Abstract
Background
The substrates for human atrial fibrillation (AF) are poorly understood but involve abnormal repolarization (action potential duration, APD). We hypothesized that beat-to-beat oscillations in APD may explain AF substrates and why vulnerability to AF forms a spectrum from control subjects without AF to patients with paroxysmal then persistent AF.
Methods and Results
In 33 subjects (12 persistent AF, 13 paroxysmal AF, 8 controls without AF), we recorded left (n=33) and right (n=6) atrial APD on pacing from cycle lengths (CL) 600–500 ms (100–120 beats/min) to AF. APD alternans required progressively faster rates for patients with persistent AF, paroxysmal AF and controls (CL 411±94 vs 372±72 vs 218±33 ms; p<0.01). In AF patients, APD alternans occurred at rates as slow as 100–120 beats/min, unrelated to APD restitution (p=NS). In this milieu, spontaneous ectopy initiated AF. At fast rates, APD alternans disorganized to complex oscillations en route to AF. Complex oscillations also arose at progressively faster rates for persistent AF, paroxysmal AF and controls (CL: 316±99 vs 266±19 vs 177±16 ms; p=0.02). In paroxysmal AF, APD oscillations amplified prior to AF (p<0.001). In controls, APD alternans arose only at very fast rates (CL < 250 ms; p<0.001 vs AF groups) just prior to AF. In n=4 AF patients in whom rapid pacing did not initiate AF, APD alternans arose transiently then extinguished.
Conclusions
Atrial APD alternans reveals dynamic substrates for AF, arising most readily (at lower rates and higher magnitudes) in persistent AF then paroxysmal AF, and least readily in controls. APD alternans preceded all AF episodes, and was absent when AF did not initiate. The cellular mechanisms for APD alternans near resting heart rates require definition.
Keywords: Atrium, Fibrillation, Action Potentials, Electrophysiology, Remodeling, Alternans, Electrical Restitution
Introduction
Human atrial fibrillation (AF) initiates when triggers 1, 2 interact with substrates 3. However, the nature of AF substrates and whether they may be detected to measure AF vulnerability remains unclear. Notably, substrates must account for AF initiation at slow sinus node rates after thoracic vein ectopy 1, 2, and also from rapid tachycardia 1, 2.
Alternans of action potential duration (APD) creates a milieu of repolarization dispersion that, in animal ventricles and in silico, may cause fibrillation directly, or by interacting with slow conduction or ectopy 4–6. In humans, right atrial APD alternans 7, 8 explains AF transitions from typical atrial flutter 9 or pacing 8, while left atrial APD alternans may explain AF initiation from pulmonary vein ectopy 10. However, atrial APD alternans has typically been reported only at fast rates, due to APD-rate dependence (restitution) 4, 5 or altered cellular calcium handling 5, 11, and it is unclear whether alternans differs between AF patients and subjects without AF.
We hypothesized that the remodeled atria of patients with persistent AF would exhibit APD alternans at slow heart rates, and that progressively faster rates would be required to elicit APD alternans in patients with paroxysmal AF or controls without AF. We tested this hypothesis by studying left and right atrial APD oscillations vis-à-vis AF initiation during incremental pacing from near-resting heart rates in patients with and without AF.
Methods
Patient Flow
We prospectively enrolled 33 patients referred for ablation to the Veterans Affairs and University of California Medical Centers in San Diego, 25 for ablation of AF (12 persistent) and 8 controls (6 with accessory pathways, 1 with atrial tachycardia, 1 with premature ventricular complexes) without AF. We approached for enrolment all consecutive patients undergoing AF ablation between December 2005 and March 2009 when research staff and catheters were available(n=54), excluding those with decompensated heart failure or coronary disease, and we report consecutive enrollees. Control subjects (recruited until November 2010) had a clinical indication for left-sided access, but no evidence for AF or atrial flutter on Holter or event monitor recordings. AF was defined as paroxysmal if it terminated ≤7 days, and persistent if it sustained >7 days or required cardioversion 12. In AF patients, atrial thrombus was excluded by transesophageal echocardiography. The study was approved by our joint Institutional Review Board, and all patients provided written informed consent. Some patients were included in our report that atrial APD restitution slope > 1 explains AF initiation from ectopic beats 10.
Catheter placement
Electrophysiology study was performed > 5 half-lives after discontinuing anti-arrhythmic medications (3 weeks after amiodarone; Table 1). A decapolar catheter was placed in the coronary sinus. After trans-septal puncture, a deflectable 7F monophasic action potential (MAP) catheter (EP technologies, Sunnyvale, CA) was advanced to record AP in the antra of the right or left superior pulmonary vein (figure 1) and high right atrium in n=6 patients(1 control), selected because tachycardias at these sites may initiate AF 1, 2.
Table 1.
Clinical Characteristics
| Characteristic | Persistent AF (n=12) | Paroxysmal AF (n=13) | Controls (n=8) | P |
|---|---|---|---|---|
| Age/years | 65±11† | 64±8† | 48±15 | 0.010 |
| Gender/M, F | 12,0 | 12,1 | 6,2 | 0.16 |
| Duration of AF/months | 71±72 | 64±132 | - | 0.87 |
| Left atrial diameter/mm | 47±5*† | 40±5 | 36±2 | <0.001 |
| LV ejection fraction/% | 54±11 | 60±7 | 62±8 | 0.19 |
| NYHA | ||||
| Class I/II | 11 | 13 | 8 | 0.41 |
| Class III | 1 | 0 | 0 | |
| Coronary Disease/n (%) | 3 (27) | 5 (46) | 1 (13) | 0.42 |
| Medications/n | ||||
| ACEI/ARB | 6† | 8† | 0 | 0.017 |
| Statins | 6 | 9 | 2 | 0.14 |
| Beta-blockers | 9† | 5 | 3 | 0.13 |
| Class I agents | 1 | 1 | 0 | 0.71 |
| Amiodarone | 1 | 0 | 0 | 0.41 |
| Sotalol | 1* | 6† | 0 | 0.017 |
| Dofetilide | 0 | 2 | 0 | 0.19 |
Key: NYHA, New York Heart Association Functional Class; ACEI, angiotensin converting enzyme inhibitors; ARB, aldosterone receptor blockers.
p<0.05 vs paroxysmal AF;
p<0.05 vs controls
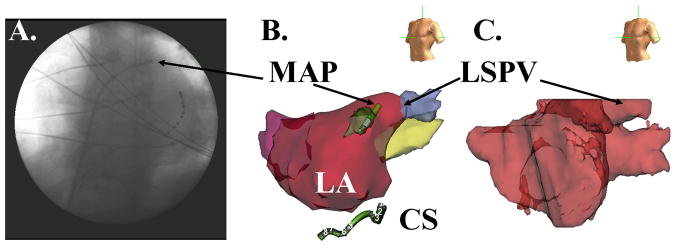
Figure 1. Left Atrial Monophasic Action Potential (MAP) recording site.
(A) Fluoroscopy (Left Anterior Oblique 30°) showing MAP catheter placed near the left superior PV (LSPV), and the coronary sinus (CS) catheter; (B) Digital Reconstruction of LA (NavX, St Jude Medical, CA). (C) Segmented 64-slice computed tomogram imported into NavX for positional reference.
Pacing Protocol
Patients in AF were electrically cardioverted to sinus rhythm and studied after 15 minutes, prior to ablation. APs were recorded from the distal poles of the MAP catheter, while pacing from the proximal poles 9 or a nearby stable position. Pacing was delivered for 74 beats at cycle lengths (CL) 500 ms, 450 ms, 400 ms, 350 ms, 300 ms, then in 10 ms steps to AF or capture failure (n=6), whichever came first. In n=5 patients, pacing started at 600 ms, 550 ms then the above sequence.
Signal filtering was 0.05–500 Hz (APs), 30–500 Hz (other intracardiac signals) and 0.05–100 Hz (ECG). Signals were digitized at 1 kHz to 16-bit resolution (Bard Pro, Billerica, MA) and exported for analysis using software written in Labview (National Instruments, Austin, TX).
Measurement of Action Potential Duration
We measured APD using validated methods 9, 10, 13–16 with manual verification. We assigned AP onset as the time of maximal computed upstroke dV/dt, and determined phase II voltage and phase IV (diastolic) voltage in the 5 ms preceding AP upstroke (figure 2A). APD90 was measured from AP onset to 90% voltage recovery from phase II. Diastolic interval (DI) spans from APD90 of the prior beat to AP onset 10.
Figure 2. APD Alternans At Slow Rates in Persistent AF, Disorganizing to Complex Oscillations Prior to AF.
This man (LA diameter 44 mm, LVEF 44 %) showed substantial APD alternans at 500 ms, (A) CL 450 ms (mean ΔAPD=120 ms for 10 beats), (B) CL 400 ms during intermittent AV block, with a phase reversal (*), (C) CL 290 ms with multiple phase reversals, and (D) CL 260 ms, with complex oscillations then AF. (E) Maximum APD restitution slope <1. (Key: AP phases II and IV marked; A, Atrial; V, Ventricular signal).
Measurement of APD Restitution
We constructed curves of APD restitution during pacing using ≥5 (DI, APD90) pairs at each pacing CL. Maximum slope was determined from linear fits for the shortest 30-ms DI segment with data (e.g. 0–30 ms or 10–40 ms)as previously described 10.
Measurement of APD Alternans and Complex Oscillations
We measured pairwise differences in APD90 (ΔAPD, figure 2A), summarized by mean absolute ΔAPD for the last 10 beats at each CL. Alternans was assigned if ΔAPD alternated in polarity with magnitude ≥5 % of mean APD 9 (baseline APD varies ≤2 % 7, 9). Since APD alternans may disorganize to complex oscillations prior to arrhythmia onset 17, 18, via ‘phase reversals’ (e.g. Long-Short-Long-Short-Long LSLSL proceeding to LSLSS*; figure 2), or AP shape changes (figure 3), we also report mean absolute ΔAPD for 10 non-alternating beats(if ≥5 % mean APD).
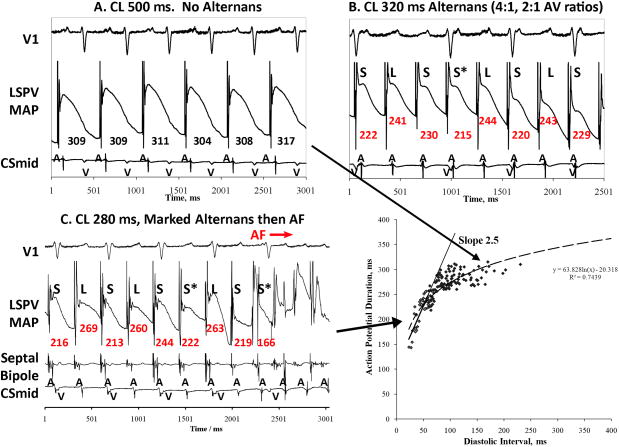
Figure 3. Intermediate Rate Alternans in Paroxysmal AF, With Rate-Dependent Onset of Complex Oscillations Prior to AF.
This 61 year old man (LA diameter 42 mm, LVEF 65 %), showed (A) No APD alternans at CL 500 ms, (B) APD alternans at CL 320 ms (LSL…) during 4:1, 2:1 AV ratios, that (C) exaggerated at CL 280 ms, with complex oscillations abruptly before AF onset. (D) APD restitution had maximum slope>1, but was <1 at time of APD alternans onset. (Key: as figure 2).
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD). ANOVA was used to compare variables between 3 patient groups, such as APD alternans magnitude or onset CL, with post-hoc Tukey-Kramer tests to identify differences between group pairs. Paired continuous variables, such as the diastolic interval of APD alternans and APD restitution slope, were compared using linear regression and the paired t-test. Group differences in APD90 at selected CLs were evaluated with separate ANOVAs using a Bonferroni correction for testing multiple CL bins. APD restitution slope was also compared between groups using a mixed effects model with an unstructured covariance matrix that incorporated all observations with diastolic intervals (DI) in the range 0ms < DI <100ms. This model adjusts for correlated observations within individuals. A log transform of DI was applied to account for the non-linear relationship over this broader DI range. The Fisher exact test was applied to contingency tables. Bi-atrial data were primarily analyzed; right versus left atrial data are presented separately. A probability of < 5 % was considered statistically significant.
Results
Our patient sample is described in Table 1. Patients with persistent AF had larger left atria than those with paroxysmal AF or controls. We performed 39 incremental pacing experiments in left (n=33) and right (n=6) atria, and their transitions to AF.
APD Alternans Preceded AF Transitions, In Different Patterns Between Groups
APD alternans preceded every AF initiation, and arose at slower rates (i.e. more easily) in patients with persistent AF than paroxysmal AF than controls (table 2). With continued pacing, APD alternans transitioned to complex oscillations(figs 2–4), also at slower rates in persistent AF than paroxysmal AF than controls (ANOVA p=0.03). Sinus rhythm rates did not differ between groups.
Table 2.
Repolarization Dynamics
| Characteristic | Persistent AF | Paroxysmal AF | Controls | P |
|---|---|---|---|---|
| No. Segments: LA/RA | 12/3 | 13/2 | 8/1 | 0.81 |
| Sinus CL, ms | 997±378 | 1067±215 | 890±73 | 0.40 |
| CL of AF Initiation, ms | 208±31 | 232±35 | 200±25 | 0.068 |
| Max S1-Rest slope | 1.1±0.5 | 1.2±0.5 | 0.8±0.2 | 0.078 |
| APD Alternans | ||||
| Onset CL, ms | 411±94† | 372±72† | 218±30 | <0.001 |
| Mean APD at Onset, ms | 223±51 | 260±37† | 181±24 | 0.008 |
| DI (pre-shorter APD), ms | 162±79*† | 100±48† | 29±12 | <0.001 |
| APDR Slope @ Onset DI | 0.29±0.17 | 0.35±0.15 | 0.44±0.10 | 0.11 |
| Magnitude@onset, ms | 24±13 | 23±13 | 13±5 | 0.16 |
| Magnitude@onset, % APD | 11±5 | 9±5 | 8±4 | 0.41 |
| Complex APD Oscillations | ||||
| Onset CL, ms | 316±99† | 266±19 | 177±16 | 0.017 |
| APD at onset, ms | 205±49 | 204±29 | 194±65 | 0.90 |
| Magnitude, ms | 23±24 | 18±26 | 7±6 | 0.43 |
| Magnitude, % APD | 10±9 | 9±12 | 4±4 | 0.56 |
p<0.05 vs paroxysmal AF;
p<0.05 vs controls
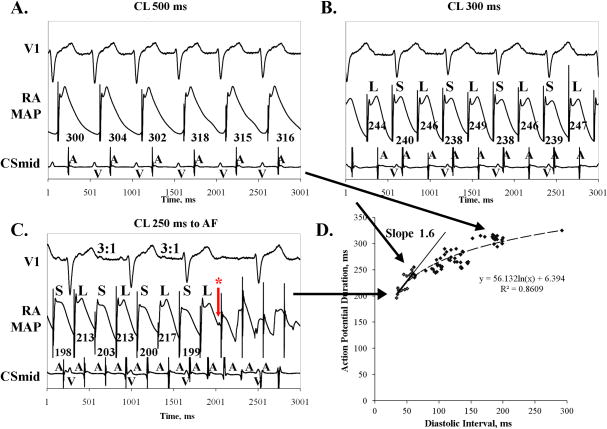
Figure 4. Amplification of Rate-Dependent APD Alternans in Paroxysmal AF Prior to AF.
This patient (LA diameter 39 mm, LVEF 59 %) showed (A) No APD alternans at CL 500 ms; (B) APD alternans at CL 300 ms; (C) marked AP Alternans at CL 250 ms(phase II/III shape), during 3:1 AV conduction prior to AF initiation. Asterisk indicates far-field AF activation or an after-depolarization triggering AF. (D) APD restitution slope was >1 at its maximum, but <1 at APD alternans onset. (Key: as figure 2).
In a patient with persistent AF, figure 2 shows left atrial APD alternans at slow rates that disorganized at faster rates to complex APD oscillations en route to AF. At CL 500 ms (120 beats/min), APD alternans had magnitude 58 ms (22 % of mean APD), increasing to 120 ms (44% of APD 272 ms, fig 2A) at CL 450 ms(130 beats/min), that continued at CL 400 ms (150 beats/min) during AV block (21% of APD; phase reversals marked *; fig. 2B) and CL 290 ms (207 beats/min; 20 % of APD, fig 2C). At CL 210 ms(286 beats/min), complex APD oscillations led directly to AF (fig 2D).
In patients with paroxysmal AF, figures3 and 4 show APD alternans at intermediate rates en route to AF. In figure 3A, pacing CL 500 ms (120 beats/min) showed APD 309±8 ms without alternans (< 5%of APD). At CL 320 ms (188 beats/min), APD alternans had magnitude 25 ms during 4:1 and 2:1 AV conduction (10 % of APD 243ms; fig 3B), increasing at CL 280 ms (214 beats/min) to 67 ms (34 % of APD 198 ms; fig 3C) immediately preceding AF. Figure 4 shows no APD alternans at CL 500 ms (120 beats/min, fig 4A), small amplitude APD alternans at CL 300 ms (200 beats/min, fig 4B) that increased at CL 250 ms (240 beats/min) to magnitude 14 ms (7 % of APD=213 ms) during 3:1 AV conduction just prior to AF. The small MAP signal (asterisk) preceding AF may indicate a far-field signal or an after depolarization.
Although paroxysmal AF patients typically present in sinus rhythm and persistent AF patients in AF, 2 paroxysmal AF patients presenting in AF developed APD alternans at CL=400 ms and 300 ms that increased with rate prior to AF. One persistent AF patient presented in sinus rhythm with APD alternans at CL 500 ms (7%of mean APD) transitioning via complex oscillations to AF.
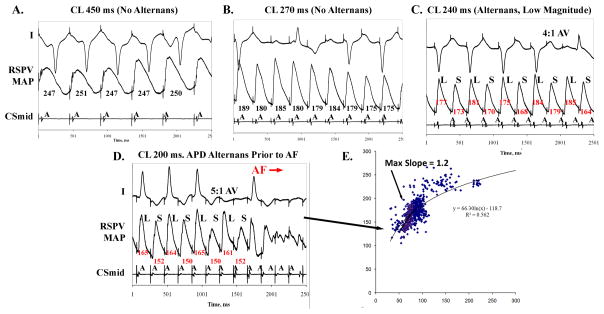
Notably, control subjects showed APD alternans of small magnitude (13±5 ms, 8±4% of APD), developing only at very rapid rates (CL 218±30 ms, p<0.001 against either AF group)just before AF. Figure 5 shows a control patient in whom APD alternans was absent at CL between 500 ms and CL 250 ms. Alternans developed at CL 240 ms (250 beats/min at small magnitude (5% of mean APD) during 2:1 and 4:1 AV ratios, then increased at CL 200 ms (300 beats/min; 8% of APD) immediately prior to AF. No control subject had APD alternans at CL ≥250 ms (p<0.001 vs either AF group).
Figure 5. Control Subject with APD Alternans Only At Very Fast Rates Preceding AF.
This 59 year old man (LA diameter 38 mm, LVEF 66%) showed no APD alternans at CL (A) 500 ms to (B) CL 240 ms. (C) APD alternans appeared at CL 210 ms during 1:1 and 2:1 AV conduction. (D) At CL=180 ms, APD alternans amplified to 6.5 % of APD immediately preceding AF initiation. (D) APD restitution slope=1 at the fast rates of APD alternans onset.(Key: as figure 2).
Onset of APD Alternans Did Not Correlate with APD Restitution Slope or AV Conduction
APD restitution did not differ significantly between groups when analyzed for maximum slope (table 2)or by a mixed-effects model for the log-linear relationship (p=0.15). Maximum restitution slope was > 1 in some patients (figures 3D, 4D) and < 1 in others (figure 2E). Importantly, at the onset of APD alternans in AF patients, APD restitution slope was always < 1 (slope 0.8 in figure 2; 0.5 at CL 300 ms in figure 3D; 0.4 at CL 300 ms in figure 4D), even if maximum slope was > 1 at faster rates. In controls, APD alternans arose both when restitution slope was near/above 1 (e.g. fig. 5E) and <1 (table 2).
Onset CL for APD alternans differed from onset CL of 2:1 AV conduction (p<0.001, paired t-test), and was longer than onset CL of 2:1 AV conduction by 79±72 ms (persistent AF), 72±62 ms (paroxysmal AF) and 18±25 ms (controls). Each of figures 2–5 show APD alternans when 2:1 AV conduction was present and absent.
Rate Dependence of APD Oscillations
Figure 6A shows the atrial APD-CL curve (figure 6A) for each group. In AF patients, APD was shorter for those with persistent than paroxysmal AF at CL 500 ms and 400 ms (p<0.0125, using Bonferroni corrections), but not CL 300 ms or 200 ms.
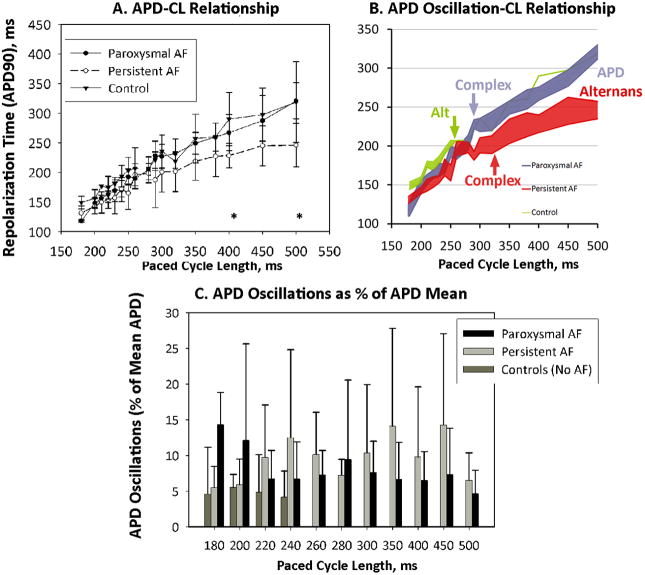
Figure 6. APD Dynamics.
(A) APD-Cycle Length had an overall trend between AF groups of p=0.08, and differed significantly at CL indicated *; (B) APD Oscillation-Cycle Length. AF patients show marked APD oscillations (alternans at slow rates transitioning to complex oscillations at faster rate), while controls show APD alternans only at very short CL; (C) APD Oscillations as a Percentage of Mean APD. In paroxysmal AF, APD oscillations increased in magnitude with rate. In persistent AF, APD oscillations onset at slow rates had modest rate-dependence. In controls, APD oscillations arise only at very fast rates.
Notably, the CL of onset and CL range for which APD oscillations was observed differed markedly between groups. Figure 6B illustrates the magnitude of APD oscillations (pair-wise APD range over 10 beats, centered at APD mean) for each CL. APD oscillations spanned all rates in persistent AF (red envelope) and paroxysmal AF (blue envelope)patients, but occurred only at fast rates in controls (green envelope). In 3 AF patients paced at 100 beats/min (CL 600 ms; not plotted), APD oscillations occurred with magnitude 13±10% of mean APD=297±74 ms. Considering only patients whose APD alternans arose without 2:1 AV conduction, alternans onset CL was 444±80 ms (persistent AF), 418±59 (paroxysmal AF)and 245±49 (controls; p=0.004, ANOVA).
Figure 6C summarizes the magnitude of APD oscillations (as a % of APD) against CL for each group. Persistent AF exhibited APD oscillations for all rates, while paroxysmal AF exhibited rate-dependent increases in APD oscillations (p<0.001). Controls showed small magnitude APD alternans only at very rapid rates.
Relative to AF initiation, patients with paroxysmal AF showed amplified APD oscillations as CL shortened to AF initiation (p<0.001); patients with persistent AF showed a weak negative relationship (p<0.05).
APD Dynamics vis-à-vis AF Vulnerability and AF Initiation
APD oscillations separated patients at different stages of remodeling (figure 6). Unlike controls, AF patients showed APD oscillations > 5 % of APD between 120 beats/min (CL 500 ms) to 240 beats/min (CL 250 ms). Paroxysmal AF patients differed from persistent AF patients by amplification in APD oscillations with rate, and APD > 303 ms at CL 500 ms.
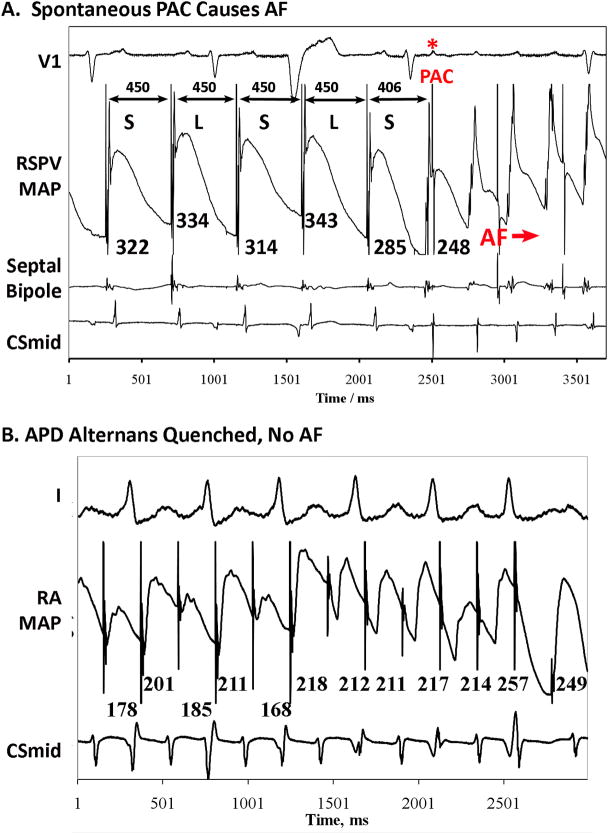
APD oscillations led to AF via 2 mechanisms. Figure 7A illustrates APD alternans at slow rates (CL 450 ms illustrated; also at CL 500 ms), when a spontaneous PAC (asterisked) triggered AF. Of note, APD restitution from single extrasystoles in this patient (S2-restitution) had maximum slope >1 (=1.79). Figures 2–5 show transitions to AF after amplified APD alternans (figs. 3–5) or via complex APD oscillations (fig. 2).
Figure 7.
A. Spontaneous Premature Atrial Complex (PAC*) Initiates AF in the Milieu of APD Alternans at relatively slow rates. This happened multiple times in this patient at various rates. B. Failure to Initiate AF: APD Alternans Quenches that slowed atrial rate and prevented AF initiation.
APD Dynamics when AF was not induced
Rapid pacing failed to initiate AF in n=4 patients. All patients initially exhibited APD alternans, although 2:1 capture then intervened to suppress alternans. This is shown for a persistent AF patient with APD restitution slope <1(figure 7B), but was also seen in paroxysmal AF and in patients with maximum APD restitution slope > 1.
Right Versus Left Atrial APD Dynamics
Quantitatively, there were no differences between atria in APD alternans onset CL (p=0.81) or magnitude as a percentage of APD (p=0.26). Qualitatively, APD alternans in the right atrium was also marked at slow rates in AF patients. For instance, at CL 450 ms, APD alternans had a range of 1.8–120 ms (0.6–44 % of APD) in left atrium, and 8.2–32 ms (3.4–14 % of APD) in right atrium. Bi-atrial APD statistics were similar when right atrial data were excluded from analysis.
Discussion
This study shows that alternans of action potential duration in human left and right atria indicate progressive substrates for, and susceptibility to, atrial fibrillation. We observed APD alternans of large amplitude near resting rate in patients with persistent AF, at intermediate amplitude and rates in patients with paroxysmal AF, and of small amplitude only at very rapid rates (>230 beats/min) just prior to induced AF in control subjects. APD oscillations preceded all transitions to AF. In patients with APD alternans at slow rates, ectopy initiated AF. In patients with APD alternans at faster rates, oscillations became complex prior to AF. In the few patients in whom AF was not induced by pacing, activation delayled to capture failure that quenched APD alternans. Thus, atrial APD alternans may be a clinical marker of susceptibility to AF and, in AF patients, arose at markedly slower rates than observed in experimental models.
Differential Onset and Dynamics of APD Alternans
The magnitude and onset rate of APD alternans paralleled the progressive susceptibility to AF observed in control subjects and patients with paroxysmal and persistent AF.
In control subjects, APD alternans occurred only at rapid rates, agreeing with a vast literature of computational and animal studies 5, 6, 11. However, marked APD alternans in AF patients near resting heart rates (fig 2, summarized in fig 6) stands in sharp contrast, and points to potentially important differences in AF between humans and prior experimental models.
At rapid rates, APD alternans may be explained by the ‘restitution hypothesis’. Restitution is the relationship of APD to rate, and a maximum slope >1 leads to APD alternans at rapid rates and wave break 5, 19. However, we found no overall relation between the onset rate of atrial APD alternans and restitution slope, as also reported in human ventricles 15. Thus, while APD restitution slope >1 may explain AF initiation from ectopy in selected patients with paroxysmal AF (fig. 7A and prior work10) or control subjects (fig. 5), it cannot explain APD alternans at slow rates in AF patients (when APD restitution is flat).
Cellular calcium overload is also a potential mechanism for APD alternans in the atrium, as it has been shown to lower the onset rate of APD alternans in remodeled versus control animal 5, 11, 20 and human 16, 21 ventricles. In animal models, diminished L-type calcium current and abnormal intracellular calcium handling from electrical remodeling causes calcium overload 22. Human atrial myocytes also exhibit altered calcium handling 23, that explains AF in computational models 6, and may explain reduced right atrial APD alternans by verapamil 8. Nevertheless, direct human evidence for calcium abnormalities as a cause for APD alternans or AF are lacking.
Atrial APD alternans may also be explained by electrical remodeling of membrane ion currents 22. In canine atria, remodeling elevates potassium currents, compounding the effects of calcium overload to shorten ERP and compress APD range 22. Myocytes from remodeled human atria also exhibit increased inward repolarizing currents that shorten APD 24, 25. This study confirms in vivo that left atrial APD has a flattened rate-response in patients with persistent AF compared to paroxysmal AF or controls (figure 6A, table 2). However, since APD shortening lengthens diastolic interval, it remains unclear how this explains APD alternans at slow rates.
Finally, atrial conduction slowing may theoretically cause APD alternans and transitions to AF 5, 26, and is a feature of structural remodeling 22. As expected, structural remodeling was more evident in patients with persistent than paroxysmal AF (table 1). However, we recently showed that AF patients show broad left atrial conduction restitution (slowing) for premature beats only for diastolic intervals <≈ 50–100 ms 10. Thus, conduction restitution may not plausibly explain APD alternans at the longer diastolic intervals in this study (table 2).
APD Alternans and Onset of Fibrillation
APD alternans preceded AF initiation across a wide range of rates, may provide a clinical index of AF susceptibility. These data are the first to characterize bi-atrial APD dynamics en route to clinical AF, and suggest that APD alternans may also be mechanistically involved in transitions to AF.
In animal ventricles and in silico, beat-to-beat repolarization dispersion (alternans) may cause wavebreak and fibrillation after ectopic beats 26 or directly 4, 5. First, during APD alternans at slow rates, spontaneous PACs were observed to trigger AF (figure 7A). In figure 4, the small MAP deflection after a long APD (asterisked) may potentially indicate an after depolarization induced by APD alternans 22 that triggers AF, although further mapping is required to exclude far-field AF signals or noise (although these signals are relatively noise-free). Second, APD alternans at fast rates in AF patients transitioned to AF via amplified alternans or complex oscillations. This may represent ‘period multupling’ in non-linear systems 17, 18, that heralds ventricular fibrillation in canine ventricles 18 or human ECG T-waves 27, 28. Complex oscillations are consistent with the findings of Mironov et al. 29 who found nodal lines during APD alternans that spanned the heart to cause phase reversals, reduce APD variability and herald fibrillation. Higher spatial resolution mapping is required to confirm whether complex APD oscillations represent nodal lines within the MAP field of view.
Clinical Implications
First, APD oscillations detected invasively, e.g. from implanted device leads, or potentially from the ECG 30 may provide a tool of dynamic susceptibility to AF, to monitor the efficacy of therapy, or to identify patients’ risk of progression from paroxysmal to persistent AF 31. Second, therapy to prevent APD alternans, as recently shown for sarcoplasmic endoplasmic reticulum Ca2+-ATPase gene therapy in guinea pig ventricles 32, may reduce AF vulnerability. Christini et al. showed that ventricular APD alternans can be controlled clinically 33 which, with our findings, opens the possibility of preventing AF initiation. Indeed, when APD alternans was suppressed in the present study, AF was non-inducible by rapid pacing (figure 7B).
Limitations
A limitation of this study is that multiple sites were studied in only n=6 patients, due to the time required to pace into AF, cardiovert, wait 15 minutes, reposition the MAP catheter and repeat the protocol. As such, we have limited statistical power to separate right and left atrial APD dynamics. Second, our sample sizes were small, due in large part to a lack of availability of MAP catheters, although this remains the largest study of human left atrial APD dynamics to date. Control subjects were younger than AF patients, because patients aged 60–65 years who required left sided access but did not have atrial fibrillation nor flutter were relatively uncommon and difficult to recruit. Third, although we focus on pacing-induced AF, this mimicks atrial tachycardias that trigger AF and reliably initiates AF without the impracticalities of waiting for spontaneous AF. Moreover, we also observed spontaneous PAC-triggered AF (figure 7A). Fourth, although pacing may distort end-repolarization, APD alternans onset occurred at slow enough rates for pacing to fall after APD (at least of the short beat, figures 2A/B, 3B/C, 4B). At faster rates, alternans often affected AP shape (figs 2, 3C, 4C) such that its detection remained robust. Fifth, although the presence (persistent AF) or absence (paroxysmal AF) of AF just prior to the protocol may influence group differences, one persistent AF patient presenting in sinus rhythm and 2 paroxysmal AF patients presenting in AF displayed APD alternans in accordance with their groups. Sixth, we cannot categorically exclude an effect of aging, since it was challenging to enroll older patients with clinical indications for left-sided mapping without AF/flutter. Nevertheless, 3 controls (aged 67, 64 and 69 years) showed similar APD dynamics to younger controls and distinct from AF patients. Seventh, statistically, although post-hoc analyses were used following omnibus ANOVA tests, no adjustments were made for testing multiple variables. Eighth, our study had few women, reflecting our Veterans’ Affairs patients. Although sex differences in AF are unclear, studies in both genders are required.
Conclusions
APD alternans reveals a spectrum of substrates for, and susceptibility to, clinical AF. Control subjects and patients with paroxysmal and persistent AF showed progressive abnormalites in the onset rate, magnitude and rate-response of APD alternans, in tandem with easier transitions to AF. When present at slow rates, APD alternans enabled ectopy to initiate AF and, at faster rates, APD alternans amplified or disorganized directly to AF. Cellular studies are required to explain the mechanisms enabling AF patients to exhibit APD oscillations at near-resting heart rates.
CLINICAL IMPACT.
Human atrial fibrillation (AF) is a highly prevalent disease whose mechanisms are poorly understood. Ectopic beats from the pulmonary veins may trigger AF, but this likely also requires substrate since ectopy rarely triggers AF in control subjects. We hypothesized that beat-to-beat oscillations in atrial repolarization (action potential duration, APD) may provide an AF substrate, and explain the spectrum of AF vulnerability from subjects without AF to those with paroxysmal and persistent AF. We found that APD alternans invariably preceded AF initiation. Notably, APD alternans arose near-resting heart rates (100–120 beats/min) in persistent AF patients, a finding that is difficult to explain by current theories and animal experiments. APD alternans onset required progressively faster rates for patients with paroxysmal AF and control subjects, in whom alternans developed only at very rapid rates (>240 beats/min) just prior to induced AF. Furthermore, in patients in whom rapid pacing failed to initiate AF, APD alternans did not develop. In conclusion, APD alternans indicates dynamic substrates for AF, and arises most readily in patients with persistent AF and least readily in control subjects without AF. APD alternans preceded every AF episode, yet was absent when AF was not induced. Accordingly, APD alternans provides a clinical tool to identify AF vulnerability, and may be useful in refining diagnosis or monitoring the effectiveness of AF therapy.
Acknowledgments
Funding Sources
Supported by grants from the Doris Duke Charitable Foundation and National Institutes of Health (HL70529, HL83359) to SMN.
Footnotes
Disclosures
Sanjiv M. Narayan: This study was supported in part by grants from the National Institutes of Health, HL70529, HL83359 and the Doris Duke Charitable Foundation. In addition, Dr. Narayan reports having received honoraria from Medtronic, St. Jude Medical and Biotronik Corporations and grant support from Biosense-Webster. His Institution has received fellowship support from Medtronic, Boston Scientific, St. Jude Medical and Biotronik. He is also the author of Intellectual property on signal processing of cardiac arrhythmias owned by the University of California.
Etienne Pruvot: None
Paul Clopton: None
Michael R. Franz: Dr. Franz was the original inventor of the MAP catheter used in this study. He reports no other conflicts.
Dr. Krummen: has received honoraria from Medtronic. His Institution has received fellowship support from Medtronic, Boston Scientific, St. Jude Medical and Biotronik.
References
- 1.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 2.Chen S-A, Hsieh M-H, Tai C-T, Tsai C-F, Prakash VS, Yu W-C, Hsu T-L, Ding Y-A, Chang MS. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins : Electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999a;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 3.Ausma J, Wijffels M, Thone F, Wouters L, Allessie MA, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 4.Walker ML, Rosenbaum DS. Cellular alternans as mechanism of cardiac arrhythmogenesis. Heart Rhythm. 2005;2:1383–1386. doi: 10.1016/j.hrthm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JN, Karma A, Shiferaw Y, Chen P-S, Garfinkel A, Qu Z. From pulsus to pulseless: The saga of cardiac alternans (review) Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 6.Gong Y, Xie F, Stein K, Garfinkel A, Culianu C, Lerman B, Christini D. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: A simulation study. Circulation. 2007;115:2094–2102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 7.Kim B-S, Kim Y-H, Hwang G-S, Pak H-N, Lee SC, Shim WJ, Oh DJ, Ro YM. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002;39:1329–1336. doi: 10.1016/s0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 8.Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M. Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circulation J. 2005;69:1368–1373. doi: 10.1253/circj.69.1368. [DOI] [PubMed] [Google Scholar]
- 9.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials as a precursor of atrial fibrillation. Circulation. 2002b;106:1968–1973. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 10.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: A mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008c;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res. 2004;94:1083–1090. doi: 10.1161/01.RES.0000125629.72053.95. [DOI] [PubMed] [Google Scholar]
- 12.Calkins H, Brugada J, Packer D, Cappato R, Chen S, Crijns H, Damiano RJ, Davies D, Haines D, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck K, Lindsay B, Marchlinski F, McCarthy P, Mont J, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin J, Shemin R. Hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. European heart rhythm association (ehra); european cardiac arrhythmia scoiety (ecas); american college of cardiology (acc); american heart association (aha); society of thoracic surgeons (sts) Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Franz MR, Kirchhof PF, Fabritz CL, Zabel M. Computer analysis of monophasic action potentials: Manual validation and clinically pertinent applications. [published erratum appears in PACE Pacing Clin Electrophysiol 1993 Jul;16(7 Pt 1):xii] 1995c;18:1666–1678. doi: 10.1111/j.1540-8159.1995.tb06988.x. [DOI] [PubMed] [Google Scholar]
- 14.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988a;82:972–979. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narayan SM, Franz MR, Kim J, Lalani G, Sastry A. T-wave alternans, restitution of ventricular action potential duration and outcome. J Am Coll Cardiol. 2007f;50:2385–2392. doi: 10.1016/j.jacc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Narayan SM, Bayer J, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: A clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008d;52:1782–1792. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritzenberg AL, Adam DR, Cohen RJ. Period multupling -evidence for nonlinear behaviour of the canine heart. Nature. 1984b;307:157–161. doi: 10.1038/307159a0. [DOI] [PubMed] [Google Scholar]
- 18.Nearing BD, Verrier RL. Progressive increases in complexity of t-wave oscillations herald ischemia-induced ventricular fibrillation. Circ Res. 2002b;91:727–732. doi: 10.1161/01.res.0000038887.17976.33. [DOI] [PubMed] [Google Scholar]
- 19.Garfinkel A, Kim Y-H, Voroshilovsky O, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci U S A. 2000;97:6061–6066. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson LD, Jeyaraj D, Wan X, Hoeker GS, Said TH, Gittinger M, Laurita KR, Rosenbaum DS. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–259. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayer JD, Narayan SM, Lalani GG, Trayanova NA. Rate-dependent action potential alternans in human heart failure implicate abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–1101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 23.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 24.Workman AJ, Kane KA, Rankin AC. Characterisation of the na, k pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovasc Res. 2003;59:593–602. doi: 10.1016/s0008-6363(03)00466-8. [DOI] [PubMed] [Google Scholar]
- 25.Caballero R, de la Fuente MG, Gomez R, Barana A, Amoros I, Dolz-Gaiton P, Osuna L, Almendral J, Atienza F, Fernandez-Aviles F, Pita A, Rodriguez-Roda J, Pinto A, Tamargo J, Delpon E. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. Journal of Cardiovascular Electrophysiology. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 27.Narayan SM, Lindsay BD, Smith JM. Demonstrating the pro-arrhythmic preconditioning of single premature extrastimuli using the magnitude, phase and temporal distribution of repolarization alternans. Circulation. 1999d;100:1887–1893. doi: 10.1161/01.cir.100.18.1887. [DOI] [PubMed] [Google Scholar]
- 28.Shusterman V, Goldberg A, London B. Upsurge in t-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113:2880–2887. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]
- 29.Mironov S, Jalife J, Tolkacheva EG. Role of conduction velocity restitution and short-term memory in the development of action potential duration alternans in isolated rabbit hearts. Circulation. 2008;118:17–25. doi: 10.1161/CIRCULATIONAHA.107.737254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Rudy Y. Electrocardiographic imaging of normal human atrial repolarization. Heart Rhythm. 2009;6:582–583. doi: 10.1016/j.hrthm.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahangir A, Lee V, Friedman PA, Trusty J, Hodge D, Kopecky S, Packer DL, Hammill SC, Shen W, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 32.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted serca2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009;2:686–694. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christini DJ, Stein KM, Markowitz SM, Mittal S, Slotwiner DJ, Scheiner MA, Iwai S, Lerman BB. Nonlinear-dynamical arrhythmia control in humans. Proc Natl Acad Sci USA. 2001;98:5827–5832. doi: 10.1073/pnas.091553398. [DOI] [PMC free article] [PubMed] [Google Scholar]