Abstract
Background
Cardiovascular disease is an increasing concern among HIV-infected persons and their providers. We determined if fatty liver disease is a marker for underlying coronary atherosclerosis among HIV-infected persons.
Methods
We performed a cross-sectional study among HIV-infected adults to evaluate the prevalence of and factors, including fatty liver disease, associated with subclinical coronary atherosclerosis. All participants underwent computed tomography for determination of coronary artery calcium (CAC; positive defined as a score >0) and fatty liver disease (defined as a liver-to-spleen ratio <1.0). Factors associated with CAC were determined using multivariate logistic regression models.
Results
We studied 223 HIV-infected adults with a median age of 43 years (IQR 36–50), 96% were male, and 49% were Caucasian. Median CD4 count was 586 cells/mm3, and 83% were receiving antiretroviral medications. Seventy-five (34%) had a positive CAC score, and 29 (13%) subjects had fatty liver disease. Among those with CAC scores of 0, 1–100, >100, the percentage with concurrent fatty liver disease was 8%, 18%, and 41%, respectively (p=0.001). In the multivariate model, CAC was associated with increasing age (OR 4.3 per 10 years, p<0.01), hypertension (OR 2.6, p<0.01), and fatty liver disease (OR 3.8, p<0.01).
Conclusions
Coronary atherosclerosis as detected by CAC is prevalent among young HIV-infected persons. The detection of fatty liver disease among HIV-infected adults should prompt consideration for assessment for underlying cardiovascular disease and risk factor reduction.
Keywords: HIV, coronary artery disease, coronary artery calcium, CAC, fatty liver disease
Background
As HIV-infected persons are experiencing longer life expectancies, there is an increasing concern regarding non-AIDS defining conditions, including cardiovascular disease [1, 2]. HIV-infected persons appear to have a higher risk of coronary artery atherosclerosis compared to the general population, which may be a result of HIV-induced inflammation, antiretroviral medications, or concurrent medical conditions, such as insulin resistance, dyslipidemia, hypertension, visceral fat deposition, and tobacco abuse [1–10]. Elevated prevalence rates of subclinical cardiovascular disease among HIV-infected persons have recently been demonstrated using computed tomography (CT) coronary artery calcium (CAC) scores [9, 11–18].
Risk factors for coronary artery atherosclerosis may be similar to those for fatty liver disease. Recent studies among HIV-uninfected persons have shown that non-alcoholic fatty liver disease (NAFLD) was independently associated with the presence and extent of coronary disease [19, 20]; however, a single study among HIV-infected persons did not found a significant relationship [21]. Given that liver test abnormalities and fatty liver disease are common among HIV infected persons [22], determining their relationship with coronary artery atherosclerosis may be helpful in the development of screening guidelines and risk stratification for underlying cardiovascular disease in this population [14]. Therefore, we evaluated the potential relationship between subclinical coronary atherosclerosis (as measured by CAC scores) and fatty liver disease among HIV infected persons.
Methods
We enrolled 223 HIV-infected adults in a cross-sectional study who underwent screening CT scans for CAC and fatty liver disease between December 9, 2008 and March 1, 2010. The primary study objective was to examine the association between fatty liver disease and CAC scores among HIV-infected persons, with secondary objectives of evaluating other factors, including metabolic and morphologic measures, with subclinical coronary atherosclerosis. Inclusion criteria for study participation included documented HIV infection (ELISA confirmed by Western Blot), age ≥18 years, and a negative pregnancy test among women. Patients with a history of coronary vessel stents were excluded as CAC scores are unreliable in this setting. Participants were military beneficiaries, including active duty members, retirees, and family members. All participants provided written informed consent; the study was approved by the governing institutional review board; and registered at ClinicalTrials.gov (NCT00889577). Data collected for this study including imaging, questionnaires, body measurements, and specimen collection occurred on the same day.
All participants underwent imaging using a single, multidetector CT scan (Siemens Definition Dual Source CT Scanner, Siemens Medical Solutions, Forsheim, Germany). Prospectively gated axial 3 mm images were obtained at 120kV during a single breath hold. The scanning protocol captured images with a 330-milisecond gantry rotation time, an individual detector width of 0.6 mm with a reconstructed section width of 3 mm, and temporal resolution of 165 milliseconds. No contrast media were administered. CAC scoring was performed on an Aquarius workstation (TeraRecon, San Mateo, California) and calculated as the sum of all lesions in each of the coronary arteries using Agatston units, as previously described [23]. A CAC score of >0 was considered positive for detectable calcium, and a score of >100 was considered clinically significant.
Limited 3 mm axial images were also obtained through the mid-liver, centered at the level of the portal vein, and spleen for evaluation for fat content estimation [24–26]. The density at three liver areas (right posterior, right anterior, and left lobe) were measured and the mean utilized. The splenic density served as an internal control, since the spleen typically contains no fat. Fatty liver disease was defined as a liver-to-spleen ratio <1.0 [27]. Images were processed on an Impax 6.3 workstation (AGFA). The total estimated radiation dose for all CT images was 3 mSv.
Clinical data were obtained from participant-administered questionnaires including demographics (age, self-reported ethnicity, and gender), history of tobacco use, alcohol use, family history of cardiovascular disease, and recent symptoms of chest pain or dyspnea. Research coordinators collected information regarding current medical conditions, medications, and HIV history from the medical records, including the use of antiretroviral medications. Highly active antiretroviral therapy (HAART) was defined as three or more drugs from at least two different classes per guidelines [28]. Cumulative exposure in months to each drug class including nucleoside reverse-transcriptase inhibitors (NRTIs), non-nucleoside reverse-transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) were recorded. In addition, the use of specific NRTIs ( abacavir and tenofovir), NNRTIs (efavirenz), and PIs (ritonavir, atazanavir) were examined based on sufficient numbers of current users. Research coordinators also determined each participant’s 10-year risk for coronary heart disease using the Framingham risk score (FRS) [29].
Each participant underwent height and a weight measurement on a calibrated scale, and body mass index (BMI) was calculated. Additional anthropometrical measurements included circumference measurements (waist, hips, and thigh) as well as skinfold thickness at four locations (biceps, triceps, subscapular, and suprailiac) on the participant’s right side using standardized calipers (Lange skinfold caliper; Beta Technology, Santa Cruz, CA) [30, 31]. All caliper measurements were performed in triplicate and the means calculated. Percent body fat was calculated from the caliper measures as previously described [30, 32]. The participant’s physician also performed a visual assessment of fat distribution in the cheeks, neck, breasts, abdomen, buttocks, and legs and graded the amount of fat in these areas from −3 to 3 (0 as normal, negative numbers as lipoatrophy, and positive numbers as lipohypertrophy) [33].
Each participant underwent a fasting (for ≥10 hours) blood sample on the day of the CT scan. Tests included a lipid panel for total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (standardized enzymatic colorimetric methods); glucose level; highly sensitive C-reactive protein (lower limit of detection <0.5 mg/dl; Particle Enhanced Immuno-Turbidimetric Assay, Roche Laboratories); erythrocyte sedimentation rate (ESR, Modified Westergren Method); D-dimer (Immuno-Turbidimetric Method, Diagnostica Stago); CD4 cell count (flow cytometry); and plasma HIV RNA level (undetectable as <50 copies/ml, Roche Amplicor).
Hypertension and diabetes were defined based on requirement for medications for these conditions. Sexual dysfunction was based on a clinical diagnosis or the use of a prescribed 5-phosphodiesterase inhibitor. The metabolic syndrome was defined by the Adult Treatment Panel III (ATP-III) criteria as the presence of ≥3 of the following abnormalities: 1) abdominal obesity (abdominal circumference >102 cm for men and >88 cm for women), 2) elevated triglyceride level (>150 mg/dl), 3) decreased HDL level (<40 mg/dl for men and <50 mg/dl for women), 4) elevated blood pressure, and 5) elevated fasting glucose (>110 mg/dl) [29].
Statistical analyses included descriptive statistics of the prevalence of subclinical coronary atherosclerosis and fatty liver disease. Categorical variables were described as numbers with proportions, and continuous variables as medians with interquartile ranges (IQR). Correlations between variables were examined using Pearson’s correlation tests. Univariate comparisons among participants with and without coronary atherosclerosis (defined by a CAC score of >0 versus 0) utilized Fisher’s exact testing and rank-sum testing for categorical and continuous variables, respectively, due to the distributions of the factors of interest. A multivariate logistic regression model was performed to evaluate factors associated with CAC. Variables with a p-value <0.10 in the univariate analyses were placed in the full multivariate model and a backward stepwise approach was used to derive the final model. Additional predefined logistic regression analyses were performed, including examining only males, and a second analysis including only participants without excessive alcohol use (defined as >140g ethanol/week for men and >70g ethanol/week for women [34]). Finally, we examined the data using linear regression models to examine factors associated with the CAC score as a continuous variable. A p-value of <0.05 was considered statistically significant. All analyses were performed using STATA 10 (College Station, TX).
Results
Baseline Study Population Characteristics
A total of 223 HIV-infected persons were evaluated with a median age of 43 (IQR 36–50) years, 96% were male, and ethnicity was Caucasian for 49%, African American for 23%, and other for 28% (Table 1). Thirty-percent of participants had hypertension, 23% had sexual dysfunction, 6% had diabetes, and 17% were current tobacco users. Only six patients (3%) had HCV coinfection reflective of the low prevalence of intravenous drug use in our population. Regarding HIV history, the median duration of HIV from diagnosis was 12 (IQR 5–19) years, the median current CD4 count was 586 (IQR 393–733) cells/mm3, the CD4 nadir was 260 (IQR 144–368) cells/mm3, 70% had an undetectable plasma RNA level, and 83% were currently receiving HAART. The median duration of NRTI use was 77 (IQR 20–149) months, NNRTI use was 17 (IQR 0–51) months, and PI use was 26 (IQR 0–75) months. Nineteen percent of participants were currently receiving abacavir.
Table 1.
Study Population Characteristics by Presence of Coronary Artery Calcium (CAC) on Computed Tomography
| Characteristica | Total Cohort | CAC | No CAC | OR (95% CI) | p- value |
|---|---|---|---|---|---|
| N=223 | N=75 | N=148 | |||
|
Demographics | |||||
| Age, years | 43 (36–50) | 49 (44–55) | 40 (31–45) | 1.2 (1.1–1.2) | <0.01 |
| Gender, male | 213 (96%) | 71 (95%) | 142 (99%) | 0.8 (0.2–2.8) | 0.74 |
| Ethnicity | |||||
| Caucasian | 110 (49%) | 48 (64%) | 62 (42%) | 2.0 (1.0–3.9) | 0.04 |
| African American | 52 (23%) | 10 (13%) | 42 (28%) | 0.6 (0.3–1.5) | 0.29 |
| Other | 61 (28%) | 17 (23%) | 44 (30%) | 1.0 (Ref) | |
|
Clinical Data | |||||
| Tobacco Use | |||||
| Current | 39 (17%) | 15(20%) | 24 (16%) | 1.29 (0.63–2.64) | 0.58 |
| Ever | 111 (50%) | 40 (53%) | 71 (48%) | 1.2 (0.7–2.2) | 0.67 |
| Years of Useb | 12 (5–20) | 18 (9–25) | 10 (5–18) | 1.1 (1.0–1.1) | <0.01 |
| Family History of Cardiovascular Disease | 75 (34%) | 31 (41%) | 44 (30%) | 1.7 (0.9–3.0) | 0.10 |
| Hepatitis C seropositive | 6 (3%) | 2 (3%) | 4 (3%) | 0.99 (0.2, 5.5) | 0.99 |
| Diabetes Mellitus | 14 (6%) | 10 (13%) | 4 (3%) | 5.5 (1.7–18.3) | <0.01 |
| Hypertension | 66 (30%) | 37 (49%) | 29 (20%) | 4.0 (2.2–7.3) | <0.01 |
| Use of Prescription Lipid Lowering Medication | 70 (31%) | 38 (51%) | 32 (22%) | 3.7 (2.0–6.8) | <0.01 |
| Metabolic Syndromec | 50 (23%) | 26 (35%) | 24 (16%) | 2.8 (1.5–5.4) | <0.01 |
| Sexual Dysfunction | 52 (23%) | 26 (35%) | 26 (18%) | 2.5 (1.3–4.7) | <0.01 |
|
Weight and Fat Distribution Measurements | |||||
| Weight | |||||
| Body Mass Index | 26.7 (24.2–29.5) | 26.2 (23.8–28.8) | 27.0 (24.3–29.8) | 0.9 (0.9–1.0) | 0.39 |
| Obese (>30 kg/m2) | 44 (19%) | 11 (15%) | 33 (22%) | 0.6 (0.3–1.3) | 0.21 |
| Waist Circumference, cm | 93 (85–99) | 94 (86–99) | 94 (86–99) | 1.0 (0.99–1.0) | 0.90 |
| Abdominal Obesityc | 54 (24%) | 22 (29%) | 32 (22%) | 1.5 (0.8–2.8) | 0.25 |
| Waist-Hip Ratio | 1.0 (0.96–1.04) | 1.01 (0.96–1.04) | 1 (0.96–1.05) | 2.8 (0.3–23.6) | 0.35 |
| Percent Body Fat | 26 (23–30) | 28 (23–33) | 26 (23–29) | 1.0 (1.0–1.1) | 0.04 |
| Thigh Circumference, cm | 56 (52–60) | 54 (51–57) | 57 (53–61) | 0.5 (0.3–0.8)d | <0.01 |
|
Physician Visual Assessment of Fat Distribution | |||||
| Cheeks | |||||
| Loss | 67 (30%) | 30 (40%) | 37 (25%) | 2.6 (1.4–4.8) | <0.01 |
| No Change | 125 (56%) | 30 (40%) | 95 (64%) | 1.0 (Ref) | |
| Gain | 31 (14%) | 15 (20%) | 16 (11%) | 3.0 (1.3–6.7) | <0.01 |
| Neck | |||||
| Loss | 4 (2%) | 2 (3%) | 2 (1%) | 2.8 (0.4–20.3) | 0.31 |
| No Change | 170 (76%) | 45 (60%) | 125 (85%) | 1.0 (Ref) | |
| Gain | 49 (22%) | 28 (37%) | 21 (14%) | 3.7 (1.9–7.2) | <0.01 |
| Breasts | |||||
| Loss | 1 (1%) | 0 (0%) | 1 (1%) | --- | --- |
| No Change | 181 (81%) | 53 (71%) | 128 (86%) | 1.0 (Ref) | |
| Gain | 41 (18%) | 22 (29%) | 19 (13%) | 2.8 (1.4–5.6) | <0.01 |
| Abdomen | |||||
| Loss | 3 (1%) | 1 (1%) | 2 (1%) | 1.6 (0.1–18.5) | 0.70 |
| No Change | 114 (51%) | 27 (36%) | 87 (59%) | 1.0 (Ref) | |
| Gain | 106 (48%) | 47 (63%) | 59 (40%) | 2.6 (1.4–4.6) | <0.01 |
| Buttocks | |||||
| Loss | 46 (21%) | 25 (33%) | 21 (14%) | 3.3 (1.7–6.5) | <0.01 |
| No Change | 159 (71%) | 42 (56%) | 117 (79%) | 1.0 (Ref) | |
| Gain | 18 (8%) | 8 (11%) | 10 (7%) | 2.2 (0.8–6.0) | 0.11 |
| Legs | |||||
| Loss | 66 (30%) | 36 (48%) | 30 (20%) | 4.0 (2.2–7.5) | <0.01 |
| No Change | 144 (64%) | 33 (44%) | 111 (75%) | 1.0 (Ref) | |
| Gain | 13 (6%) | 6 (8%) | 7 (5%) | 2.9 (0.9–9.2) | 0.07 |
|
Laboratory Measures | |||||
| Hyperglycemiac | 21 (9%) | 11 (15%) | 10 (7%) | 2.4 (1.0–5.9) | 0.06 |
| Hypercholesterolemia (> 200 mg/dl) | 71 (32%) | 30 (40%) | 41 (28%) | 1.7 (1.0–3.1) | 0.06 |
| High LDL (≥160 mg/dl) | 25 (11%) | 11 (15%) | 14 (9%) | 1.6 (0.7–3.8) | 0.25 |
| Low HDLc | 102 (46%) | 34 (45%) | 68 (46%) | 1.0 (0.6–1.7) | 1.0 |
| Hypertriglyceridemiac | 106 (48%) | 45 (60%) | 61 (41%) | 2.1 (1.2–3.8) | <0.01 |
| C-Reactive Protein | |||||
| >0.5 mg/dl | 31 (14%) | 15 (20%) | 16 (11%) | 1.8 (1.0–3.2) | 0.05 |
| ESR | |||||
| >20 mm/hour | 53 (24%) | 20 (27%) | 33 (22%) | 1.3 (0.7–2.4) | 0.51 |
| D-Dimer | |||||
| >0.4 μg/ml | 34 (15%) | 14 (19%) | 20 (14%) | 1.5 (0.7–3.1) | 0.33 |
|
CT Results | |||||
| Fatty Liver Disease Presente | 29 (13%) | 17 (23%) | 12 (8%) | 3.4 (1.5–7.5) | <0.01 |
|
HIV-Specific Factors | |||||
| HIV Duration, years | 12 (5–19) | 18 (9–22) | 9 (4–16) | 1.1 (1.1–1.1) | <0.01 |
| Current CD4 Cell Count, cells/mm3 | 586 (393–733) | 571 (328–795) | 600 (456–726) | 1.0 (0.0–1.1)f | 0.30 |
| <200 | 13 (6%) | 7 (9%) | 6 (4%) | 2.8 (0.9–8.9) | 0.13 |
| 200–500 | 73 (33%) | 28 (37%) | 45 (30%) | 1.5 (0.8–2.7) | 0.18 |
| >500 | 137 (61%) | 40 (53%) | 97 (66%) | 1.0 (Ref) | 0.08 |
| Nadir CD4 Cell Count, cells/mm3 | 260 (144–368) | 184 (79–323) | 285 (183–390) | 0.7 (0.6–0.9)f | <0.01 |
| HIV RNA Level <50 copies/ml | 157 (70%) | 55 (73%) | 102 (69%) | 1.2 (0.7–2.3) | 0.50 |
| Current HAART Use | 185 (83%) | 70 (93%) | 115 (78%) | 4.0 (1.5–10.8) | <0.01 |
| Abacavir Use | |||||
| Current | 42 (19%) | 21 (28%) | 21 (14%) | 2.4 (1.2–4.7) | 0.01 |
| Ever | 78 (35%) | 40 (53%) | 38 (26%) | 3.3 (1.8–5.9) | <0.01 |
| Ritonavir Use | |||||
| Current | 92 (41%) | 38 (51%) | 54 (36%) | 1.8 (1.02, 3.1) | 0.04 |
| Ever | 110 (49%) | 52 (69%) | 58 (39%) | 3.5 (1.9, 6.4) | <0.01 |
| NRTI, months of use | 77 (20–149) | 143 (66–182) | 49 (10–122) | 1.1 (1.1–1.2)g | <0.01 |
| NNRTI, months of use | 17 (0–51) | 33 (1–71) | 12 (0–41) | 1.2 (1.1–1.3)g | <0.01 |
| PI, months of use | 26 (0–75) | 53 (10–106) | 3 (0–68) | 1.1 (1.1–1.2)g | <0.01 |
Categorical variables expressed as number (percentage) and continuous variables as median (interquartile range)
Among those with a history of tobacco use
The definitions are based on the ATP III guidelines: abdominal obesity (abdominal circumference >102 cm for men and >88 cm for women), elevated triglyceride level (>150 mg/dl), decreased HDL level (<40 mg/dl for men and <50 mg/dl for women), elevated blood pressure, and elevated fasting glucose (>110 mg/dl). The presence of 3 or more defined the metabolic syndrome [Expert Panel]
OR calculated for 10 mm increase
Two participants did not have fatty liver assessment performed; one due to splenectomy and one due to error in obtaining liver imaging
OR calculated per 100 cells
OR calculated for 12 months of use
Prevalence of Coronary Atherosclerosis and Fatty Liver Disease
Seventy-five (34%) participants had a positive CAC score and 17 (8%) had a CAC score of >100, indicating significant atherosclerotic disease (Figure 1). Fatty liver disease on CT imaging was diagnosed among 29 (13%) HIV-infected persons. The prevalence of fatty liver disease among those without CAC, those with a CAC score of 1–100, and those with a score >100 was 8%, 18%, and 41%, respectively (p=0.001). Of those with fatty liver disease, 59% (17/29) also had coronary atherosclerosis as detected by CAC >0, and these two conditions were significantly correlated (r=0.21, p=0.002).
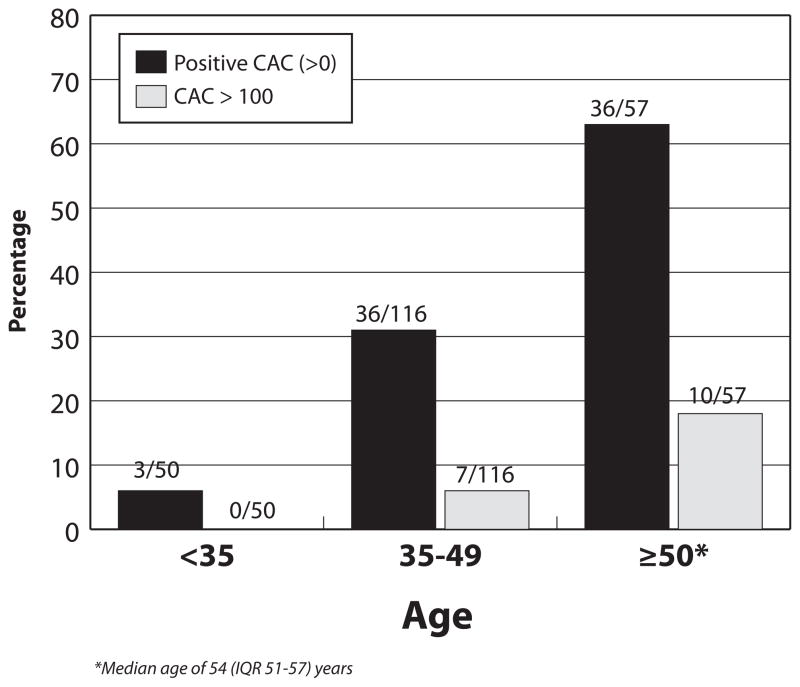
Figure 1.
Prevalence of Coronary Artery Calcium (CAC) among HIV-Infected Persons by Age Group
The prevalence of a positive CAC score among those 35–49 years of age in our cohort was 31% (36/116), with 6% having a CAC score of >100 (Figure 1). Similar relationships between fatty liver disease and a positive CAC score were also noted in this age group.
Clinical Risk Assessments and CAC Scores
Regarding clinical symptoms, participants with a positive CAC score were not significantly more likely to report a history of chest pain or dyspnea compared to those without CAC (21% vs. 17%, p=0.46). HIV-infected persons with a low (<10%), moderate (10–20%), or high (>20%) FRS had a positive CAC scan in 27%, 63%, and 60% of patients, respectively (p<0.01) (Table 2). The median FRS for those with a positive CAC was 8 (IQR 3–12) compared to those without CAC who had a score of 3 (IQR 1–6), p<0.01. Of note, the majority (64%) of those with a positive CAC score had a low FRS. We assessed the FRS in predicting positive CAC scores; of note, CAC is a non-invasive test for detecting calcified coronary disease, and may miss noncalcified plaque compared to the gold standard diagnostic test, coronary catheterization. The sensitivity, specificity, positive and negative predictive value of the FRS in predicting a positive CAC score among HIV-infected persons was 36%, 89%, 63%, and 73%, respectively.
Table 2.
Framingham Risk Score (FRS) and Detection of Coronary Artery Calcium (CAC) on Computed Tomography
| Framingham Risk Scorea | CAC Score | |
|---|---|---|
| Positive | Negative | |
| Mild (<10%) | 48 (27%) | 132 (73%) |
| Moderate (10–20%) | 24 (63%) | 14 (37%) |
| High (>20%) | 3 (60%) | 2 (40%) |
Risk of coronary heart disease in 10 years [29].
CAC, coronary artery calcium
Factors Associated with Coronary Artery Disease among HIV-Infected Persons
HIV-infected persons with CAC compared to those without CAC in the univariate analyses were older (49 vs. 40 years, OR 1.2, p<0.01), Caucasian (64% vs. 42%, OR 2.0, p=0.04), had a longer duration of tobacco use (18 vs. 10 years, OR 1.1 per year, p<0.01), more likely receiving a lipid lowering medication (51% vs. 22%, OR 3.7, p<0.01), and more likely to have diabetes (13% vs. 3%, OR 5.5, p<0.01), hypertension (49% vs. 20%, OR 4.0, p<0.01), the metabolic syndrome (35% vs. 16%, OR 2.8, p<0.01), and sexual dysfunction (35% vs. 18%, OR 2.5, p<0.01) (Table 1). Those with CAC were more likely to have fatty liver disease than those without CAC (23% vs. 8%, OR 3.4, p<0.01). Regarding body measurements, the thigh circumference, the physician visual assessments of body fat at six locations, and the percent of body fat as calculated by caliper measurements were univariately associated with CAC (Table 1). No other circumference or individual skinfold measurement was associated with CAC (data not shown).
HIV-specific factors that were significantly associated with CAC in the univariate analyses included a longer HIV duration (18 vs. 9 years, OR 1.1 per year, p<0.01), lower CD4 nadir (184 vs. 285 cells/mm3, OR 0.7, <0.01), and current HAART use (93% vs. 78%, OR 4.0, p<0.01). Duration of each of the three main drug classes were also positively associated with CAC in the univariate models. In addition, individual use (current or ever) of abacavir and ritonavir were each associated with CAC (Table 1). Current receipt of tenofovir, efavirenz, or atazanavir was not associated with CAC (data not shown).
In the multivariate analyses, older age (OR 4.3 per 10 year increase, p<0.01), fatty liver disease (OR 3.8, p<0.01), and hypertension (OR 2.6, p<0.01) were significantly associated with the presence of coronary atherosclerosis by CAC score (Table 3). There were no significant associations with body measurements or HIV-specific factors, including antiretroviral medication use (evaluated as months of use, current use, and ever use), in the multivariate model. The multivariate model was replicated excluding those with HCV seropositivity (n=6) with no significant differences noted in the association of fatty liver disease and CAC (OR 4.2, 95% CI 1.6–11.1, p<0.01). Finally, in order to evaluate the relationship of fatty liver disease and CAC independently of the metabolic syndrome, we repeated the model examining only participants without the metabolic syndrome (n=173); fatty liver disease remained associated with a positive CAC score in this subset (OR 5.4, 95% CI 1.5–19.2, p<0.01).
Table 3.
Final Multivariate Model for Factors Associated with Coronary Artery Calcium on Computed Tomography
| Factor | OR (95% CI) | p-value |
|---|---|---|
| Age, per 10 years | 4.3 (2.6, 6.9) | <0.001 |
| Fatty Liver Disease by CT imaging | 3.8 (1.5, 9.6) | 0.004 |
| Hypertension | 2.6 (1.3, 5.4) | 0.009 |
CI, confidence interval; OR, odds ratio.
We performed sensitivity analyses to evaluate the robustness of our findings. Since fatty liver disease can be due to either NAFLD or alcohol overuse, we excluded patients with excessive alcohol use (n= 12) and noted similar findings. Since the risk factors for coronary atherosclerosis may vary by gender, we also performed the analyses among only males and found the same associations. Finally, using multivariate linear regression modeling, we evaluated the association between the CAC score as a continuous variable and found that age (Coef 4.4, 95% CI 2.3 – 6.4, p<0.01) and fatty liver disease (Coef 88.1, 95% CI 30.2 – 146.1, p<0.01) were associated with CAC, with a trend for hypertension (Coef 39.2, 95% CI -5.3 – 83.7, p=0.08).
Discussion
In our study, HIV-infected persons, despite their relatively young age, had a high prevalence of subclinical heart disease with elevated rates compared to historical age-matched HIV-uninfected persons [35–37]. These data, and that of other studies, emphasize the importance of cardiovascular disease among HIV-infected patients and suggest that addressing underlying heart disease may be an important component of further normalizing the life expectancy of this group [9, 11–14, 16–18, 38].
The etiology of the higher prevalence rates of coronary atherosclerosis in HIV-infected persons is likely multifactorial. In our study, increasing age was strongly associated with subclinical coronary atherosclerosis. Both the elevated prevalence of heart disease and its significant association with increasing age could suggest that HIV patients may be experiencing accelerated vascular aging, although this requires further study since mechanisms unrelated to aging may be occurring. One prior study showed that the vascular age of HIV-patients may be increased a mean of 15 years over chronological age [16]. However, further studies on the potential premature senescence of HIV-infected persons as well as the impact of medications, such as HAART and anti-inflammatory agents, on aging in this population are needed.
Our study found a significant association between fatty liver disease and CAC. To our knowledge, only one other study among HIV-infected persons has been performed to examine this potential relationship, but failed to demonstrate a significant association [21]. The reasons for the divergent results may be due to differing population characteristics (the prior study had more tobacco users and lower rates of obesity [21]) or differing sensitivities of detecting fatty liver disease (e.g., the prior study noted a prevalence of fatty liver of 37% vs. the 13% in our study). Our results are concordant with investigations in the general population showing that fatty liver disease is independently associated with coronary artery disease [19, 39]. Furthermore, in our study, fatty liver disease was increasingly present as the extent of coronary atherosclerosis increased. Although the precise relationship of these two conditions remains unclear, recent studies have suggested that hepatic steatosis may not be a direct cause cardiovascular disease, but that the systemic, inflammatory state in which fatty liver disease develops is also a risk factor for atherosclerotic disease [40]. Although our study did not detect a role of HIV medications in this relationship, either the direct or indirect effects of some antiretrovirals cannot be definitively excluded.
Since fatty liver disease has been shown to predict future cardiovascular events [20, 41], our data have potentially important clinical implications for HIV-infected persons. Foremost, fatty liver disease may be a novel surrogate marker for underlying heart disease beyond that of the ‘metabolic syndrome’. As such, HIV-infected persons with fatty liver disease may warrant early cardiovascular assessments and institution of risk factor reduction methods; further studies are needed.
Regarding scores to predict heart disease, we found that although a higher Framingham risk score was associated with an increased prevalence of CAC, the majority of the HIV-infected persons in our study with a positive CAC had a ‘low’ FRS. Furthermore, despite a “low-risk” FRS, nearly 30% had a positive CAC score, and 6% had significant plaque burden (i.e., CAC >100). We acknowledge the comparison of the FRS using CAC as the comparator is limited since the gold standard in diagnosing coronary artery disease is coronary catheterization, which was not performed in our study. The low sensitivity of FRS in detecting coronary calcification in our study, as well as another study in HIV patients [42], suggests that better clinical screening tools beyond the FRS are needed for this population. Of note, our study did not investigate clinical outcomes; however, a recent study demonstrated that FRS may underestimate myocardial infarctions among those receiving HAART [43].
These data suggest that novel equations that encompass additional factors may be useful for HIV-infected persons. Higher risk scores for increasing age (given concerns for accelerated vascular aging), elevated inflammatory markers, and inclusion of novel factors such as fatty liver disease and antiretroviral use should be considered. Since cardiovascular disease is a leading cause of death among HIV-infected persons [38, 44], clinical trials investigating the predictiveness of novel equations are advocated.
Our study had potential limitations. First, due to the cross-sectional study design, we could not ascertain the temporal association between development of fatty liver disease and CAC. We advocate for longitudinal studies to confirm the associations between fatty liver disease and coronary atherosclerosis in HIV-infected persons; in addition, diagnostic tests including MRI for evaluating fatty liver disease, transient elastography for assessing associated hepatic fibrosis, and carotid intima-media thickness for estimating arterial atherosclerosis by ultrasonography should be considered in future studies. Second, the diagnoses of fatty liver and coronary disease relied on CT imaging; although studies have supported the use of CT scans in diagnosing these conditions, it may underestimate the prevalence of liver steatosis and overlook non-calcified coronary plaques [23, 45, 46]. Third, although we evaluated the relationship of body measurements and visual lipodystrophy scores with CAC, objective and reproducible measurements of body fat composition by DEXA were not performed. Finally, we were unable to specifically examine cardiovascular disease among women owing to our predominantly male population.
Our study had several strengths. It is one of the first studies among HIV-infected persons to examine the potential association between fatty liver disease and CAC scores. In addition, a comprehensive evaluation of anthropometric, clinical, and laboratory data were simultaneously collected from all participants. Finally, our study cohort consisted of a well-characterized population and adds to the existing literature on cardiovascular disease among HIV-infected persons.
In summary, despite their young age, HIV-infected persons have a high prevalence of subclinical coronary atherosclerosis. Fatty liver disease is associated with underlying cardiovascular disease and should be considered as a novel marker for risk stratification among HIV-infected persons.
Acknowledgments
Support for this work (IDCRP-018) was provided by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense (DoD) program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072.
Footnotes
Conflict of Interest: None. The authors have no financial interest in this work. All authors contributed to the content of the manuscript and concurred with the decision to submit it for publication.
The content of this publication is the sole responsibility of the authors and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, the DoD or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
This work is original and has not been published elsewhere. Part of these data will be presented at the XVIII International AIDS Conference, July 18-23, 2010, Vienna, Austria.
References
- 1.Boccara F. Cardiovascular complications and atherosclerotic manifestations in the HIV-infected population: type, incidence and associated risk factors. AIDS. 2008;22:S19–26. doi: 10.1097/01.aids.0000327512.76126.6e. [DOI] [PubMed] [Google Scholar]
- 2.d’Arminio A, Sabin CA, Phillips AN, Reiss P, Weber R, Kirk O, et al. Writing Committee of the D:A:D: Study Group. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18:1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 3.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, et al. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [Google Scholar]
- 4.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 5.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 6.DAD Study Group. Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum A, Hadas V, Burke M, Yust I, Kessler A. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. Clin Cardiol. 2005;28:149–153. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kingsley LA, Cuervo-Rojas J, Muñoz A, Palella FJ, Post W, Witt MD, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22:1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 11.Mangili A, Gerrior J, Tang AM, O’Leary DH, Polak JK, Schaefer EJ, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43:1482–1489. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 12.Acevedo M, Sprecher DL, Calabrese L, Pearce GL, Coyner DL, Halliburton SS, et al. Pilot study of coronary atherosclerotic risk and plaque burden in HIV patients: ‘a call for cardiovascular prevention’. Atherosclerosis. 2002;163:349–354. doi: 10.1016/s0021-9150(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 13.Meng Q, Lima JA, Lai H, Vlahov D, Celentano DD, Strathdee SA, et al. Coronary artery calcification, atherogenic lipid changes, and increased erythrocyte volume in black injection drug users infected with human immunodeficiency virus-1 treated with protease inhibitors. Am Heart J. 2002;144:642–648. doi: 10.1067/mhj.2002.125009. [DOI] [PubMed] [Google Scholar]
- 14.Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44:1368–1374. doi: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talwani R, Falusi OM, Mendes de Leon CF, Nerad JL, Rich S, Proia LA, et al. Electron beam computed tomography for assessment of coronary artery disease in HIV-infected men receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;30:191–195. doi: 10.1097/00042560-200206010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, et al. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009;49:1756–1762. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 17.Robinson FP, Hoff JA, Kondos GT. Coronary artery calcium in HIV-infected men treated with highly active antiretroviral therapy. J Cardiovasc Nurs. 2005;20:149–154. doi: 10.1097/00005082-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Lai S, Lima JA, Lai H, Vlahov D, Celentano D, Tong W, et al. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:690–695. doi: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 19.Arslan U, Türkoğlu S, Balcioğlu S, Tavil Y, Karakan T, Cengel A. Association between nonalcoholic fatty liver disease and coronary artery disease. Coron Artery Dis. 2007;18:433–436. doi: 10.1097/MCA.0b013e3282583c0d. [DOI] [PubMed] [Google Scholar]
- 20.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191:235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 22.Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, et al. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183–191. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 24.Cho CS, Curran S, Schwartz LH, Kooby DA, Klimstra DS, Shia J, et al. Preoperative radiographic assessment of hepatic steatosis with histologic correlation. J Am Coll Surg. 2008;206:480–488. doi: 10.1016/j.jamcollsurg.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology. 2007;244:479–485. doi: 10.1148/radiol.2442061177. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs JE, Birnbaum BA, Shapiro MA, Langlotz CP, Slosman F, Rubesin SE, et al. Diagnostic criteria for fatty infiltration of the liver on contrast-enhanced helical CT. AJR Am J Roentgenol. 1998;171:659–664. doi: 10.2214/ajr.171.3.9725292. [DOI] [PubMed] [Google Scholar]
- 27.Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 28.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Dec 1, 2009. [Accessed December 10, 2009.]. pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 29.National Cholesterol Education Program. Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 30.Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 1 to 72 years. Br J Nutr. 1974;32:77–97. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 31.Pierson RN, Jr, Wang J, Heymsfield SB, Russell-Aulet M, Mazariegos M, Tierney M, et al. Measuring body fat: calibrating the rulers. Intermethod comparisons in 389 normal Caucasian subjects. Am J Physiol. 1991;261:e103–8. doi: 10.1152/ajpendo.1991.261.1.E103. [DOI] [PubMed] [Google Scholar]
- 32.Mayo Clinic Diet Manual. 5. Philidelphia: W. B. Saunders Company; 1981. [Google Scholar]
- 33.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, et al. HIV Outpatient Study Investigators. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 34.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 35.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 36.Bild DE, Folsom AR, Lowe LP, Sidney S, Kiefe C, Westfall AO, et al. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–857. doi: 10.1161/01.atv.21.5.852. [DOI] [PubMed] [Google Scholar]
- 37.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 38.Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 40.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103:3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picardi A, Vespasiani-Gentilucci U. Association between non-alcoholic fatty liver disease and cardiovascular disease: a first message should pass. Am J Gastroenterol. 2008;103:3036–3038. doi: 10.1111/j.1572-0241.2008.02196.x. [DOI] [PubMed] [Google Scholar]
- 42.Rossi R, Nuzzo A, Guaraldi G, Orlando G, Squillace N, Ligabue G, et al. The role of the Framingham risk score to predict the presence of subclinical coronary atherosclerosis in patients with HIV infection. J Acquir Immune Defic Syndr. 2009;52:303–304. doi: 10.1097/QAI.0b013e3181b18c19. [DOI] [PubMed] [Google Scholar]
- 43.Law MG, Friis-Møller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A, et al. D:A:D Study Group. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 44.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 45.Oliva MR, Mortele KJ, Segatto E, Glickman JN, Erturk SM, Ros PR, Silverman SG. Computed tomography features of nonalcoholic steatohepatitis with histopathologic correlation. J Comput Assist Tomogr. 2006;30:37–43. doi: 10.1097/01.rct.0000193818.31749.84. [DOI] [PubMed] [Google Scholar]
- 46.Lester SJ, Eleid MF, Khandheria BK, Hurst RT. Carotid intima-media thickness and coronary artery calcium score as indications of subclinical atherosclerosis. Mayo Clin Proceed. 2009;84:229–233. doi: 10.4065/84.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]