Abstract
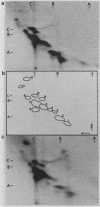
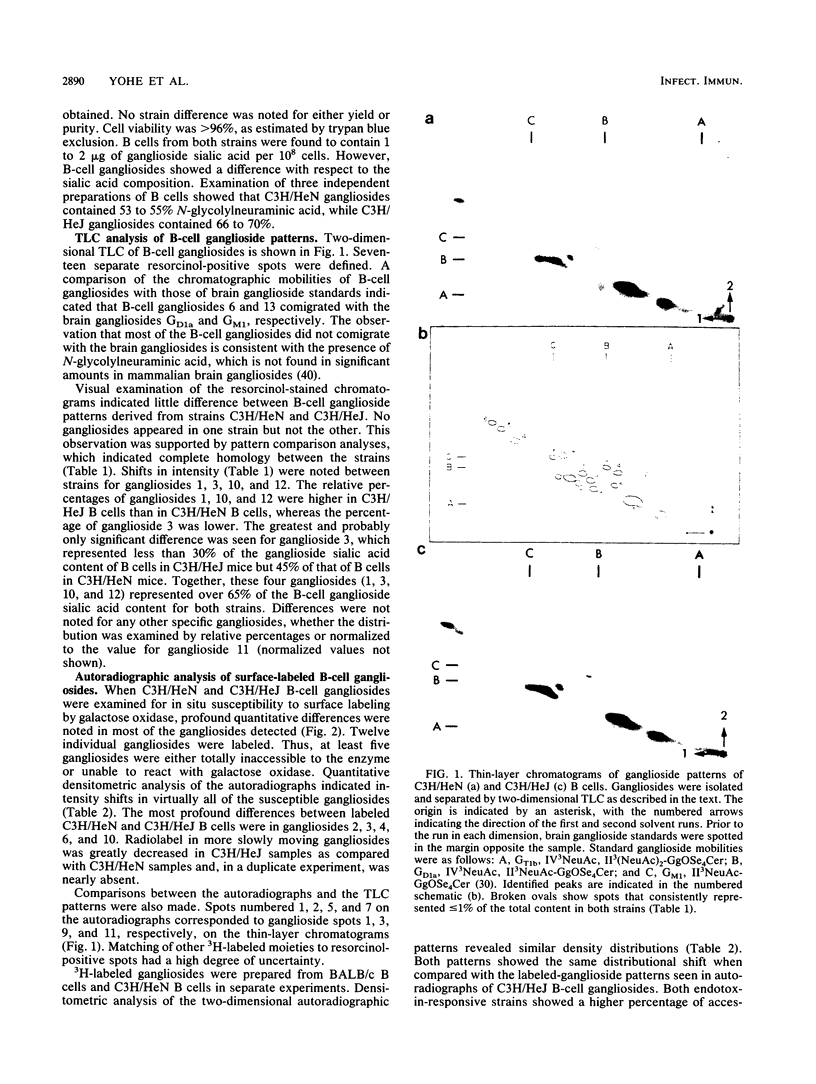
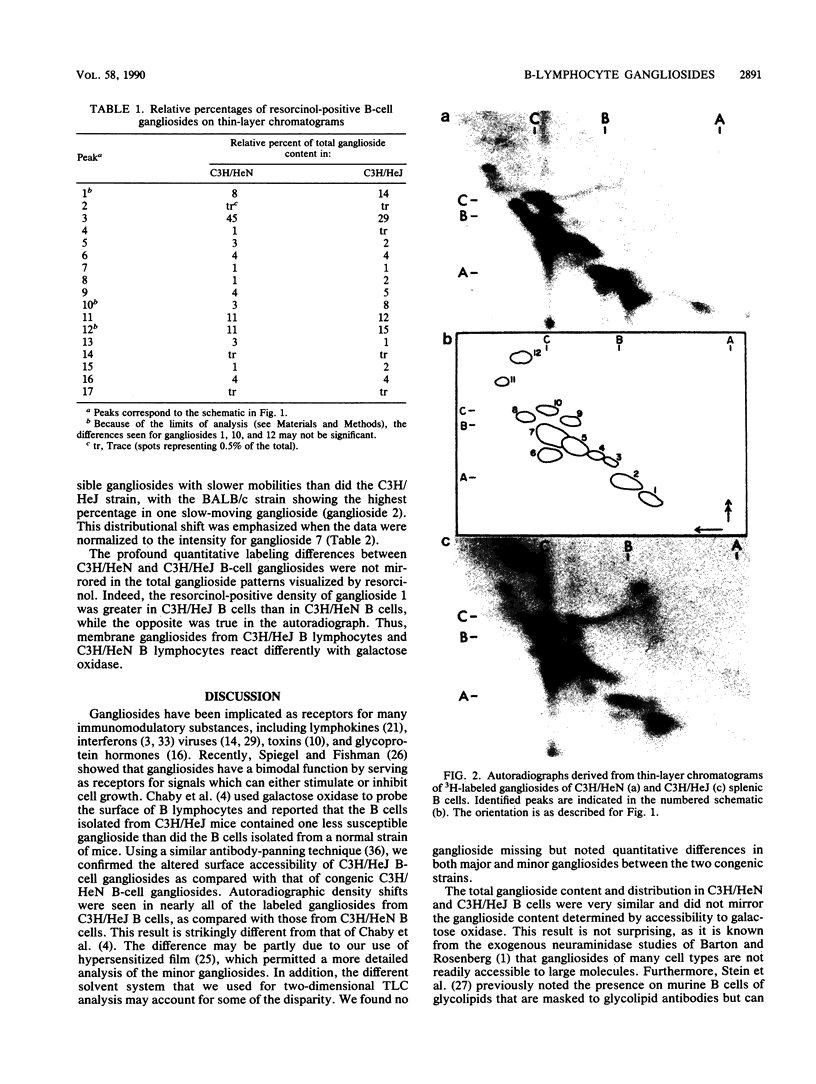
We have analyzed both the total ganglioside composition and the surface accessibility of C3H/HeN B lymphocytes and C3H/HeJ B lymphocytes. Seventeen individual resorcinol-positive moieties were visualized by two-dimensional thin-layer chromatography of the purified gangliosides from both strains. Complete homology between strains was seen in the patterns of total gangliosides purified from the endotoxin-responsive and -hyporesponsive strains, with only minor differences in the relative concentrations of four gangliosides. In comparison, only 12 individual gangliosides were accessible to surface labeling following galactose oxidase treatment in these same strains, suggesting that some gangliosides are masked at the cell surface in both strains. However, labeling of the more polar components was greatly reduced in the endotoxin-hyporesponsive (C3H/HeJ) strain, suggesting that these gangliosides have decreased accessibility to galactose oxidase at the cell surface. Therefore, while the total ganglioside compositions of the two strains were nearly equivalent, there were dramatic differences in ganglioside surface accessibility. These findings indicate that an alteration in membrane structure that is associated with the endotoxin hyporesponsiveness observed in C3H/HeJ B lymphocytes exists.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton N. W., Rosenberg A. Action of Vibrio cholerae neuraminidase (sialidase) upon the surface of intact cells and their isolated sialolipid components. J Biol Chem. 1973 Nov 10;248(21):7353–7358. [PubMed] [Google Scholar]
- Berenson C. S., Yohe H. C., Ryan J. L. Factors mediating lipopolysaccharide-induced ganglioside expression in murine peritoneal macrophages. J Leukoc Biol. 1989 Mar;45(3):221–230. doi: 10.1002/jlb.45.3.221. [DOI] [PubMed] [Google Scholar]
- Besancon F., Ankel H., Basu S. Specificity and reversibility of interferon ganglioside interaction. Nature. 1976 Feb 19;259(5544):576–578. doi: 10.1038/259576a0. [DOI] [PubMed] [Google Scholar]
- Chaby R., Morelec M. J., Ensergueix D., Girard R. Membrane glycolipid and phospholipid composition of lipopolysaccharide-responsive and -nonresponsive murine B lymphocytes. Infect Immun. 1986 Jun;52(3):777–785. doi: 10.1128/iai.52.3.777-785.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M., Miller H. C., Esselman W. J. Antigen induced modulation by shed lymphocyte membrane gangliosides. Immunol Commun. 1980;9(6):543–557. doi: 10.3109/08820138009052994. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Forni L., Watanabe T. Genetic and functional characterization of an antiserum to the lipid A-specific triggering receptor on murine B lymphocytes. Eur J Immunol. 1978 Jan;8(1):63–67. doi: 10.1002/eji.1830080113. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Larrick J. W., Ryan J. L., Szoka F. C. Incorporation of LPS in liposomes diminishes its ability to induce tumoricidal activity and tumor necrosis factor secretion in murine macrophages. J Leukoc Biol. 1988 May;43(5):436–444. doi: 10.1002/jlb.43.5.436. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., Mellors J. W., Ryan J. L., Szoka F. C. Modulation of the biological activity of bacterial endotoxin by incorporation into liposomes. J Immunol. 1987 Apr 15;138(8):2663–2670. [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Flebbe L., Vukajlovich S. W., Morrison D. C. Immunostimulation of C3H/HeJ lymphoid cells by R-chemotype lipopolysaccharide preparations. J Immunol. 1989 Jan 15;142(2):642–652. [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Raetz C. R. Lipid A binding sites in membranes of macrophage tumor cells. J Biol Chem. 1988 Oct 15;263(29):14802–14807. [PubMed] [Google Scholar]
- Holmgren J., Svennerholm L., Elwing H., Fredman P., Strannegård O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Sharon N., Zan-Bar I. Acquisition of mitogenic responsiveness by nonresponding lymphocytes upon insertion of appropriate membrane components. J Exp Med. 1982 Oct 1;156(4):1274–1279. doi: 10.1084/jem.156.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn L. D., Aloj S. M., Beguinot F., Vitti P., Yavin E., Yavin Z., Laccetti P., Grollman E. F., Valente W. A. Molecular interactions at the cell surface: role of glycoconjugates and membrane lipids in receptor recognition processes. Prog Clin Biol Res. 1982;97:55–83. [PubMed] [Google Scholar]
- Kuus-Reichel K., Ulevitch R. J. Partial restoration of the lipopolysaccharide-induced proliferative response in splenic B cells from C3H/HeJ mice. J Immunol. 1986 Jul 15;137(2):472–477. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- Miller H. C., Chaney W. G., Klinman N. R., Esselman W. J. Regulation of B cell tolerance by murine gangliosides. Cell Immunol. 1982 Mar 1;67(2):390–395. doi: 10.1016/0008-8749(82)90230-1. [DOI] [PubMed] [Google Scholar]
- Parker J., Caldini G., Krishnamurti C., Ahrens P. B., Ankel H. Binding of interleukin 2 to gangliosides. FEBS Lett. 1984 May 21;170(2):391–395. doi: 10.1016/0014-5793(84)81351-4. [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Arbeit R. D., Dickler H. B., Henkart P. A. Inhibition of lymphocyte mitogenesis by immobilized antigen-antibody complexes. J Exp Med. 1975 Oct 1;142(4):814–826. doi: 10.1084/jem.142.4.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Spiegel S., Fishman P. H. Gangliosides as bimodal regulators of cell growth. Proc Natl Acad Sci U S A. 1987 Jan;84(1):141–145. doi: 10.1073/pnas.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein K. E., Schwarting G. A., Marcus D. M. Glycolipid markers of murine lymphocyte subpopulations. J Immunol. 1978 Feb;120(2):676–679. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Matsunaga M., Matsumoto M. N-Acetylneuraminyllactosylceramide, GM3-NeuAc, a new influenza A virus receptor which mediates the adsorption-fusion process of viral infection. Binding specificity of influenza virus A/Aichi/2/68 (H3N2) to membrane-associated GM3 with different molecular species of sialic acid. J Biol Chem. 1985 Feb 10;260(3):1362–1365. [PubMed] [Google Scholar]
- Vengris V. E., Reynolds F. H., Jr, Hollenberg M. D., Pitha P. M. Interferon action: role of membrane gangliosides. Virology. 1976 Jul 15;72(2):486–493. doi: 10.1016/0042-6822(76)90177-x. [DOI] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohe H. C., Coleman D. L., Ryan J. L. Ganglioside alterations in stimulated murine macrophages. Biochim Biophys Acta. 1985 Aug 8;818(1):81–86. doi: 10.1016/0005-2736(85)90141-5. [DOI] [PubMed] [Google Scholar]
- Yohe H. C., Cuny C. L., Berenson C. S., Ryan J. L. Comparison of thymocyte and T lymphocyte gangliosides from C3H/HeN and C3H/HeJ mice. J Leukoc Biol. 1988 Dec;44(6):521–528. doi: 10.1002/jlb.44.6.521. [DOI] [PubMed] [Google Scholar]
- Yohe H. C., Ryan J. L. Ganglioside expression in macrophages from endotoxin responder and nonresponder mice. J Immunol. 1986 Dec 15;137(12):3921–3927. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gas--liquid chromatographic assay of lipid-bound sialic acids: measurement of gangliosides in brain of several species. J Lipid Res. 1970 Nov;11(6):506–516. [PubMed] [Google Scholar]