Total Smad2/3 (t-smad2/3), total p38 (t-p38), and actin served as loading controls. Shown are representative immunoblots of three independent experiments. (B) Quiescent D2.OR and D2.A1 cells were stimulated with TGF-β1 as in (B) for 30 min before being processed for Smad2/3 immunofluorescence (400×). Data are representative of 10 random fields of view over two independent experiments. (C) D2.OR and D2.A1 cells were transfected with CMV-control and TGF-βdriven 3TP reporter constructs, and subsequently stimulated with TGF-β1 (5 ng/ml) for 18 h. Data are the mean (±SE) of three independent experiments completed in triplicate. (D) D2.OR, D2.A1, and NMuMG cells were incubated for 48 h in the absence or presence of increasing concentrations of TGF-β1 as indicated. Incorporation of [3H]thymidine was determined as a measure of DNA synthesis. Data are normalized to untreated controls and are the mean (±SE) of three independent experiments completed in triplicate. ***, p < 0.001. (E) D2.OR or D2.A1 cells were inoculated into the top well of an invasion assay in the absence or presence of TGF-β1 (5 ng/ml). Data are normalized to serum-free medium (line) and are the mean (±SE) of two independent experiments completed in triplicate. n.s., not significant. (F) 3D outgrowth of D2.OR and D2.A1 cells under compliant and rigid conditions was quantified by bioluminescence. Where indicated, the cells were grown in the presence of TGF-β1 (5 ng/ml) or the TβR-I inhibitor, SB431542 (SB431, 10 μM). Data are the mean (±SE) of at least two independent experiments completed in triplicate.
Expression of E-cad is sufficient to block the initiation of pulmonary outgrowth
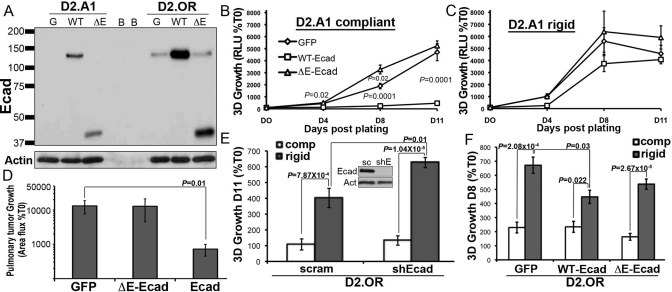
Given the differential expression of E-cad observed between D2.OR and D2.A1 cells, and the inability of D2.OR cells to down-regulate E-cad expression in response to TGF-β (Figure 4, A and B, and Supplemental Figure S3A), we next sought to investigate the mechanism by which loss of E-cad initiates metastatic outgrowth. In doing so, we engineered both D2-HAN derivatives to stably express either wild-type (WT) E-cad or a mutant E-cad molecule that lacked its extracellular domain (ΔE-Ecad) and functions as a dominant-negative protein through its ability to bind and sequester β-catenin in the cytoplasm (Dahl et al., 1996; Onder et al., 2008). Importantly, expression of WT E-cad, but not its ΔE-Ecad mutant, blocked the growth of D2.A1 organoids in 3D cultures (Figure 6B). Thus the homotypic binding properties of E-cad appear essential to its suppression of pulmonary outgrowth, whereas its ability to bind and sequester β-catenin appears dispensable for these events. Interestingly, the ability of E-cad to inhibit the 3D outgrowth of D2.A1 cells was circumvented by increased matrix rigidity (Figure 6C), suggesting that decreased tissue compliance may inactivate the tumor-suppressing activities of E-cad. Heterologous expression of E-cad in D2.A1 cells was resistant to administration of TGF-β or changes in matrix compliance and, more importantly, was able to elicit an epithelial morphology that prevented D2.A1 cells from undergoing EMT in response to TGF-β (Supplemental Figures S2 and S5). More importantly, Figure 6D shows that the expression of WT E-cad, but not its ΔE-Ecad mutant counterpart, effectively inhibited the initiation of metastatic outgrowth of D2.A1 cells in the lungs of BALB/c mice. Overexpression of WT or ΔE-Ecad had no effect on the dormancy of D2.OR cells under compliant pulmonary organotypic culture (Figure 6F); however, the enhanced growth of D2.OR cells on rigid matrices was 1) stimulated by depletion of endogenous E-cad (Figure 6E), 2) inhibited by the overexpression of WT E-cad (Figure 6F), and 3) unaffected by expression of ΔE-Ecad (Figure 6F). Taken together, these findings indicate that the extracellular domain of E-cad is sufficient to inhibit the initiation of pulmonary metastatic outgrowth by breast cancer cells.
FIGURE 6:
E-cad expression is sufficient to inhibit the initiation of pulmonary outgrowth. (A) Immunoblot showing recombinant expression of WT or ΔE-Ecad (ΔE) in the D2.OR and D2.A1 cells, and in GFP-expressing cells as a control (G). Blank wells are denoted as B. (B) The growth of control (GFP) or E-cad variant D2.A1 cells was quantified by bioluminescence in 3D outgrowth assays. (C) D2.A1 E-cad variant cells as in (B) were grown on 3D matrices that included type I collagen to increase its rigidity (Rigid). (D) Control (GFP) or E-cad variant D2.A1 cells were injected into the lateral tail vein of BALB/c mice, and pulmonary tumor growth was quantified using bioluminescence. Data are the mean (±SE; n = 10 mice per group) area flux values normalized to the injected values (%T0) 2 wk postinoculation. (E) E-cad deficiency significantly enhanced the growth of D2.OR cells in rigid 3D cultures. Inset, E-cad expression was stably depleted in D2.OR cells using shRNAs. sc, scrambled control; shE, Ecad-directed shRNA. Actin is shown as a loading control. (F) Overexpression of WT E-cad in D2.OR cells significantly inhibited their growth in rigid 3D cultures. Data for (B, C, E, and F) are the mean (±SE) of at least two independent experiments completed in triplicate.
E-cad regulates the expression of β1 integrin during 3D outgrowth
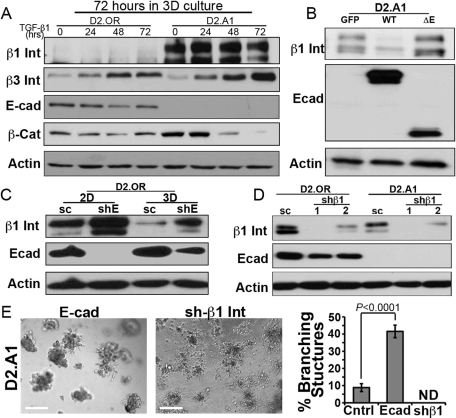
Our findings that increased matrix rigidity overcomes the ability of E-cad to suppress the metastatic outgrowth of breast cancer cells is supported by several recent studies that implicate β1 integrin as an essential mediator for the outgrowth of D2-HAN derivatives (Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). Unfortunately, D2.OR and D2.A1 cells express similar levels of β1 integrin (Figure 4B; Barkan et al., 2010), and, as such, D2.OR cells would be expected to be similarly proficient in metastatic outgrowth as compared with their D2.A1 counterparts. Given these conflicting results, we instead hypothesized that E-cad may cross-talk with and/or regulate the expression and activity of β1 integrin in dormant breast cancer cells. To address this hypothesis, we first confirmed that the differential expression of E-cad was retained in the D2-HAN derivatives following their propagation in 3D cultures. Although TGF-β administration had no effect on E-cad expression in either D2-HAN derivative, this experimental condition did reduce β-catenin expression in D2.A1 cells, suggesting that dysregulated β-catenin signaling does not underlie the outgrowth of D2.A1 organoids (Figure 7A). Interestingly and consistent with a recent report by Green and colleagues (Barkan et al., 2008), we found β1 integrin expression to be diminished in D2.OR cells propagated in 3D cultures (Figure 7A). More importantly, we observed heterologous expression of E-cad to suppress β1 integrin expression in D2.A1 cells (Figure 7B) and, conversely, depletion of E-cad expression in D2.OR cells to prevent their loss of β1 integrin expression in 3D cultures (Figure 7C). Equally intriguing, we found endogenous E-cad expression to be up-regulated significantly in dormant D2.OR cells upon their growth in 3D cultures (Figure 7C). These findings are in stark contrast to the down-regulated expression of E-cad observed in fully metastatic 4T1 cells (Figure 1, C and D), as well as in the outgrowth-proficient 4T07 cells (Figure 2D). Furthermore, the enhanced expression and/or stability of E-cad in D2.OR cells may explain our inability to reinitiate proliferation signals in D2.OR cells transduced with E-caddirected shRNAs. Along these lines, reciprocal depletion of β1 integrin failed to alter E-cad expression in D2.A1 cells (Figure 7D); however, this cellular condition completely prevented these D2.A1 cells from forming any multicellular organoids, which contrasted sharply with the acquisition of branched cellular aggregates formed by E-cadexpressing D2.A1 cells (Figure 7E, Supplemental Figure S6). Therefore our findings have identified a novel mechanism whereby the extracellular domain of E-cad promotes the down-regulation of β1 integrin expression in breast cancer cells, an event coupled to MEC differentiation and metastatic dormancy. These data are supported by prior findings pointing to an extracellular interaction between E-cad and β1 integrin, an event that inhibits β1 integrin function and down-regulates its expression (Whittard et al., 2002; Zhang et al., 2006). Thus down-regulation of E-cad in metastatic cells permits their expression and activation of β1 integrin, which facilitates the initiation of metastatic outgrowth.
FIGURE 7:
E-cad regulates β1 integrin expression during 3D outgrowth. (A) D2.OR and D2.A1 cells were propagated in 3D cultures for 72 h in the absence (0) or presence of TGF-β1 (5 ng/ml), and subsequently were analyzed for the expression of the indicated proteins by immunoblotting. Data are representative of at least three experiments. Int, integrin; Cat, catenin. (B) Control (GFP), WT E-cad (E-cad), or ΔE-Ecad (ΔE)-expressing D2.A1 cells were grown in 3D culture before visualizing the expression of β1 integrin (β1 Int), E-cad, and actin by immunoblotting. Data are representative of at least three experiments. (C) D2.OR cells expressing either a scrambled (sc) or an E-cadspecific (shE) shRNA were cultured in 2D or 3D conditions, and subsequently were analyzed for the expression of β1 integrin (β1 Int), E-cad, and actin as a loading control. (D) D2.OR and D2.A1 cells expressing either a scrambled (sc) or two distinct β1 integrin shRNAs (shβ1#1 and #2) were analyzed by immunoblotting to visualize the expression of β1 integrin (β1 Int), E-cad, and actin as a loading control. (E) D2.A1 cells expressing E-cad (E-cad) or depleted for β1 integrin (shβ1) were grown under compliant 3D cultures and imaged using phase-contrast microscopy (100×). Afterward, the percentage of branching structures was quantified. Data are the mean (±SE) of nine random fields of view over three independent experiments. ND, none detected.
Twist is sufficient to elicit an outgrowth initiation competent phenotype
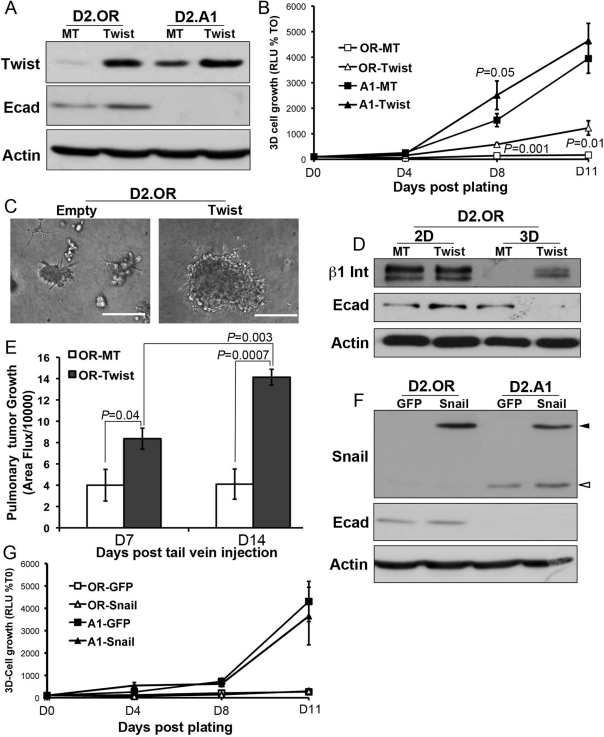
Having established E-cad as a molecular determinant of β1 integrin expression and metastatic outgrowth, we next sought to examine the role of known transcriptional regulators of E-cad expression in 1) governing the ability of TGF-β to regulate E-cad in systemically dormant breast cancer cells and 2) driving metastatic outgrowth in a TGF-βdependent or -independent manner. In doing so, we first monitored the expression of Twist, which is a master suppressor of E-cad expression (Yang et al., 2004). Figure 8A shows that Twist expression was indeed significantly higher in metastatic D2.A1 cells as compared with their dormant D2.OR counterparts. Accordingly, transgenic expression of Twist in D2.OR cells was sufficient to initiate their outgrowth in 3D cultures (Figure 8B). The acquisition of 3D outgrowth by Twist-expressing D2.OR cells was accompanied by their abandonment of branched mammary structures in favor of dense metastatic cell spheroids (Figure 8C). Interestingly, although Twist expression failed to decrease that of E-cad in D2.OR cells propagated in traditional 2D cultures (Figure 8, A and D), this transcription factor readily inactivated E-cad expression in D2.OR cells propagated in 3D cultures (Figure 8D). Consistent with our findings in Figure 7, Twist-mediated down-regulation of E-cad expression in 3D cultures stabilized β1 integrin expression and created an E-cad(low)/β1 integrin(hi) phenotype necessary for the initiation of 3D outgrowth (Figure 8D). Importantly, Figure 8E shows that Twist expression supported the initial metastatic outgrowth of D2.OR cells in the lungs of BALB/c mice. Finally, we also observed D2.A1 cells to express Snail (Figure 8F), which mediates EMT and down-regulates E-cad expression (Cano et al., 2000). Overexpression of a green fluorescent protein (GFP)-Snail fusion protein, however, failed to down-regulate the expression of E-cad (Figure 8F) and, more importantly, was unable to initiate 3D outgrowth by D2.OR organoids (Figure 8G). Taken together, our findings suggest that elevated expression of the EMT transcription factor Twist, but not that of Snail, is sufficient to initiate pulmonary outgrowth.
FIGURE 8:
Twist expression is sufficient to initiate pulmonary outgrowth. (A) Immunoblot analysis of Twist and E-cad (Ecad) expression in D2.OR and D2.A1 cells transduced with Twist or empty vector (MT). (B) The 3D outgrowth values of the cells described in (A) were quantified using bioluminescence and are the mean (±SE) of three independent experiments completed in triplicate. (C) Representative photomicrographs (200×) of control (Empty) and Twist-expressing D2.OR cells propagated in 3D cultures. (D) Control (MT) and Twist-expressing D2.OR cells were propagated for 5 d under 2D or 3D culture conditions before analyzing their expression of β1 integrin (β1 Int), E-cad, or actin as a loading control. (E) Control (MT) and Twist-expressing D2.OR cells were injected into the lateral tail vein of BALB/c mice, and pulmonary tumor formation was quantified by bioluminescence. Data are the mean (±SE) area flux values of five mice per group at the indicated time points. (F) Immunoblot analysis showing Snail and E-cad (Ecad) expression in D2.OR and D2.A1 cells stably transfected with GFP-Snail or with GFP as a control. Open arrowhead indicates endogenous Snail; closed arrowhead indicates the GFP-Snail fusion protein. (G) The 3D outgrowth of the cells described in (E) was quantified using bioluminescence assays. Data are the mean (±SD) of triplicate wells from a representative experiment that was performed three times with similar results.
DISCUSSION
EMT is a normal physiological process essential for proper development and wound healing (Taylor et al., 2010); however, aberrant initiation of oncogenic EMT can promote the acquisition of invasive phenotypes in developing and progressing carcinomas, thereby driving their systemic dissemination (Wendt et al., 2009a; Taylor et al., 2010; Tian et al., 2010). More recently, TGF-β stimulation of EMT was shown to establish a population of MECs that possess stem celllike properties (Mani et al., 2008). Thus the ability of individual breast cancer cells to undergo EMT in response to TGF-β may represent the molecular crux that endows TGF-β with oncogenic activity. Indeed, we recently found EMT induced by TGF-β to bestow EGF with oncogenic activity in breast cancers, as well as to enhance pulmonary tumor formation by breast cancer cells typically unable to undergo metastatic outgrowth (Wendt et al., 2010). In the current study, we used a 3D organotypic culture system to investigate the molecular mechanisms of metastatic dormancy and its potential regulation by TGF-β and EMT. In doing so, we established down-regulated E-cad expression as a critical event in EMT-driven initiation of metastatic outgrowth. Moreover, characterization of the EMT status of the D2-HAN model of pulmonary outgrowth revealed that dormant breast cancer cells expressed abundant levels of E-cad, which was notably absent in their metastatic proficient counterparts, suggesting that an EMT event had transpired (Rak et al., 1992; Morris et al., 1994; Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). Accordingly, heterologous E-cad expression significantly inhibited the outgrowth of metastatic D2.A1 and MDA-MB-231 cells propagated in 3D cultures. Finally, unlike metastatic breast cancer cells that do express E-cad constitutively (e.g., 4T1 cells), systemically dormant breast cancer cells were incapable of down-regulating E-cad expression when propagated in 3D cultures or when treated with TGF-β. Importantly, we demonstrated that combining Twist expression with compliant 3D cultures did result in the down-regulation of E-cad expression and the initiation of 3D outgrowth.
Recent studies using the D2-HAN system to model metastatic dormancy versus proliferative outgrowth have demonstrated the necessity of β1 integrin in mediating 3D outgrowth of these cells (Barkan et al., 2008, 2010; Shibue and Weinberg, 2009). Indeed, propagation of dysmorphic, malignant MEC organoids in 3D cultures requires aberrant expression and activity of β1 integrin (Weaver et al., 1997). Unfortunately, similar levels of β1 integrin between the D2-HAN derivatives suggest that its expression and activity cannot solely account for metastatic outgrowth failure (D2.OR) or success (D2.A1). Given the dramatic differences in E-cad expression between the D2 cell lines, we hypothesized that this adherens molecule is a key determinant in regulating β1 integrin action under compliant 3D growth conditions. Along these lines, the extracellular domain of E-cad can regulate β1 integrin function by acting as a heterotypic binding partner (Whittard et al., 2002) and by down-regulating β1 integrin expression (Zhang et al., 2006). Moreover, ectopic E-cad expression in the MDA-MB-231 cells, which significantly reduced their 3D outgrowth (Figure 1), effectively inhibited their expression of β1 integrin and activation of FAK (Wu et al., 2006). These findings wholly support our conclusion that the heterotypic interaction of E-cad with β1 integrin results in a loss of β1 integrin expression coupled to metastatic dormancy. In contrast to β1 integrin, we observed D2.OR cells to express markedly higher levels of β3 integrin as compared with D2.A1 cells. Given the essential role of β3 integrin in regulating TGF-β stimulation of breast cancer EMT and metastasis (Galliher and Schiemann, 2006, 2007; Galliher-Beckley and Schiemann, 2008; Wendt and Schiemann, 2009; Wendt et al., 2010), we suspect that elevated β3 integrin expression underlies the 1) enhanced invasive and migratory capacity of D2.OR cells, and 2) elevated invasion of D2.A1 cells in response to TGF-β. Thus β3 integrin expression represents one of the most sensitive and robust markers of TGF-β signaling during the invasive progression of metastatic mammary tumors. Collectively these findings highlight the dynamic interactions that transpire between carcinoma cells and their microenvironments in dictating metastatic proficiency of breast cancers; they also suggest that the delivery of extracellular E-cad mimetics may prevent the initiation of metastatic outgrowth by disseminated breast cancers through interaction and suppression of β1 integrin.
We recently demonstrated the ability of EMT induced by TGF-β to stabilize EGFR expression, an event that conferred oncogenic and invasive capacity to EGF (Wendt et al., 2010). Following chronic TGF-β treatment of NM-E cells, however, we observed their subsequent withdrawal and recovery from TGF-β to elicit a mesenchymal-epithelial transition (MET) that generated a population of MECs that lacked EGFR expression. This intriguing finding is consistent with the 1) malfunction of D2.A1 cells to express EGFR (Figure 4); 2) selection of highly mesenchymal E-cad(neg)/EGFR(neg) subpopulation of cells in response to TGF-β (Wendt et al., 2010); and 3) initiation of “RTK (receptor tyrosine kinase) switching” in post-EMT breast and lung cancer cells that acquire resistance to EGFR-directed therapies (Barr et al., 2008; Thomson et al., 2008). Future studies need to determine the molecular mechanisms whereby TGF-β, EMT, and E-cad dictate the repertoire of RTKs expressed in systemically disseminated breast cancer cells. This knowledge, together with enhanced understanding of how metastatic microenvironments and niches govern RTK switching, will enable the development of specialized therapies against dormant micrometastases harbored within vital tissues.
FAK is an essential signaling node targeted by E-cad, various RTKs, and β1 and β3 integrins. We (Wendt and Schiemann, 2009) and others (Cicchini et al., 2008) established FAK as an essential mediator of EMT induced by TGF-β and of metastatic outgrowth by D2.A1 cells (Shibue and Weinberg, 2009). We now show that FAK activity is absolutely essential to the initiation of 3D organotypic outgrowth but not to the maintained growth of established 3D organoids (Figure 4). Collectively these findings coalesce to support the notion that 1) EMT is required for the initiation of micrometastatic outgrowth, which is clearly FAK-dependent, and 2) MET is required for the continued proliferation and expansion of macrometastastic growth, which may be FAK-independent (Figure 4; Hugo et al., 2007; Chao et al., 2010). These findings have important therapeutic ramifications because FAK inhibitors are currently being evaluated in clinical trials (Lim et al., 2008); however, our present findings do not address the potential role of nuclear FAK and its FERM (Band 4.1/Ezrin/Radixin/Moesin homology) domain to promote carcinoma growth and survival, independent of its PTK (protein tyrosine kinase) activity (Lim et al., 2008). Indeed, we have recently observed TGF-β to promote the proliferation of breast cancer cells in part by stimulating the nuclear accumulation of FAK and its FERM domain, findings that are currently under investigation (M. K. Wendt, B. J. Schiemann, and W. P. Schiemann, unpublished data). Finally, Twist expression, but not that of Snail, initiated the pulmonary outgrowth of D2.OR cells. Despite this initial proliferative event, Twist-expressing D2.OR cells ultimately failed to form lethal macroscopic pulmonary lesions in the lungs of mice (unpublished data). These findings, together with those detecting the reexpression of E-cad in fully formed 3D organoids (Figure 2), strongly suggest that MET is required for the maintenance and continued outgrowth of pulmonary metastases, a reaction that fails to occur in cells engineered to constitutively express master EMT drivers such as Twist (Yang et al., 2004). Furthermore, these data suggest that Twist and Snail mediate distinct and nonredundant functions during EMT and metastasis. Indeed, recent findings delineate unique functions for these transcription factors in regulating carcinoma invasion, metastasis, and chemoresistance (Gupta et al., 2009; Casas et al., 2011). Future studies need to further delineate the downstream effectors of Twist and its control over EMT that are operant in overcoming senescence to generate metastatic initiating cells, and how these processes then convert to a MET to support the formation of lethal macroscopic pulmonary lesions (Ansieau et al., 2008; Mani et al., 2008). Experiments are currently under way to address these important questions.
MATERIALS AND METHODS
Cell lines and reagents
D2-HAN (D2.OR and D2.A1) and 4T1 derivatives (67NR, 168FARN, 66cl4, 4T07, and 4T1) were obtained from Fred Miller (Wayne State University, Detroit, MI) and cultured in DMEM supplemented with 10% fetal bovine serum and 1% Pen/Strep as described previously (Wendt et al., 2010). Bioluminescent D2-HAN and 4T1 derivatives were engineered to stably express luciferase by transfection with pNifty-CMV-luciferase as described (Wendt et al., 2008; Wendt and Schiemann, 2009). Dual bioluminescent 4T1 cells were generated by transfection with pcDNA3.1-renilla luciferase, followed by hygromycin selection. Afterward, renilla luciferase-expressing 4T1 cells were transfected with firefly luciferase the expression of which was driven by the human E-cad promoter (pGL4.20, 108 to +125 base pairs), followed by selection with puromycin. Expression of WT or a dominant-negative E-cad mutant lacking its extracellular domain (ΔE-Ecad; Dahl et al., 1996; Onder et al., 2008) was achieved by vesicular stomatitis virus-glycoprotein (VSVG) retroviral transduction of pWZL and selection with blastocidin (210 μg/ml). Cellular depletion of E-cad and β1 integrin expression was achieved by VSVG lentiviral transduction of pLKO.1 shRNA vectors (Open Biosystems, Huntsville, AL; Supplemental Table S2) as described (Wendt and Schiemann, 2009). Expression of Twist was achieved by VSVG retroviral transduction of pBabe and selected with puromycin. A pEGFP-C2 construct encoding GFP-Snail was provided by Thomas T. Egelhoff (Cleveland Clinic Foundation, Cleveland, OH), and breast cancer cells stably expressing this fusion protein were selected with G418.
In vivo bioluminescence imaging
WT D2.A1 and D2.OR (1 × 106 cells), E-cadexpressing D2.A1 (1 × 105 cells), or Twist-expressing D2.OR (1 × 106 cells) were injected into the lateral tail vein of 4-wk-old BALB/c mice, and pulmonary tumor development was assessed by weekly bioluminescence imaging normalized to an initial reading conducted immediately after inoculation. Bioluminescence imaging was performed on an IVIS-200 (Caliper Life Sciences, Hopkinton, MA) as described (Wendt and Schiemann, 2009; Wendt et al., 2009b, 2010), and in accordance with the Institutional Animal Care and Use Committees for the University of Colorado Denver and Case Western Reserve University.
3D organotypic growth assays
Malignant MECs were diluted in complete medium supplemented with 5% Cultrex (Trevigen, Gaithersburg, MD) and seeded onto solidified Cultrex cushions (50 μl/well) contained in 96-well plates (5000 cells/cm2). Where indicated, the cells were grown in the presence of 1) EGF (50 ng/ml); 2) the dual FAK/Pyk2 inhibitor, PF562271 (10 μM; Pfizer, Groton, CT); and 3) the FAK-specific inhibitor, PF573228 (10 μM; Pfizer). The medium/Cultrex mixtures were replaced every 4 d, and cellular outgrowth was detected by the addition of D-luciferin potassium salt (Caliper Life Sciences) to induce bioluminescence, which was quantified using a GloMax-Multi detection system (Promega, Madison, WI). Longitudinal cell growth was normalized to an initial reading taken 18 h after cell plating. Where indicated, collagen type I (3 mg/ml; BD Biosciences, Bedford, MA) was incorporated into Cultrex cushions to increase their rigidity. For long-term (514 d) morphology experiments, the cells were propagated on 175 μl Cultrex cushions contained within 48-well plates (5 × 104 cells/cm2), and short-term (24 h) cellular aggregation experiments transpired on 400 ml Cultrex cushions contained within 12-well dishes (7.5 × 104 cells/cm2). Time-lapse microscopy was performed using a Leica DMI6000 over a span of 18 h with the resulting images being captured once every 10 min.
Immunoblot assays
Lysates generated from 3D cultures were prepared by removing the medium/Cultrex overlay and adding lysis buffer directly to the organoids grown on top of Cultrex cushions. The resulting mixtures were incubated and shaken continuously for 60 min before harvesting the solubilized extract away from the remaining intact Cultrex cushion. Afterward, the whole cell extracts were processed for immunoblotting as described (Wendt and Schiemann, 2009; Wendt et al., 2009b, 2010). Antibodies used herein are described in Supplemental Table S1.
Cell biological assays
The ability of TGF-β1 (5 ng/ml) to alter serum-induced invasion of D2.OR and D2.A1 cells was analyzed using a Matrigel-coated transwell assay as described (Galliher and Schiemann, 2006). Incorporation of [3H]thymidine into cellular DNA to monitor DNA synthesis was accomplished as described (Wendt et al., 2009b), as was the detection luciferase reporter gene expression regulated by TGF-β and by 3D cultures (Wendt et al., 2010). For real-time PCR analysis, D2.OR and D2.A1 cells were stimulated with TGF-β1 (5 ng/ml) for 24 or 48 h, at which point total RNA was isolated using an RNeasy Plus Kit (Qiagen, Valencia, CA). Afterward, total RNA was reverse transcribed using the iScript cDNA Synthesis System (Bio-Rad, Hercules, CA), and semiquantitative real-time PCR was conducted using iQ SYBR Green (Bio-Rad) as described previously (Wendt and Schiemann, 2009). The oligonucleotide primer pairs used are provided in Supplementary Table S2. Indirect immunofluorescence of Smad2/3 subcellular localization or direct phalloidin fluorescence to visualize the actin cytoskeleton was accomplished as described previously (Wendt and Schiemann, 2009; Wendt et al., 2009b)
Statistical analyses
Statistical values were defined using an unpaired Student's t test, where a p value < 0.05 was considered significant. Values of p for all experiments analyzed are indicated.
Supplementary Material
Acknowledgments
We thank Pfizer for generously providing the small molecule inhibitors against FAK and Pyk2. W.P.S. was supported in part by grants from the National Institutes of Health (CA129359), the Susan G. Komen for the Cure Foundation (BCTR0706967), and the Department of Defense (BC084561). M.K.W. was supported by a fellowship from the American Cancer Society (PF-09120-01).
Abbreviations used:
- 2D
two-dimensional
- 3D
three-dimensional
- CMV
cytomegalovirus
- E-cad
epithelial cadherin
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial-mesenchymal transition
- ERK1/2
extracellular signal-regulated kinase 1/2
- FAK
focal adhesion
- GFP
green fluorescent protein
- HAN
hyperplastic alveolar nodule
- MEC
mammary epithelial cell
- NM-E
NMuMG cells transformed by EGFR
- RTK
receptor tyrosine kinase
- TGF-β
transforming growth factor-β
- TβRI
TGF-β receptor type I
- VSVG
vesicular stomatitis virus-glycoprotein
- WT
wild-type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-04-0306) on May 25, 2011.
REFERENCES
- Ansieau S, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- Barkan D, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr S, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis. 2008;25:685–693. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battula VL, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J. Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res. 2011;71:245–254. doi: 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao YL, Shepard CR, Wells A. Breast carcinoma cells reexpress E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9:179. doi: 10.1186/1476-4598-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L, Tripodi M. TGF-beta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314:143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Cowin P, Welch DR. Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia. 2007;12:99–102. doi: 10.1007/s10911-007-9041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Semb H. Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development. 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Mol Biol Cell. 2009;20:2207–2217. doi: 10.1091/mbc.E08-10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-beta. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG. Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem. 2000;275:2727–2732. doi: 10.1074/jbc.275.4.2727. [DOI] [PubMed] [Google Scholar]
- Graff JR, Greenberg VE, Herman JG, Westra WH, Boghaert ER, Ain KB, Saji M, Zeiger MA, Zimmer SG, Baylin SB. Distinct patterns of E-cadherin CpG island methylation in papillary, follicular, Hurthle's cell, and poorly differentiated human thyroid carcinoma. Cancer Res. 1998;58:2063–2066. [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. Epithelialmesenchymal and mesenchymalepithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Lim ST, Mikolon D, Stupack DG, Schlaepfer DD. FERM control of FAK function: implications for cancer therapy. Cell Cycle. 2008;7:2306–2314. doi: 10.4161/cc.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaerts M, et al. E-cadherin transcriptional down-regulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 1996;56:4063–4070. [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VL, Koop S, MacDonald IC, Schmidt EE, Grattan M, Percy D, Chambers AF, Groom AC. Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin Exp Metastasis. 1994;12:357–367. doi: 10.1007/BF01755879. [DOI] [PubMed] [Google Scholar]
- Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, Graff JR. Aberrant methylation of the estrogen receptor and E-cadherin 5′ CpG islands increases with malignant progression in human breast cancer. Cancer Res. 2000;60:4346–4348. [PubMed] [Google Scholar]
- Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- Rak JW, McEachern D, Miller FR. Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia. Br J Cancer. 1992;65:641–648. doi: 10.1038/bjc.1992.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WG, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Tian M, Neil JR, Schiemann WP. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal. 2010;23:951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen MJ, Verkoeijen S, van Buren L, Burg D, van de Water B. Requirement for focal adhesion kinase in the early phase of mammary adenocarcinoma lung metastasis formation. Cancer Res. 2005;65:4698–4706. doi: 10.1158/0008-5472.CAN-04-4126. [DOI] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt M, Schiemann W. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Research. 2009;11:R68. doi: 10.1186/bcr2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol. 2009a;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2008;27:1461–1471. doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- Wendt MK, Smith JA, Schiemann WP. p130Cas is required for mammary tumor growth and transforming growth factor-beta-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem. 2009b;284:34145–34156. doi: 10.1074/jbc.M109.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-beta-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010;29:6485–6498. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittard JD, Craig SE, Mould AP, Koch A, Pertz O, Engel JR, Humphries MJ. E-cadherin is a ligand for integrin alpha2-beta1. Matrix Biol. 2002;21:525–532. doi: 10.1016/s0945-053x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Wu H, Liang YL, Li Z, Jin J, Zhang W, Duan L, Zha X. Positive expression of E-cadherin suppresses cell adhesion to fibronectin via reduction of alpha5-beta1 integrin in human breast carcinoma cells. J Cancer Res Clin Oncol. 2006;132:795–803. doi: 10.1007/s00432-006-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yoshida BA, Sokoloff MM, Welch DR, Rinker-Schaeffer CW. Metastasis-suppressor genes: a review and perspective on an emerging field. J Natl Cancer Inst. 2000;92:17171730. doi: 10.1093/jnci/92.21.1717. [DOI] [PubMed] [Google Scholar]
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase. Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- Zhang W, Alt-Holland A, Margulis A, Shamis Y, Fusenig NE, Rodeck U, Garlick JA. E-cadherin loss promotes the initiation of squamous cell carcinoma invasion through modulation of integrin-mediated adhesion. J Cell Sci. 2006;119:283–291. doi: 10.1242/jcs.02738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.