Abstract
The exaggerated expression of chitinase-like protein YKL-40, the human homologue of breast regression protein–39 (BRP-39), was reported in a number of diseases, including chronic obstructive pulmonary disease (COPD). However, the in vivo roles of YKL-40 in normal physiology or in the pathogenesis of specific diseases such as COPD remain poorly understood. We hypothesized that BRP-39/YKL-40 plays an important role in the pathogenesis of cigarette smoke (CS)–induced emphysema. To test this hypothesis, 10-week-old wild-type and BRP-39 null mutant mice (BRP-39−/−) were exposed to room air (RA) and CS for up to 10 months. The expression of BRP-39 was significantly induced in macrophages, airway epithelial cells, and alveolar Type II cells in the lungs of CS-exposed mice compared with RA-exposed mice, at least in part via an IL-18 signaling–dependent pathway. The null mutation of BRP-39 significantly reduced CS-induced bronchoalveolar lavage and tissue inflammation. However, CS-induced epithelial cell apoptosis and alveolar destruction were further enhanced in the absence of BRP-39. Consistent with these findings in mice, the tissue expression of YKL-40 was significantly increased in the lungs of current smokers compared with the lungs of ex-smokers or nonsmokers. In addition, serum concentrations of YKL-40 were significantly higher in smokers with COPD than in nonsmokers or smokers without COPD. These studies demonstrate a novel regulatory role of BRP-39/YKL-40 in CS-induced inflammation and emphysematous destruction. These studies also underscore that maintaining physiologic concentrations of YKL-40 in the lung is therapeutically important in preventing excessive inflammatory responses or emphysematous alveolar destruction.
Keywords: YKL-40/BRP-39, COPD, emphysema, cigarette smoke
CLINICAL RELEVANCE.
Recent studies suggest that the chitinase-like breast regression protein–39 (BRP-39) plays a significant role in airway inflammation and tissue remodeling. However, the in vivo function of BRP-39 in the pathogenesis of chronic obstructive pulmonary disease has not been defined. This study demonstrates that the expression of BRP-39 or its human homologue YKL-40 is regulated by cigarette smoke and plays an important role in the pathogenesis of chronic obstructive pulmonary disease.
Although mammals cannot synthesize or metabolize chitin, a number of chitinolytic enzymes (true chitinases, e.g., chitotriosidases and acidic mammalian chitinase) or chitin-binding proteins (chitinase-like proteins [CLPs], e.g., Ym-1, Ym-2, breast regression protein–39 [BRP-39], and chondrocyte protein–39) that belong to the family of 18 glycosyl–hydrolases were discovered in mammals (1). Both chitinases and CLP are constitutively expressed in macrophages and epithelial cells of the lung and digestive tract, consisting of the body's first line of defense against exogenous agents (2–5).
BRP-39 and its human homologue YKL-40 are regarded as a prototype of CLP in mammals. A variety of inflammatory cells (e.g., neutrophils, macrophages, and differentiating monocytes) and structural cells (e.g., differentiated smooth muscle cells, chondrocytes, synovial cells, endothelial cells, and tumor cells) endogenously express this protein. Intriguingly, increased concentrations of YKL-40 protein and mRNA were noted in a number of diseases characterized by inflammation, tissue remodeling, and aberrant cell growth. They include rheumatoid arthritis, osteoarthritis, giant-cell arthritis, sarcoidosis, sclerosis, diabetes, atherosclerosis, inflammatory bowel disease, liver fibrosis, and several malignancies (6, 7). Recently, elevated concentrations of YKL-40 in the bronchoalveolar lavage (BAL) and serum of smokers with chronic obstructive pulmonary disease (COPD) were reported (8). These significant associations of YKL-40 with the development or progression of disease make YKL-40 a useful prognostic or diagnostic biomarker and potential therapeutic target (9, 10).
Recent studies from our laboratory demonstrated that serum and tissue concentrations of YKL-40 also correlated with the frequency and severity of asthma (11). In addition, the genetic variations of YKL-40 correlated with the risk of asthma and with patients' lung function (12). These studies suggest a proactive role of BRP-39/YKL-40 in the pathogenesis of disease, rather than being a simple biomarker. Several in vitro studies also demonstrated antiapoptotic and proinflammatory roles of YKL-40. YKL-40 protein that was purified from culture supernatants of human articular chondrocytes stimulated the proliferation of connective-tissue cells by activating extracellular signal–regulated kinase and protein kinase-B–mediated signaling pathways (13). YKL-40 was also shown to stimulate alveolar macrophages and fibroblasts to release inflammatory and fibrogenic mediators such as IL-8, monocyte chemotactic protein-1, macrophage inflammatory protein-1 alpha, and matrix metalloproteinase 9 (8, 14). However, the in vivo roles of BRP-39/YKL-40 in normal physiology or in the pathogenesis of disease remain poorly understood.
Pulmonary emphysema is a major manifestation of COPD, and is characterized by the accumulation of inflammatory cells in bronchioles and alveolar structures (15). Alveolar destruction with small airway fibrosis is another pathologic hallmark in the lungs of patients with COPD (16). Although a number of mechanisms were proposed for the development of emphysema, its molecular pathogenesis is not yet clearly understood (17–22). These pathologic features of emphysema and the dysregulated expression of YKL-40 in smokers with COPD (8, 16) led us to hypothesize that BRP-39/YKL-40 may play an important role in the development of cigarette smoke (CS)–induced inflammation and emphysema.
To define the in vivo roles of BRP-39 in CS-induced emphysema, we investigated the regulation of BRP-39 expression in CS-exposed mice compared with room air (RA)–exposed mice. In particular, we evaluated inflammatory and tissue responses in wild-type and BRP-39 null mutant mice after exposure to RA or CS. These studies demonstrated that CS significantly induced the expression of BRP-39 in macrophages, airways, and alveolar epithelial cells in the lungs in an IL-18 signaling–dependent pathway. In mice with BRP-39 null mutation, CS-induced BAL and tissue inflammation was significantly decreased. On the other hand, the CS-induced macrophage and epithelial cell apoptosis and alveolar destruction were further enhanced in mice with the BRP-39 null mutation compared with wild-type mice. Consistent with our murine findings, the levels of YKL-40 expression in the lung tissues of smokers and sera of smokers with COPD were significantly higher than in nonsmoking control subjects. These studies demonstrate a novel regulatory role of BRP-39/YKL-40 in CS-induced inflammation, apoptosis, and alveolar remodeling. They further demonstrate that BRP-39/YKL-40 is an essential regulator for the induction of CS-induced inflammation and maintenance of alveolar integrity, suggesting that the maintenance of physiologic concentrations of BRP-39/YKL-40 may be important in therapeutic interventions for COPD.
MATERIALS AND METHODS
Genetically Modified Mice
C57BL/6 wild-type (WT) mice and IL-18 receptor null mice (IL-18R−/−) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice with null mutations in the BRP-39 loci (BRP-39−/−) were generated from a mixed 129/C57BL/6 background and were bred for over 10 generations onto a C57BL/6 background (23). All animal studies were approved by the Institutional Animal Care and Use Committee of Yale University.
Exposure to Cigarette Smoke
Ten-week-old C57BL/6J mice were exposed to RA or the CS from research cigarettes (2R4; University of Kentucky, Lexington, KY), using a custom-made smoking apparatus described by Shapiro (24). During the first week, mice received a half cigarette twice a day to allow for acclimation, and then two cigarettes per day (one cigarette/session, two sessions/day) for up to 10 months.
BAL and Lung Inflammation
Lung inflammation was assessed by BAL, as described previously (25). In brief, animals were anesthetized, a median sternotomy was performed, the trachea was dissected free from the underlying soft tissues, and BAL was performed by perfusing the lungs in situ with 0.9 ml of PBS and gently aspirating the fluid back. This procedure was repeated twice. Samples were then pooled and centrifuged, and total cell numbers and differentials were assessed. The cell-free BAL fluid was stored at −70°C until used.
Lung Morphometry
The right main bronchus was ligated, and the left lung was inflated with 0.5% low temperature–melting agarose in STRECK (diazolidinyl urea, 2-bromo-2-nitropropane-1,3-diol, zinc sulfate) fixative at a constant pressure of 30 cm. This allowed for the homogenous expansion of lung parenchyma, as described by Halbower and colleagues (26). The lungs were then fixed, paraffin-embedded, and stained with hematoxylin and eosin. Ten random fields were evaluated by microscopic projection onto the Image program, version 1.63 (National Institutes of Health, Bethesda, MD), and alveolar size was estimated from the mean chord length of the air space, as described previously by our laboratory (27).
TUNEL Analysis
End labeling of exposed 3′-OH ends of DNA fragments in paraffin-embedded tissue was undertaken with the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) In Situ Cell Death Detection Kit (Roche Diagnostics), using the instructions provided by the manufacturer. Staining specificity was assessed by comparing the signals when terminal transferase was included and then excluded from the reaction.
Immunoblot Analysis
BAL (35 μL from 2 ml BAL fluid) and lung lysate (50 μg) were subjected to immunoblot analysis, using a polyclonal rabbit antiserum against BRP-39. The antiserum was raised against a peptide containing amino acids 224–243 of mouse BRP-39 (fqgqkdtrfdrysnvnyavq), conjugated with keyhole limpet hemocyanin (KLH) according to standard protocol. BAL fluid protein or lung lysates were fractionated by PAGE, transferred to a membrane, and evaluated according to procedures previously described (27).
Immunohistochemistry
The immunohistochemistry (IHC) of BRP-39 was performed as described by our laboratory (28), using a rabbit anti–BRP-39 polyclonal antibody (MedImmune, Inc., Gaithersburg, MD). The specificity of the staining was tested in experiments in which the primary antiserum was not employed, and tissue samples from BRP-39−/− animals were tested as negative controls. Double-labeled IHC was undertaken, using a modification of procedures described previously by our laboratory (28). Antibodies against pro–SP-C (Chemicon International, Inc., Temecula, CA), CC10 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and F4/80 (e-Bioscience, San Diego, CA) were used to identify alveolar Type II cells, airway epithelial cells, and macrophages, respectively. Anti–pro–SP-C, anti-CC10, and anti-F4/80 were diluted at 1:500, 1:1,000, and 1:500, respectively, and were incubated overnight at 4°C. Fluorescent images were photographed using a LEICA DMIRB fluorescent microscope (LEICA Microsystems, Wetzlar, Germany).
Quantification of BRP-39/YKL-40 and IL-18
The concentrations of BRP-39 and IL-18 in BAL or lung lysates were evaluated by ELISA. ELISA plates that were coated with anti–BRP-39 rabbit polyclonal IgG (Medimmune, Inc.), biotinylated anti–BRP-39 antibody (Medimmune, Inc.), and horseradish peroxidase–labeled streptavidin (Amersham, GE Healthcare, Piscataway, NJ) were used for signal detection. This assay detects as little as 50 pg/ml of recombinant BRP-39. Concentrations of YKL-40 and IL-18 were quantitated using commercially available ELISA Kits. The specificity of the ELISA assay of BRP-39 or YKL-40 was confirmed using recombinant acidic mammalian chitinase and chitotriosidase.
mRNA Analysis
mRNA concentrations were assessed using real-time RT-PCR, as described by our laboratory (29). The sequences for the primers used in real-time RT-PCR were obtained online from PrimerBank (http://pga.mgh.harvard.edu/primerbank).
FACS Analysis
Cells from BAL fluids and lungs were subjected to FACS analysis. BAL was performed as already described. Whole-lung cell suspensions were obtained by a modified method according to Rice and colleagues (30). In brief, lung tissue was digested using Dispase (5 mg/ml; Stem Cell Technologies, Vancouver, British Columbia, Canada), collagenase (0.04%; Sigma-Aldrich, St. Louis, MO) and 100 U/ml DNase I (Sigma-Aldrich). After several centrifugations (300 × g for 10 min.) and hemolysis (precooled hemolysis solution containing 11 mM KHCO3 and 152 mM NH4Cl; washing for 5 minutes, 400 g at 4°C), cells were strained through progressively smaller cell strainers (100 μm to 20 μm) and nylon gauze, and were finally resuspended in FACS buffer (PBS, 2% BSA, and 2% FCS) supplemented with 10 U/ml DNase I. Cells were then incubated for 30 minutes at room temperature with purified rat anti-mouse CD16/CD32 monoclonal antibody (1 μg per 105 cells; BD Biosciences, San Diego, CA) to prevent nonspecific binding of antibodies to Fc receptors. Cells were then incubated for 30 minutes at 4°C with specific antibodies for surface-marker staining for F4/80, CD45, Gr-1, and CD11b to identify macrophages or neutrophils. Lung epithelial cells were characterized according to a gating algorithm described previously (31). All antibodies and FACS reagents were from BD Biosciences. Saturating concentrations of the antibodies were determined by titration experiments before the study. Ten thousand cells per sample were analyzed. Isotype controls were subtracted from the respective specific antibody expression, and the results are reported as mean fluorescence intensity. Calculations were performed with Cell Quest analysis software (Becton-Dickinson, Franklin Lakes, NJ). All experiments were performed in triplicate.
Evaluation of YKL-40 Concentrations in Human Lungs and Sera
Studies were undertaken to evaluate the association between the expression of YKL-40 and smoking and the development of COPD in humans. First we evaluated the tissue expression of YKL-40 in patients who had undergone lung resection at Yale University School of Medicine from 1995–1999. Clinical data on samples were extracted, using a standardized chart abstraction form by an investigator blinded to the results of tissue staining. The characteristics of the study samples are described in Table 1. IHC was performed using the antibody against human YKL-40 (Rb204; MedImmune, Inc.), according to the protocols described above. Sections were scored on a scale from 0–4 (0, no staining; 4, strong, diffuse staining), based on a global assessment of staining (intensity per cell and number of cells stained) by a pathologist blinded to the clinical information associated with each slide. A minimum of 10 nonoverlapping fields was evaluated for each patient. Serum concentrations of YKL-40 in humans were also evaluated in patients with COPD and in control subjects recruited from Yale University Hospital into the Yale Center for Asthma and Airways Disease, in accordance with the guidelines of the Global Initiative for Obstructive Lung Disease (GOLD) (32). Smokers were defined as individuals with more than 10 pack years of smoking history. The patients with COPD were all smokers with clinical symptoms and decreased lung function (FEV1/FVC < 0.7). The characteristics of the study population are summarized in Table 2. All human studies were approved by the Human Investigations Committee of our institution, and written, informed consent was obtained from all subjects. Serum concentrations of YKL-40 were evaluated using ELISA, as already described.
TABLE 1.
CHARACTERISTICS OF PATIENTS UNDERGOING LUNG RESECTION ACCORDING TO SMOKING STATUS
| Ex-Smokers (n = 20) | Current Smokers (n = 15) | Never-Smokers (n = 6) | P* | |
|---|---|---|---|---|
| Age (years), median | 70 (64–75) | 60 (56–63) | 59 (50–72) | ns |
| Male (%) | 10 (50) | 7 (47) | 2 (33) | |
| Race (%) | ||||
| Caucasian | 17 (35) | 14 (93) | 6 (100) | |
| African American | 3 (15) | 1 (7) | 0 | |
| Hispanic | 0 | 0 | 0 | |
| Pack-years of smoking (median) | 58 (24–79) | 75 (40–80) | 0 | <0.0001 |
| Comorbidities | ||||
| Non–small-cell lung cancer | 16 (80%) | 14 (93%) | 2 (25%) | |
| Chronic obstructive pulmonary disease | 9 (45%) | 10 (67%) | 0 |
Definition of abbreviation: ns, nonsignificant.
Medians are given with interquartile ranges.
Comparison between all three groups, according to Kruskal-Wallis analysis.
TABLE 2.
DEMOGRAPHIC CHARACTERISTICS OF CONTROL SUBJECTS, SMOKERS WITHOUT COPD, AND SMOKERS WITH COPD
| Control Subjects (n = 12) | Smokers/COPD-Negative (n = 11) | Smokers/COPD-Positive (n = 18) | P* | |
|---|---|---|---|---|
| Age (years), median | 50 (45–63) | 53 (40–55) | 56 (49–70) | ns |
| Male sex (%) | 3 (25) | 3 (27) | 8 (44) | |
| Race (%) | ||||
| Caucasian | 9 (75) | 10 (91) | 15 (84) | |
| African American | 0 (0) | 0 (0) | 1 (5) | |
| Hispanic | 0 (0) | 1 (9) | 2 (11) | |
| Asian | 3 (25) | 0 (0) | 0 (0) | |
| Pack years of smoking (median) | 0 | 20 (12–33) | 32 (16–68) | <0.0001 |
Definition of abbreviation: ns, non significant.
Medians are given with interquartile ranges.
Comparison between all three groups, according to Kruskal-Wallis analysis.
Statistical Analysis
Statistical evaluations were undertaken with Prism software, version 5.0c (GraphPad Software, Inc., La Jolla, CA). Normally distributed data are expressed as mean ± SEM, and were assessed for significance using a Student t test or ANOVA, as appropriate. Data that were not normally distributed were assessed for significance using the Kruskal-Wallis test, followed by the Dunn post hoc test for multiple comparisons or the Mann-Whitney test for two-group comparisons. The nonparametric correlation between two variables was evaluated by Spearman rank test.
RESULTS
CS Stimulation of BRP-39 via an IL-18–Dependent Pathway
To begin to understand the relationship between BRP-39 and CS in the lung, studies were undertaken to determine if CS stimulated the production of BRP-39. These studies demonstrated that CS was an important stimulator of BRP-39 protein accumulation. This effect was evident after as few as 2 weeks of exposure to CS, was most prominent after 1 month of exposure to CS, and was still appreciable after 10 months of exposure to CS (Figure 1A and data not shown). At all time points, alterations in BRP-39 protein were associated with comparable alterations in BRP-39 mRNA (Figures 1B and 1C). IHC demonstrated that, in CS-exposed mice, BRP-39 accumulated most prominently in airway epithelial cells, alveolar macrophages, and alveolar Type II cells (Figure 1D). Thus CS stimulates BRP-39 mRNA and protein accumulation in macrophages and epithelial cells in the murine lung. Consistent with previous studies from our laboratory (27), a proinflammatory cytokine, IL-18, was significantly induced in the lungs of CS-exposed mice, and the induction of IL-18 was independent of BRP-39 (Figure 1E). To determine the induction mechanism of BRP-39, we further evaluated the level of BRP-39 expression in lungs from wild-type and IL-18 receptor (R) null mice after exposure to RA or CS. The expression of BRP-39 was significantly reduced in the IL-18 R null mice (Figure 1F). These studies demonstrate that CS induces BRP-39 expression in the lung via an IL-18–dependent pathway.
Figure 1.
Effects of exposure to cigarette smoke (CS) on the expression of breast regression protein–39 (BRP-39). Ten-week-old wild-type (WT) mice were exposed to room air (RA) or CS. (A) Protein concentrations of BRP-39 in lung lysates at indicated time points were evaluated by ELISA. (B and C) The expression of BRP-39 protein and mRNA at 1 month after RA or CS exposure was assessed by immunoblot assay and real-time PCR, respectively. (D) Tissue expression of BRP-39 was localized in the lungs of mice after 1 month of RA and CS exposure, using immunohistochemical staining (IHC) (solid arrows, airway epithelial cells; arrowheads, alveolar Type II cells; open arrow, alveolar macrophage; ×40 original magnification; inset, ×80 original magnification). (E and F) Concentrations of IL-18 or BRP-39 in the lungs from WT (+/+), and BRP-39 or IL-18 receptor (R) null mice (−/−) were evaluated by ELISA. Values in A, C, E, and F represent the mean ± SEM of evaluations in a minimum of five animals. B and D are representative of at least two similar evaluations (*P < 0.05, **P < 0.01, ***P < 0.001; ns, not significant).
Role of BRP-39 in CS-Induced Inflammation
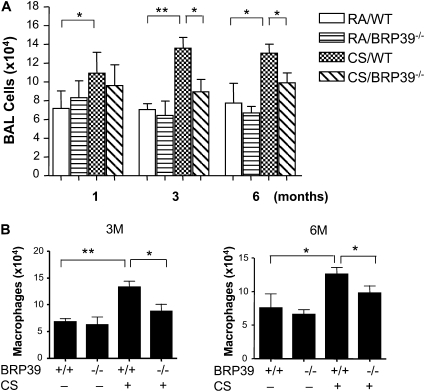
BAL and tissue inflammation was increased in CS-exposed mice compared with RA-exposed control mice (Figure 2). After 3–6 months of exposure to CS, total inflammatory cells were significantly increased in the BAL, and a differential analysis further revealed that the numbers of macrophages were significantly higher in the mice exposed to CS compared with RA-exposed control mice (Figures 2A and 2B). However, in the absence of BRP-39, CS-induced BAL total cells and macrophages were significantly reduced compared with wild-type control mice (Figures 2A and 2B). Similar but less significant cellular changes were evident in BAL lymphocytes and neutrophils (data not shown).
Figure 2.
BRP-39 regulation of CS-induced inflammation. Ten-week-old WT and BRR-39 null mice (BRP-39−/−) were exposed to CS or RA for 1, 3, and 6 months. The mice were killed, and bronchoalveolar lavage (BAL) total cells (A) and differential counts of macrophages at 3-month (3M) and 6-month (6M) time points (B) were measured. Values in A and B represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05, **P < 0.01).
Role of BRP-39 in CS-Induced Emphysema
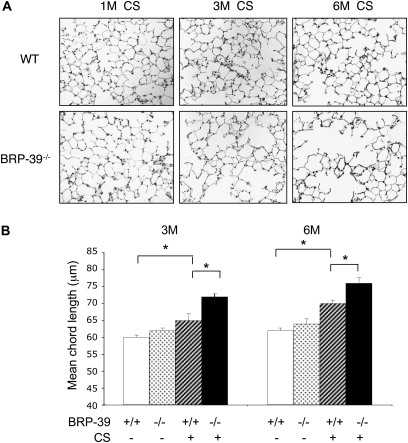
Studies were next undertaken to define the role of BRP-39 in the pathogenesis of the pathologic effects of CS in the lung. This was achieved by comparing the ability of CS to induce emphysema in wild-type mice and BRP-39−/− mice. As previously reported (27), chronic exposure to CS caused modest increases in histologically appreciable and morphometrically apparent alveolar enlargement (Figure 3). This effect was not evident after 1 month, but was evident after 3–6 months of exposure to CS (Figure 3). Alveolar size was not significantly altered in BRP-39−/− mice exposed to RA. In contrast, in BRP-39−/− mice, the emphysematous response was enhanced. Emphysema was appreciable after 1 month of exposure to CS, and enhanced emphysema was evident after 3–6 months of exposure to CS, compared with wild-type mice (Figures 3A and 3B). Thus these studies demonstrate that BRP-39 plays an important role in controlling CS-induced emphysema.
Figure 3.
BRP-39 regulation of CS-induced alveolar destruction. Ten-week-old WT (+/+) and BRR-39 null mice (BRP-39−/−) were exposed to CS or RA for 1 month (1M), 3 months (3M), and 6 months (6M). The mice were killed, and hematoxylin-and-eosin stains in lung-tissue sections (×20 original magnification) (A) and mean chord length (B) were evaluated. A represents the composite of a minimum of five mice from each group. Values in B represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05).
Regulation of Apoptosis by BRP
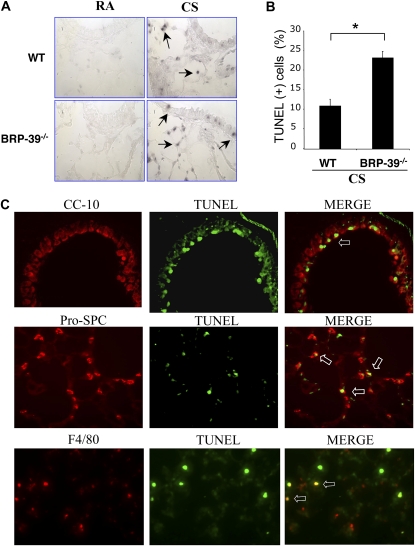
Structural cell apoptosis plays an important role in the pathogenesis of emphysema. Thus, in an attempt to understand the enhanced emphysema of BRP-39−/− mice, we compared levels of apoptosis in CS-exposed wild-type and BRP-39−/− animals. In accordance with our previous observations (27, 33), a significant number of TUNEL-positive cells was not evident in lungs from wild-type mice or BRP-39−/− mice breathing RA (Figure 4A). In addition, modest numbers of TUNEL-positive cells were evident in lungs from wild-type mice exposed to CS (Figure 4A). Importantly, enhanced levels of TUNEL staining were evident in tissues of CS-exposed BRP-39−/− animals (Figure 4A). Double-labeling IHC demonstrated that the majority of these cells were epithelial (airway and alveolar Type II cells) and macrophages (Figure 4B). FACS analysis with propidium iodide/annexin V staining confirmed these observations, highlighting the enhanced apoptosis of epithelial cells, alveolar macrophages, and neutrophils in CS-exposed BRP-39−/− animals (Figures 5A and 5B). Interestingly, basal increases occurred in the apoptotic responses of cells isolated from BRP-39−/− animals compared with wild-type animals, even without exposure to CS. In all cases, these alterations were associated with enhanced levels of Fas expression and enhanced concentrations of activated caspase-3 (Figures 5C and 5D). When viewed in combination, these studies demonstrate that BRP-39 is an important inhibitor of both epithelial-cell and inflammatory-cell apoptosis in the CS-exposed lung.
Figure 4.
Role of BRP-39 in CS-induced apoptosis in the lung. (A) Representative TUNEL stains on lung-tissue sections from 3-month RA-exposed or CS-exposed wild-type and BRP-39−/− mice are illustrated (arrows, TUNEL-positive cells; ×40 original magnification). (B) TUNEL-positive cells were scored and (C) further localized in lungs from CS-exposed BRP-39−/− mice, as detected by double-labeled immunohistochemistry (IHC). Top row, Clara cell 10 kD (CC-10) and TUNEL stains and merged image (MERGE; open arrow, double-positive cells; ×40 original magnification); middle row, pro-surfactant C (Pro-SPC) and TUNEL stains and merged image (open arrow, double-positive cells; ×40 original magnification); bottom row, F4/80 and TUNEL stains and merged image (open arrow, double-positive cells; ×40 original magnification). A and B are representative of three similar evaluations. Values in B represent the mean ± SEM of evaluations in a minimum of four animals (*P < 0.01).
Figure 5.
BRP-39 regulation of CS-induced cellular apoptosis and death. Ten-week-old WT (+) and BRR-39 null mice (−) were exposed to CS or RA for 3 months. The apoptosis/necrosis responses in airway epithelial cells, alveolar macrophages, and neutrophils were evaluated by FACS analysis after staining with annexin V (A), propidium iodide (PI) (B), active caspase-3 (C), and Fas (D). Values represent the mean ± SEM of evaluations in a minimum of five animals (*P < 0.05). MFI, mean fluorescence intensity.
YKL-40 Tissue Expression in Human Smokers
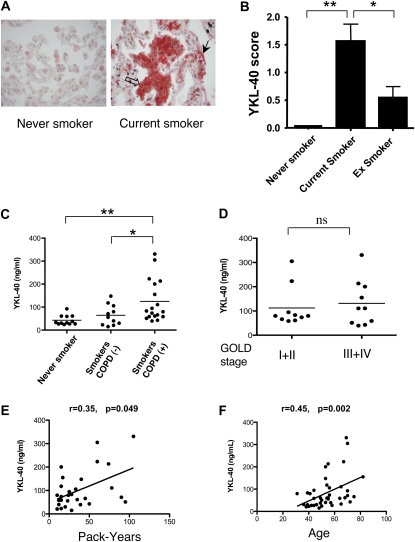
To evaluate the applicability of our murine findings to human disease, studies were undertaken to determine if YKL-40 was expressed in an exaggerated fashion in CS-exposed human lungs. This was accomplished by comparing the concentrations of immunoreactive YKL-40 in lung tissues from current smokers, former smokers, and never-smokers (Figures 6A and 6B). The clinical characteristics of these patients are listed in Table 1. Modest concentrations of immunoreactive YKL-40 were evident in the lungs of never-smokers (Figure 6A). In contrast, increased concentrations of immunoreactive YKL-40 were readily appreciated in the alveolar macrophages and epithelial cells of current-smoker tissues (Figure 6A). Pairwise comparisons showed significantly higher concentrations of YKL-40 in tissues from current smokers compared with never-smokers (P < 0.01) and current smokers versus former smokers (P < 0.05) (Figure 6B). Former smokers exhibited concentrations of YKL-40 between those observed in never-smokers and current smokers (Figure 6B).
Figure 6.
Expression of YKL-40 in human lungs and sera. (A) After immunostaining lung tissue sections obtained from never-smokers, current smokers, and ex-smokers with anti-human YKL-40 antibody, representative tissue staining was performed (open arrow, YKL-40–positive macrophages in the inflamed region; solid arrow, YKL-40–positive epithelial cells; ×40 original magnification), and (B) an IHC score was assigned. (C) Concentrations of circulating YKL-40 were assessed by ELISA in age-matched never-smokers, smokers without COPD, and smokers with COPD (*P < 0.05, **P < 0.01; ns, no significance). Concentrations of YKL-40 were further stratified according to GOLD stages (D), and correlations between serum YKL-40 concentrations and pack-years of smokers (E) or the ages of individuals (F) were plotted and tested. r, Spearman rank correlation coefficient.
Concentrations of Serum YKL-40 in Smokers and Patients with COPD
To understand further the regulation of YKL-40 in humans, we also compared the concentrations of immunoreactive YKL-40 in the serum of age-matched controls, active smokers without COPD, and smokers with COPD. The demographics of these populations are described in Table 2. These populations were similar with respect to other comorbidities, yet more subjects among the smokers with COPD had smoking histories with a greater number of overall pack-years than the control subjects. As shown in Figure 6C, YKL-40 was readily apparent in the circulation of our control patients. No significant difference was evident in YKL-40 concentrations when stratifying for other comorbidities such as coronary artery disease, hypertension, diabetes mellitus, asthma, and gastroesophageal reflux disease (data not shown). The YKL-40 serum concentrations were, however, significantly higher in the smokers with COPD compared with nonsmoking control subjects or smokers without COPD (Figure 6C). The serum concentrations of YKL-40 were not significantly correlated with the severity of COPD when patients with COPD were compared after disease stratification according to the GOLD classification (Stages I + II versus Stages III + IV, P = 0.68; Mann Whitney test) (Figure 6D). Borderline significance was evident between YKL-40 concentrations and pack-years in the smokers (P = 0.049) (Figure 6E), and significant correlation was evdient between overall age and serum YKL-40 concentrations in this study population, although the different groups were well age-controlled (P < 0.01) (Figure 6F).
DISCUSSION
BRP-39/YKL-40 is a member of the CLPs, and belongs to glycohydrolase family 18. BRP-39/YKL-40 is a phylogentically highly conserved chitin-binding, heparin-binding, and collagen-binding protein, with homologues in vertebrates and invertebrates (1). Elevated concentrations of YKL-40 were demonstrated in a variety of diseases that are pathologically characterized by tissue inflammation and remodeling. Because of its strong association with disease, YKL-40 is regarded as a useful prognostic or diagnostic marker and potential therapeutic target (9, 10). However, the molecular processes governing the induction of YKL-40 and the roles of YKL-40 in normal physiology and in the pathogenesis of diseases remain poorly understood. This is partly attributable to the lack of appropriate animal models, such as specific gene-targeted null or overexpressing transgenic mice. In this study, we demonstrated that BRP-39, the mouse homologue of human YKL-40, plays an important role in the development of CS-induced inflammation and emphysema, by using BRP-39 null mutant mice recently generated in our laboratory (23).
The mRNA and protein expression of BRP-39 was significantly induced in CS-exposed mice compared with RA-exposed controls. We further demonstrated that macrophages, airways, and alveolar epithelial cells are the major BRP-39–expressing cells in the lungs of CS-exposed mice. Previous in vitro studies showed that proinflammatory cytokines IL-1β and TNF-α efficiently stimulated articular chondrocytes to produce YKL-40 (34). Because these proinflammatory cytokines are elevated in the lungs of patients with COPD (27, 35) and in CS-exposed animal models of COPD (18, 36), CS-induced proinflammatory cytokines could play a role in the induction of BRP-39 in the lungs of CS-exposed mice. Although we could not detect a significant induction of IL-1β and TNF-α, the expression of proinflammatory cytokine IL-18 was significantly induced in mice exposed to CS, which is consistent with previous findings from our laboratory (27). Interestingly, the CS-stimulated induction of BRP-39 was dependent on the IL-18–signaling pathway, at least in part, because the expression of BRP-39 was substantially decreased in mice with the IL-18 receptor null mutation. On the other hand, the induction of IL-18 was not significantly modulated in the absence of BRP-39, suggesting that BRP-39 is a downstream mediator of IL-18. The presence of the IL-18 receptor in bronchoalveolar epithelial cells and macrophages (37) further supports the potential regulatory role of IL-18 in the induction of BRP-39 in these cells after exposure to CS. However, we cannot exclude the possibility of BRP-39 induction through CS-induced proinflammatory cytokines other than IL-18, such as TNF-α or IL-1β, because previous studies demonstrated that these cytokines regulate the expression of YKL-40 in differentiated macrophages or articular chondrocytes (38, 39). Interestingly, we did not observe increased expression of TNF-α, IL-6, or IL-1β in the lungs of IL-18R−/− after exposure to CS (data not shown). On the other hand, when we overexpressed IL-18 in the lung, we noted significant increases in the expression of TNF-α, IL-6, and IL-1β, together with BRP-39 (unpublished data). These studies suggest that IL-18 is an upstream mediator that regulates the expression of multiple proinflammatory cytokines together with BRP-39. The specific contributions of these proinflammatory cytokines in the induction by CS of BRP-39 remain to be determined. Recent studies from our laboratory and others suggest a potential regulatory role of BRP-39/YKL-40 in inflammation. The administration of recombinant YKL-40 can directly stimulate alveolar macrophages and fibroblasts to release proinflammatory and profibrogenic mediators such as IL-8, MCP-1, and MIP-1α to levels comparable with those obtained upon cell activation by TNF-α (8, 14). Those studies raised the possibility that BRP-39/YKL-40 could mediate or synergize the effects of IL-18 or other proinflammatory cytokines for the generation of optimal inflammatory responses by exposure to CS. In addition, our laboratory demonstrated the direct role of BRP-39/YKL-40 in the regulation of lung inflammation in an animal model of allergen-induced Th2 inflammation (23). BRP-39, as a downstream mediator of IL-13, directly regulates allergen-induced BAL and tissue inflammation through the inhibition of inflammatory cell apoptosis (23). Similar to the induction of BRP-39 and the regulatory mechanism in Th2 inflammatory responses, the expression of BRP-39 was also induced by a proinflammatory cytokine (IL-18), and endogenous BRP-39 was required for CS-induced inflammation. However, BRP-39/YKL-40 itself seems insufficient to induce inflammation, because the lung-specific overexpression of YKL-40 only enhanced the allergen-induced inflammatory response, but did not induce an inflammatory response without additional challenge (23). In combination, these studies demonstrate that BRP-39 is induced in macrophages or epithelial cells after exposure to CS, and plays a proinflammatory role through regulating cellular apoptosis.
Several mechanistic pathways were suggested for the pathogenesis of COPD (17, 18). They include protease/antiprotease imbalance, oxidative stress, and deregulated inflammatory and immune responses. Recent data from both animal models of COPD and human studies strongly suggest another mechanism of COPD pathogenesis, that is, the disruption of the balance between apoptosis and the replenishment of structural cells in the lung (22). Specifically, the critical role of epithelial-cell apoptosis in the development of emphysema is supported by a number of studies. The treatment of vascular endothelial cell growth factor (VEGF) receptor blockers was shown to induce alveolar septal-cell apoptosis, resulting in emphysematous lung destruction (21, 40–43). Studies from our laboratory demonstrated that epithelial-cell apoptosis was significantly augmented in animal models of emphysema (28, 44, 45). Intervention with apoptotic tissue responses by nonspecific pan-caspase inhibitors, such as Z-VAD-fmk, significantly reduced IFN-γ–stimulated or TGF-β–stimulated airway and alveolar remodeling, further supporting the critical role of apoptosis in alveolar and airway remodeling processes (29, 45). In the present study, we also observed significantly increased TUNEL-positive and annexin V-positive apoptotic macrophages and alveolar and airway epithelial cells after chronic exposure to CS. However, the CS-induced structural cell death response and emphysematous destruction were significantly enhanced in the absence of BRP-39. These results suggest a protective role of endogenous BRP-39 from CS-induced apoptosis or cell-death response. In previous studies, the proactive role of BRP-39/YKL-40 in cellular proliferation and tissue remodeling was demonstrated. YKL-40 was shown to act as a growth factor for fibroblasts and chondrocytes in synergy with insulin-like growth factor–1 (13), and to limit the catabolic effects of TNF-α and IL-1β (14, 38). YKL-40 is also synthesized by vascular smooth muscle cells, and it promotes their migration and attachment (46–48). In addition, YKL-40 was shown to stimulate endothelial cells directly, and to increase angiogenesis in vivo and in vitro (49). Interestingly, the absence of BRP-39 did not significantly alter epithelial-cell proliferation in the lungs of mice after exposure to RA or CS (Figure 1E in the online supplement). This result suggests that the deregulation of BRP-39 contributes to CS-induced emphysema, mainly via the regulation of the CS-induced cell death response rather than through epithelial-cell proliferation. The present study further reveals that BRP-39 regulates the CS-induced cell death response via the modulation of Fas expression in inflammatory and epithelial cells. When these findings are viewed in combination, we can envision that BRP-39/YKL-40 plays an important role in structural cell survival, and that the endogenous expression of BRP-39/YKL-40 is essential for the maintenance of normal alveolar structures in the lung.
In the present study, we noted that a deficiency of BRP-39 diminishes CS-induced inflammation while augmenting tissue destruction. On superficial analysis, this may appear confusing. However, these and other studies make it very clear that BRP-39/YKL-40 exerts important effects on both inflammatory and structural cell death (23). Specifically, superphysiologic levels of BRP-39 and YKL-40 appear to inhibit inflammatory cell death (23). In contrast, physiologic concentrations of BRP-39 appear to inhibit oxidant injury and maintain the survival of structural cells such as epithelial cells and endothelial cells (50). Thus, in the absence of BRP-39, we can envision heightened levels of CS-induced injury and heightened levels of structural cell apoptosis. Simultaneously, we would also see heightened levels of inflammatory cell apoptosis, and thus a diminished inflammatory response. Thus, not only are these results consistent with each other, but they represent a heightened level of understanding of the biologic roles of BRP-39 in the setting of CS-induced injury. This study also highlights the importance of maintaining physiologic levels of BRP-39 or YKL-40 in the lungs, to prevent both excessive inflammatory responses and excessive alveolar destruction. In this regard, the physiologic balance of YKL-40 should be considered in future therapeutic applications of YKL-40 in diseases associated with an elevated concentration of YKL-40, such as asthma or COPD.
To evaluate the applicability of our murine findings to human diseases, studies were undertaken to investigate the expression of YKL-40 in human lung tissues. Corresponding to the murine data, the tissue expression of YKL-40 was highest in smokers, followed by former smokers and never-smokers, representing a significant impact of CS on the expression of YKL-40 in the lung. In addition, the major YKL-40–expressing cells in the lungs of smokers were also mostly macrophages, airway cells, and alveolar epithelial cells. Letuve and colleagues reported that YKL-40–expressing macrophages and interstitial cells were significantly increased in bronchial biopsies from smokers with COPD compared with never-smokers (8). In contrast to our findings, they did not directly correlate YKL-40 expression in the lungs with a history of CS. However, a higher percentage of cells expressing YKL-40 in alveolar macrophages was isolated from smokers than from never-smokers (8), indicating substantial effects of CS on the expression of YKL-40 in the lung.
To see whether correlations exist between the expression of YKL-40 and the development of COPD in humans, serum concentrations of YKL-40 in nonsmoking control subjects, smokers, and smokers with COPD were evaluated. These studies demonstrated that only smokers with COPD had significantly higher serum YKL-40 concentrations compared with smokers without COPD or never-smokers. These findings are consistent with those reported by Levute and colleagues (8). Interestingly, only a borderline correlation existed between serum concentrations of YKL-40 and smoking history (P = 0.049). Although the levels of CS exposure (pack-years) were higher in COPD patients with higher YKL-40 concentrations than with lower YKL-40 concentrations (58.43 ± 12.35 versus 34.82 ± 7.09 pack-years [mean ± SEM] for patients with > 100 ng/ml versus < 100 ng/ml, respectively), these reults did not reach statistical significance. On the other hand, serum YKL-40 concentrations were significantly correlated with the age of individuals, irrespective of smoking history, as demonstrated in a previous study (51). These results suggest that concentrations of circulatory YKL-40 in patients with COPD are profoundly affected by other local or systemic factors in addition to CS exposure. Because activated inflammatory cells express YKL-40, concentrations of serum YKL-40 could be influenced by the presence of low-grade systemic inflammation in patients with COPD (52, 53). In the present study, the severity of COPD according to GOLD stages did not indicate a significant difference in serum concentrations of YKL-40. In contrast to our studies and others, Agapov and colleagues found no significant differences in serum concentrations of YKL-40 between simple smokers and smokers with COPD, or between stratified groups based on GOLD stages (54). As suggested Agapov and colleagues (54), the differences between studies may be attributed to the heterogeneity of a complex disease. Additional investigations on the potential source or other modulating factors of serum YKL-40 concentrations and their clinical implications in a larger cohort of patients with COPD are warranted.
In conclusion, these studies demonstrate that BRP-39/YKL-40 is accumulated during exposure to CS, and plays a significant role in the pathogenesis of CS-induced inflammation and emphysema. These studies also underscore that maintaining the balance of physiologic levels of YKL-40 will be therapeutically important in preventing excessive inflammatory responses and emphysematous alveolar destruction.
Supplementary Material
This work was supported by National Institutes of Health grants HL-081639 and HL-093027 (J.A.E.) and HL-084225 (C.G.L.) and a grant from Bioneer, Korea (C.G.L.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0081OC on July 23, 2010
Author Disclosure: G.L.C. has received lecture fees from AstraZeneca ($10,001–$50,000) and GlaxoSmithKline ($1,001–$5,000), and has received industry-sponsored grants from Gilead ($10,001–$50,000) and Genetech ($10,001–$50,000). J.A.E. has received consultancy fees from Promedior, Inc. ($5,001–$10,000), has served on the board of Intermune, Inc. ($1,001–$5,000), has patents pending/received with Yale University on chitinases in lung inflammation, mir-1 in VEGF tissue responses, IL-18 in COPD, and VEGF in asthma, and holds stock ownership/options with Merck, Inc. ($1,001–$5,000), and Intermune, Inc. ($5,001–$10,000). None of the other authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(–like) members of family 18 glycosyl hydrolases. Genetics 2007;177:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol 2006;291:L502–L511. [DOI] [PubMed] [Google Scholar]
- 3.Mizoguchi E. Chitinase 3–like–1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 2006;130:398–411. [DOI] [PubMed] [Google Scholar]
- 4.Lee CG, Elias JA. Role of breast regression protein–39/YKL-40 in asthma and allergic responses. Allergy Asthma Immunol Res 2010;2:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CG, Da Silva CA, Lee JY, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol 2008;20:684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J 2009;50:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawada M, Hachiya Y, Arihiro A, Mizoguchi E. Role of mammalian chitinases in inflammatory conditions. Keio J Med 2007;56:21–27. [DOI] [PubMed] [Google Scholar]
- 8.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol 2008;181:5167–5173. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Ripa RS, Johansen JS, Gabrielsen A, Steinbruchel DA, Friis T, Bindslev L, Haack-Sorensen M, Jorgensen E, Kastrup J. YKL-40: a new biomarker in patients with acute coronary syndrome or stable coronary artery disease. Scand Cardiovasc J 2008;42:295–302. [DOI] [PubMed] [Google Scholar]
- 10.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40: a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev 2006;15:194–202. [DOI] [PubMed] [Google Scholar]
- 11.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med 2007;357:2016–2027. [DOI] [PubMed] [Google Scholar]
- 12.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, Radford S, Parry RR, Heinzmann A, Deichmann KA, et al. Effect of variation in Chi3l1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med 2008;358:1682–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recklies AD, White C, Ling H. The chitinase 3–like protein human cartilage glycoprotein 39 (HC-GP39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase– and protein kinase B–mediated signalling pathways. Biochem J 2002;365:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling H, Recklies AD. The chitinase 3–like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor–alpha. Biochem J 2004;380:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2008;4:435–459. [DOI] [PubMed] [Google Scholar]
- 16.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 17.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke–induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 2008;294:L612–L631. [DOI] [PubMed] [Google Scholar]
- 19.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest 2004;125:626–632. [DOI] [PubMed] [Google Scholar]
- 20.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 2003;28:555–562. [DOI] [PubMed] [Google Scholar]
- 21.Tuder RM, Zhen L, Cho CY, Taraseviciene-Stewart L, Kasahara Y, Salvemini D, Voelkel NF, Flores SC. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol 2003;29:88–97. [DOI] [PubMed] [Google Scholar]
- 22.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3–like–1 in Th2 and IL-13–induced tissue responses and apoptosis. J Exp Med 2009;206:1149–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro SD. Animal models for chronic obstructive pulmonary disease: age of Klotho and Marlboro mice. Am J Respir Cell Mol Biol 2000;22:4–7. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Homer RJ, Hong L, Cohn L, Lee CG, Jung S, Elias JA. Il-11 selectively inhibits aeroallergen-induced pulmonary eosinophilia and Th2 cytokine production. J Immunol 2000;165:2222–2231. [DOI] [PubMed] [Google Scholar]
- 26.Halbower AC, Mason RJ, Abman SH, Tuder RM. Agarose infiltration improves morphology of cryostat sections of lung. Lab Invest 1994;71:149–153. [PubMed] [Google Scholar]
- 27.Kang MJ, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, et al. Il-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke–induced pulmonary emphysema and inflammation. J Immunol 2007;178:1948–1959. [DOI] [PubMed] [Google Scholar]
- 28.Zheng T, Zhu Z, Wang Z, Homer RJ, Ma B, Riese RJ Jr, Chapman HA Jr, Shapiro SD, Elias JA. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase– and cathepsin-dependent emphysema. J Clin Invest 2000;106:1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, Yehualaeshet T, Lu B, Flavell RA, Milbrandt J, et al. Early growth response gene 1–mediated apoptosis is essential for transforming growth factor beta1–induced pulmonary fibrosis. J Exp Med 2004;200:377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse Type II cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 2002;283:L256–L264. [DOI] [PubMed] [Google Scholar]
- 31.Hartl D, He CH, Koller B, Da Silva CA, Homer R, Lee CG, Elias JA. Acidic mammalian chitinase is secreted via an ADAM17/epidermal growth factor receptor–dependent pathway and stimulates chemokine production by pulmonary epithelial cells. J Biol Chem 2008;283:33472–33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 33.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, Elias JA. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest 2008;118:2771–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johansen JS, Olee T, Price PA, Hashimoto S, Ochs RL, Lotz M. Regulation of YKL-40 production by human articular chondrocytes. Arthritis Rheum 2001;44:826–837. [DOI] [PubMed] [Google Scholar]
- 35.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 2008;118:3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor–alpha drives 70% of cigarette smoke–induced emphysema in the mouse. Am J Respir Crit Care Med 2004;170:492–498. [DOI] [PubMed] [Google Scholar]
- 37.Kitasato Y, Hoshino T, Okamoto M, Kato S, Koda Y, Nagata N, Kinoshita M, Koga H, Yoon DY, Asao H, et al. Enhanced expression of interleukin-18 and its receptor in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2004;31:619–625. [DOI] [PubMed] [Google Scholar]
- 38.Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of Chi3l1 by articular chondrocytes. J Biol Chem 2005;280:41213–41221. [DOI] [PubMed] [Google Scholar]
- 39.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, Krause SW. Transcriptional regulation of Chi3l1, a marker gene for late stages of macrophage differentiation. J Biol Chem 2003;278:44058–44067. [DOI] [PubMed] [Google Scholar]
- 40.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 42.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566, discussion 1549. [DOI] [PubMed] [Google Scholar]
- 44.Ma B, Kang MJ, Lee CG, Chapoval S, Liu W, Chen Q, Coyle AJ, Lora JM, Picarella D, Homer RJ, et al. Role of CCR5 in IFN-gamma–induced and cigarette smoke–induced emphysema. J Clin Invest 2005;115:3460–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng T, Kang MJ, Crothers K, Zhu Z, Liu W, Lee CG, Rabach LA, Chapman HA, Homer RJ, Aldous D, et al. Role of cathepsin S–dependent epithelial cell apoptosis in IFN-gamma–induced alveolar remodeling and pulmonary emphysema. J Immunol 2005;174:8106–8115. [DOI] [PubMed] [Google Scholar]
- 46.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. GP38K, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res 1999;250:168–173. [DOI] [PubMed] [Google Scholar]
- 47.Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (GP38K) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem 1995;270:13076–13083. [DOI] [PubMed] [Google Scholar]
- 48.Nishikawa KC, Millis AJ. GP38K (Chi3l1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res 2003;287:79–87. [DOI] [PubMed] [Google Scholar]
- 49.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene 2009;28:4456–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Lee CG, Elias JA. The chitinase-like proteins breast regression protein–39 and YKL-40 regulate hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2010; (In Press). [DOI] [PMC free article] [PubMed]
- 51.Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL-40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol 2009;143(1):35–42. [DOI] [PubMed] [Google Scholar]
- 52.Yanbaeva DG, Dentener MA, Creutzberg EC, Wouters EF. Systemic inflammation in COPD: is genetic susceptibility a key factor? COPD 2006;3:51–61. [DOI] [PubMed] [Google Scholar]
- 53.McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med 2009;180:692–700. [DOI] [PubMed] [Google Scholar]
- 54.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, Atkinson JJ, Holtzman MJ. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol 2009;41:379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.