Abstract
Induction of nitric oxide synthase (NOS)–2 and production of nitric oxide (NO) are common features of allergic airway disease. Conditions of severe asthma are associated with deficiency of airway S-nitrosothiols, a biological product of NO that can suppress inflammation by S-nitrosylation of the proinflammatory transcription factor, NF-κB. Therefore, restoration of airway S-nitrosothiols might have therapeutic benefit, and this was tested in a mouse model of ovalbumin (OVA)-induced allergic inflammation. Naive or OVA-sensitized animals were administered S-nitrosoglutathione (GSNO; 50 μl, 10 mM) intratracheally before OVA challenge and analyzed 48 hours later. GSNO administration enhanced lung tissue S-nitrosothiol levels and reduced NF-κB activity in OVA-challenged animals compared with control animals, but did not lead to significant changes in total bronchoalveolar lavage cell counts, differentials, or mucus metaplasia markers. Administration of GSNO also altered the activation of hypoxia-inducible factor (HIF)–1, leading to HIF-1 activation in naive mice, but suppressed HIF-1 activation in OVA-challenged mice. We assessed the contribution of endogenous NOS2 in regulating NF-κB and/or HIF-1 activation and allergic airway inflammation using NOS2−/− mice. Although OVA-induced NF-κB activation was slightly increased in NOS2−/− mice, associated with small increases in bronchoalveolar lavage neutrophils, other markers of allergic inflammation and HIF-1 activation were similar in NOS2−/− and wild-type mice. Collectively, our studies indicate that instillation of GSNO can suppress NF-κB activation during allergic airway inflammation, but does not significantly affect overall markers of inflammation or mucus metaplasia, thus potentially limiting its therapeutic potential due to effects on additional signaling pathways, such as HIF-1.
Keywords: nitric oxide, asthma, S-nitrosothiols, nuclear factor–κB, hypoxia inducible factor−1
Nitric oxide (NO) is a ubiquitous signaling molecule that is continuously produced within the airways of healthy individuals, primarily originating from constitutive expression of the inducible NO synthase (NOS)–2 (iNOS) within the respiratory epithelium (1, 2). Airway NO production is increased under conditions of infection or inflammation due to induction of NOS2. Indeed, induction of airway NOS2 and increased airway production of NO are common symptoms of allergic asthma, an inflammatory disease of the airways characterized by airway hyperreactivity, smooth muscle thickening, and mucus hypersecretion. Analysis of exhaled human breath has demonstrated higher levels of NO in patients with asthma compared with healthy control subjects, with increases in epithelial NOS2 being the primary site of its production (1, 2). Induction of NOS2 is critical in innate antimicrobial or antiviral host defense, but its contribution in conditions of airway inflammation, including allergic asthma, is still controversial, and includes both pro- and anti-inflammatory properties, based on studies with NOS2 inhibitors or genetic NOS2 deletion (3–7). Variable metabolism of NO under these conditions to diverse mediators with opposing actions may be a major reason for these inconclusive results. NO may promote inflammatory signaling by conversion to more reactive intermediates through interaction with reactive oxygen metabolites. However, its anti-inflammatory properties may include suppressed activation of NF-κB, a central regulator of innate and adaptive immune responses, and an important factor in development of allergic airway inflammation (8), by S-nitrosylation of several specific proteins within the NF-κB signaling pathway (9, 10). Because NOS inhibition would minimize both events, the overall outcome during chronic airway inflammation is not always clear.
Intriguingly, in spite of enhanced NOS2 expression and airway NO production in subjects with asthma, levels of S-nitrosothiols in tracheal aspirates or bronchoalveolar lavage (BAL) fluids from these patients are commonly reduced (11). In addition to potentially increased S-nitrosothiol breakdown by reactive oxygen species, recent studies also indicate increased enzymatic S-nitrosothiol degradation within asthmatic airways by S-nitrosoglutathione (GSNO) reductase (GSNOR) (12). Based on these observations and the demonstrated ability of S-nitrosothiols to suppress inflammation, S-nitrosothiol supplementation (e.g., by aerosolized GSNO) has been proposed as a therapy for asthma and cystic fibrosis (13, 14). Indeed, administration of ethyl nitrite was recently found to enhance airway S-nitrosothiol levels and suppress acute inflammation by LPS in association with inhibition of NF-κB (15). Our previous studies indicated that S-nitrosothiols can inhibit NF-κB signaling by S-nitrosylation (10), and that NF-κB is also a critical mediator of allergic airway inflammation and mucus metaplasia (8). The present studies were designed to explore whether administration of GSNO could be employed to suppress NF-κB activation, markers of allergic inflammation, and mucus metaplasia in an ovalbumin (OVA) model of allergic airway disease in mice. In addition, in light of recent studies demonstrating a potential role for the transcription factor, hypoxia-inducible factor (HIF)–1, in allergic airway inflammation (16), and its known regulation by NO and S-nitrosothiols (17), we also explored the impact of GSNO on activation of HIF-1 during allergic inflammation. Finally, we determined the contribution of NOS2, a presumed major source of endogenous NO and S-nitrosothiols during inflammation, in regulating these transcription factors in this model of allergic asthma. Collectively, our results indicate that GSNO administration is capable of suppressing signaling by NF-κB during allergic airway inflammation, but does not significantly affect allergic airway inflammation, presumably because of additional effects of GSNO on other signaling pathways, such as HIF-1, which may oppose the effects on NF-κB.
MATERIALS AND METHODS
Model of Allergic Airway Disease
Weight-matched, male, 6-week-old C57BL/6J or Nos2tm1Lau/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and housed in the University of Vermont Animal Facility (Burlington, VT). Mice were subjected to OVA-induced allergic airway inflammation, as described previously (8). Mice were administered OVA (20 μg, grade V; Sigma, St. Louis, MO) with aluminum hydroxide (Alum) (2.25 mg, Imject Alum; Pierce, Rockford, IL) via a 100-μl total volume intraperitoneal injection on Days 0 and 7 (OVA/OVA). Nonsensitized control animals received an injection of alum alone (Alum/OVA). Mice were challenged with aerosolized 1% OVA in PBS for 30 minutes on Days 21–23. Select mice received GSNO (50 μl; Calbiochem, San Diego, CA) (10 mM in PBS), by oropharyngeal administration, 30 minutes before OVA challenge (designated Alum/OVA and OVA/OVA GSNO). The concentration of GSNO was chosen based on similar doses proposed in treatment of patients with cystic fibrosis (14). Control mice received 50 μl PBS (designated Alum/OVA and OVA/OVA PBS). Mice were killed by a lethal dose of pentobarbital via intraperitoneal injection on Day 25. All animal procedures were reviewed and approved by the institutional animal care and use committee.
Lung Lavage/Tissue Collection and Analysis of Airway Inflammation and Injury
BAL fluid was collected immediately upon death by three consecutive installations of 500 μl sterile PBS. Combined BAL fluids were centrifuged (420 × g, 15 min), cell pellets were resuspended in 1 ml of PBS for total and differential cell counts, and supernatants were stored at −80°C until further analysis. Protein concentrations in lung lavage fluids were quantified by a bicinchoninic acid (BCA) method (Pierce) as an indicator of airway epithelial permeability. Total NO production was determined by measuring accumulation of the metabolite nitrite in the BAL fluids using the Griess reagent (6). Lung tissues were removed subsequent to lavage and prepared for lung histology or biochemical analysis. Right lobes were fixed in 4% paraformaldehyde for 24 hours and transferred to 70% ethanol before embedding in paraffin. Sections (5 μm) were prepared on glass microscope slides and deparaffinized with xylene, followed by rehydration with a series of ethanols. They were then stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) for analysis by light microscopy using an Olympus BX50 light microscope (Center Valley, PA) with a QImaging Retiga 2000R digital camera (Surrey, British Columbia, Canada) using 40× and 60× objectives, respectively. Postimage capture, brightness, and contrast were adjusted to enhance visualization of the staining. Left lung lobes were snap frozen in liquid N2 and stored at −80°C until subsequent analysis, or placed in RNAlater (Ambion, Austin, TX) for 24 hours before storage at −20°C and subsequent RNA extraction.
Analysis of Lung Histology and Mucus Metaplasia
Analysis of H&E– and PAS-stained sections was performed as previously described (18, 19). Stained tissue sections were evaluated by one single-blinded and two double-blinded reviewers using a numerical rating system (18). Tissue inflammation was scored using a scale of 1 to 5 (1, absence of infiltrates surrounding airways or vasculature; 2, small infiltrates surrounding low number of airways or vasculature; 3, large infiltrates surrounding only some of the airways or vasculature; 4, large infiltrates around most airways or vasculature; and 5, severe structural damage to airways or vasculature). Mucus metaplasia was evaluated by PAS staining, scored on a scale of 1 to 4 (0, no staining; 1, less than 50% stained; 2, 50% stained; 3, more than 50% stained; 4, completely stained). A total of 10 airways were evaluated per mouse, and the average scores were calculated.
Serum Collection and Ig Analysis
Blood was collected from the inferior vena cava using a 26-gauge needle attached to a 1-ml syringe, centrifuged, and serum kept frozen at −80°C. For determination of OVA-specific serum Ig by ELISA, plates were coated with 2 μg/ml monoclonal anti-mouse IgE antibody (clone R35-72) or IgG1 (clone A85-1; BD Pharmingen, San Jose, CA) in PBS overnight at 4°C. Plates were washed and serum samples incubated in duplicate at dilutions of 1:2–1:250 in 100 μl/well PBS/1% BSA for 1 hour at room temperature. Plates were washed and incubated with a 1:2,500 dilution of digoxigenin-coupled OVA (Roche, Madison, WI) in PBS/1% BSA for 1 hour at room temperature. Plates were then washed and incubated with a 1:2,000 dilution of anti-digoxigenin Fab fragments coupled to peroxidase (Roche) in PBS/1% BSA for 30 minutes. Upon washing, plates were developed using reagents from R&D Systems (Minneapolis, MN), and stopped with 100 μl/well of 1 N H2SO4. OD was read using a Biotek Synergy HT plate reader (Biotek, Winooski, VT) at 450 nm, with background subtraction at 570 nm.
Analysis of NF-κB and HIF-1 Activation
Nuclear proteins were extracted from ground frozen lung tissues using a nuclear extraction kit (Chemicon International, Billerica, MA), and protein concentrations were quantified using the BCA method. NF-κB p65 and HIF-1 DNA binding were quantified with 10 and 5 μg of nuclear protein, respectively, using Active Motif TransAM assays (Active Motif, Carlsbad, CA), according to the manufacturer's instructions. The data are expressed as absorbance at 450 nm corrected for blank wells. Nuclear extracts containing equivalent amounts of protein (10–50 μg, determined using the BCA method; Pierce) were also analyzed by SDS-PAGE and Western blot for NF-κB p65 (1:1,000, Clone C-20; Santa Cruz, CA), and stabilization of HIF-1α was verified in whole-lung tissue protein lysates by Western blot (1:20,000, NB100-479; Novus Biologicals, Littleton, CO). After SDS-PAGE, proteins were transferred to nitrocellulose membranes and blotted overnight (4°C) with primary antibodies, and detected with horseradish peroxidase–conjugated secondary antibodies (Cell Signaling, Danvers, MA) and enhanced chemiluminescence (Pierce).
PCR Array of Transcription Factor Activation
A Mouse Signal Transduction PathwayFinder RT2 Profiler PCR Array (SuperArray Bioscience Corp., Frederick, MD), comprised of 84 transcripts representing 18 distinct signaling pathways, was used to identify general signaling pathways that might be affected by GSNO and/or OVA challenge. RNA was extracted, and the integrity number was measured for each sample using an Agilent 2,100 (Agilent Technologies, Santa Clara, CA). All RNA used for analysis had an integrity number of 8.8 or greater. RNA (1 μg) pooled in equal amounts from five replicates was converted to first-strand complementary DNA (cDNA) using the RT2 first strand kit (SuperArray Bioscience Corp.), and the cDNA was amplified according to the manufacturer's instructions using SuperArray RT2 qPCR Master Mix (SuperArray Bioscience Corp.) on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA).
Quantitative RT-PCR Analysis
Total mRNA was extracted from whole-lung tissue using TRIzol (Invitrogen, Carlsbad, CA) and an RNeasy mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was prepared from 1 μg of total RNA with M-MLV reverse transcriptase and Oligo(dT)12–16 primer (Invitrogen). A 1-μl aliquot of a 1:10 dilution of the cDNA was used for amplification by SYBR Green methods (Applied Biosystems). Transcripts were amplified using the primers listed in Table E1 in the online supplement, with GAPDH (FAM channel) assay on demand (Applied Biosystems) as a housekeeping gene. The amplification reaction was run for 35 cycles, and relative quantity was calculated using ΔΔCt analysis, as described previously (20).
Analysis of S-Nitrosylation of NF-κB and HIF-1
Detection of protein S-nitrosylation was achieved by performing the biotin switch technique as previously described (21, 22), with minor modifications. All steps were conducted with minimal exposure to light. A minimum of 750 mg of mouse lung tissue was homogenized on ice in 1 ml ice-cold 250 mM Hepes (pH 7.7), 1 mM EDTA, and 0.1 mM neocuproine (HEN buffer), and centrifuged at 1,500 rpm for 10 minutes at 4°C. The supernatant was adjusted to 2.5% SDS, blocked by the addition of 3 mM S-methyl methanethiosulfonate (Pierce), and incubated at 50°C for 30 minutes with frequent vortexing. Proteins were precipitated with 2 vol of acetone at −20°C for 30 minutes and recovered by centrifugation at 14,000 rpm at 4°C for 10 minutes, followed by rinsing of the pellet with 4 × 1 ml of 70% acetone/H2O. The pellet was resuspended in 500 μl HEN/10 (HEN diluted 10-fold in H2O) containing 1% SDS, and labeled by the addition of 400 μM biotin-HPDP (Pierce) and 5 mM sodium ascorbate. Negative control samples were prepared by the omission of sodium ascorbate. Samples were incubated in the dark for 1 hour at room temperature, and proteins were precipitated and washed as described previously here. The air-dried pellet was resuspended in 100 μl HEN/10 plus 1% SDS, and protein concentrations were quantified using the BCA method.
For analysis of changes in global protein S-nitrosylation, equal amounts of derivatized proteins were loaded onto SDS-PAGE gels and immunoblotted with streptavidin–horseradish peroxidase (1:5,000; Sigma). To determine changes in S-nitrosylation in specific proteins, derivatized proteins (250 μg) were incubated overnight (4°C) in 750 μl neutralization buffer (25 mM Hepes, 100 mM NaCl, 1 mM ETDA, 1% Triton X-100 [pH 7.5]) with 40 μl NeutrAvidin agarose beads (Pierce). The beads were washed with 4 × 1 ml wash buffer (neutralization buffer plus 500 mM NaCl), followed by 2 × 1 ml neutralization buffer, and eluted by boiling at 95°C in Laemmli buffer for 10 minutes. Recovered biotinylated proteins, negative controls, and input protein lysates were then separated by SDS-PAGE, followed by immunoblotting for NF-κB p65 (1:1,000, Clone C-20; Santa Cruz), inhibitory κB kinase (IKKβ) (1:500, Clone 2C8; Cell Signaling), or HIF-1α (1:20,000, NB100-479; Novus Biologicals). Ratios of S-nitrosylated p65 (SNO-p65) to total p65 were determined by band densitometry analysis using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
All quantitative data are presented as means (±SEM). Statistical significance was evaluated using GraphPad Prism (GraphPad Software, La Jolla, CA), and results were considered significant with a P value less than 0.05 using one-way ANOVA, followed by Tukey's post test for multiple comparisons.
RESULTS
Oropharyngeal Instillation of GSNO Increases Lung Tissue Protein S-Nitrosylation
To assess if supplementation of GSNO was efficacious in increasing levels of S-nitrosylated proteins, including proteins involved in NF-κB signaling, C57Bl6/J mice were administered GSNO (10 mM, 50 μl) via oropharyngeal instillation, and lung tissues were collected at 15 minutes, 1 hour, and 4 hours for analysis of protein S-nitrosothiols using the biotin switch technique. As shown in Figure 1A, administration of GSNO resulted in marked increases in global protein S-nitrosylation, determined by biotin switch labeling, as early as 15 minutes after GSNO instillation, and persisting for 4 hours. Similarly, analysis of NF-κB p65 revealed significantly elevated S-nitrosylation of p65 in lungs of animals supplemented with GSNO (Figure 1B). Similar analysis of IKKβ, a putative target for S-nitrosylation (10), did not show detectable S-nitrosylated IKKβ in lungs from GSNO-treated mice (data not shown). Analysis of GSNO in BAL fluids by HPLC (23) indicated that instilled GSNO was degraded rapidly, and was below the detection limit (<0.5 μM) within 30 minutes after instillation. Overall, these findings indicate that instillation of GSNO readily enhances lung tissue protein SNO levels, including NF-κB p65.
Figure 1.
Instillation of S-nitrosoglutathione (GSNO) enhances protein S-nitrosylation in the mouse airway. Naive C57BL/6J mice were administered oropharingeal GSNO (50 mM; 50 μl), and lung tissues were collected after various time periods (15 min, 1 h, and 4 h) for analysis of protein S-nitrosylation. Whole lung tissue was analyzed by the biotin switch assay for changes in global protein S-nitrosylation (A) and S-nitrosylation of NF-κB p65 (SNO-p65) (B). Ctl, control.
GSNO Administration Reduces NF-κB Activation during Allergic Airway Inflammation
To assess the major signaling pathways that are modulated in allergen-sensitized and -challenged animals, we performed a Signal Tranduction PathwayFinder PCR Array (SuperArray) on lung tissues from OVA-challenged, -sensitized (OVA/OVA) and unsensitized (Alum/OVA) mice. A total of 19 genes were found to be up-regulated (≥1.5-fold) in OVA/OVA mice compared with Alum/OVA mice, 7 of which were transcriptionally activated by NF-κB, including Ccl20 (up 14.2-fold) and Cxcl1 (up 7.2-fold). In addition, other up-regulated genes are associated with Jak-STAT (three) and LDL pathways (three genes, including Ccl2 [up 16.8-fold]), whereas down-regulated genes included those indicative of the p53 (four genes) and hedgehog (four genes) pathways (Table E2). The prominent role of NF-κB activation in the lungs of OVA/OVA mice compared with Alum/OVA mice was confirmed by DNA binding analysis of lung nuclear extracts for the NF-κB p65 subunit, and by Western blot analysis (Figures 2A and 2B), and confirms previous reports (8, 24, 25).
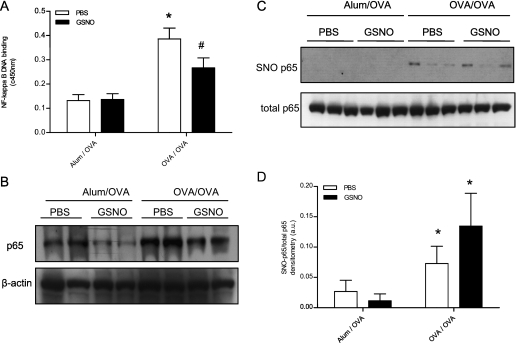
Figure 2.
Inhibition of NF-κB activity in ovalbumin (OVA)-sensitized and -challenged mice by administration of GSNO. Naive or sensitized C57BL/6J mice were challenged with OVA on 3 consecutive days, and, where indicated, mice were also administered oropharingeal GSNO (10 mM, 50 μl; 30 minutes before OVA challenge). Lung tissues were collected 48 hours after the last OVA challenge, and nuclear extracts were prepared for analysis of NF-κB p65 DNA binding assay (A) and Western blot (B). *P < 0.01 compared with aluminum hydroxide (Alum)/OVA PBS group; #P < 0.05 compared with the OVA/OVA PBS group using ANOVA (n = 8). (C) S-Nitrosylated NF-κB p65 (SNO-p65) was assessed by biotin switch assay in whole lung tissue 48 hours after the latest OVA challenge. (D) Densitometric analysis of S-nitrosylated (SNO)-p65 to total p65 ratio. *P < 0.05 compared with the corresponding Alum/OVA control group using ANOVA (n = 6).
We next investigated whether administration of GSNO into the airways was capable of diminishing OVA-induced NF-κB signaling and allergic inflammation. Administration of GSNO immediately before OVA challenge did not affect basal lung NF-κB DNA binding in nonsensitized control animals (Alum/OVA GSNO), but significantly reduced NF-κB DNA binding in OVA-challenged and -sensitized mice (OVA/OVA GSNO) compared with PBS-treated control animals (Figure 2A). Similarly, analysis of NF-κB p65 by Western blot revealed decreased levels of this protein in lung nuclear extracts generated from OVA/OVA GSNO animals compared with OVA/OVA PBS animals (Figure 2B). Inhibition of NF-κB activation by GSNO was also confirmed by SuperArray analysis (Table E3). Of the 84 genes analyzed, 28 (3 up, 25 down) were found to be differentially regulated (≥1.5-fold) between the two groups. Of the 25 genes with decreased expression, which included matrix metalloproteinase–7 (down 6.7-fold) and leptin (down 2.4-fold), a large number (eight) are associated with NF-κB signaling (Table E3).
To determine whether the observed inhibition of NF-κB by GSNO resulted from S-nitrosylation of proteins within the NF-κB family, a biotin switch assay was performed on mouse lung tissue. Levels of lung p65 SNO were found to be significantly elevated in allergen-sensitized and -challenged animals compared with control animals (Figures 2C and 2D). Although administration of GSNO did not affect these levels in unsensitized control animals, moderate increases in p65 SNO were detected in OVA/OVA GSNO mice compared with OVA/OVA PBS mice (Figures 2C and 2D; P = 0.15), suggesting the direct S-nitrosylation of p65 by GSNO. Collectively, these results are consistent with earlier findings (9, 10, 26), and show that in vivo instillation of GSNO is capable of suppressing NF-κB activation by S-nitrosylation.
GSNO Administration Does Not Suppress Markers of Allergic Airway Inflammation or Mucus Metaplasia
We next questioned whether inhibition of lung NF-κB by GSNO administration corresponded to reduced allergic airway inflammation. As expected, OVA challenge in sensitized animals caused marked allergic airway inflammation, as indicated by increased total BAL cell counts (Figure 3A), dominated primarily by increased eosinophils, compared with nonsensitized control animals (Figure 3B). However, administration of GSNO to OVA/OVA mice did not significantly alter total BAL cell counts or differentials compared with OVA/OVA mice that received PBS (OVA/OVA PBS) (Figure 3B). Similarly, administration of GSNO to nonsensitized mice did not significantly alter BAL cell counts compared with PBS control animals (Alum/OVA PBS), indicating that GSNO administration, on its own, did not cause considerable airway inflammation.
Figure 3.
Effects of GSNO administration on airway inflammation, bronchoalveolar lavage (BAL) protein content, and Ig levels. Naive or sensitized C57BL/6J mice were challenged with OVA and/or GSNO on 3 consecutive days, and bronchoalveolar lavage (BAL) fluid was collected 48 hours after the last aerosolized OVA challenge for the assessment of total (A) and differential (B) cell counts. (C) Sections generated from paraffin-embedded lungs were stained with hematoxylin and eosin (H&E) and evaluated by light microscopy to visualize inflammation by assessing the extent of infiltrates around airways or vasculature. (D) Protein concentrations were measured in the BAL and assessed as a marker of vascular permeability. OVA-specific IgE (E) and IgG1 (F) were analyzed from serum by ELISA. *P < 0.05 compared with the corresponding Alum/OVA control group using ANOVA (n = 8).
Analysis of lung histopathology by H&E staining revealed prominent cellular infiltration in OVA/OVA mice compared with Alum/OVA control animals, but this was not significantly affected by GNSO administration before OVA challenge (Figure 3C). As a marker of epithelial permeability and pulmonary edema, we measured protein levels in the BAL. As expected, protein levels were increased in OVA/OVA mice compared with Alum/OVA control animals, but this was not altered by treatment with GSNO (Figure 3D). Allergic airway inflammation in response to OVA sensitization and challenge was also evaluated by analysis of serum levels of OVA-specific IgE (Figure 3E) and IgG1 (Figure 3F). As shown, GSNO administration did not significantly affect levels of these Igs in either Alum/OVA or OVA/OVA mice.
Another major feature of allergic airway inflammation is the induction of mucus metaplasia (27), and our present studies confirm that OVA sensitization and challenge resulted in enhanced mucus metaplasia, as evaluated by PAS stain (Figure 4) and expression of IL-13, MUC5ac, and GOB5 (Table 1). GSNO administration did not affect mucus metaplasia in Alum/OVA mice, but tended to enhance PAS staining in OVA/OVA mice, although this did not reach statistical significance (Figure 4B; P = 0.18). GSNO administration also did not significantly alter OVA-induced induction of IL-13, MUC5ac, or GOB5 mRNA (Table 1). Another feature of allergic asthma is the induction of NOS2, which is regulated by NF-κB. As shown in Table 1, lung NOS2 mRNA levels were slightly increased in OVA/OVA mice (2.06 ± 0.43-fold) compared with control animals, with comparable results observed in the GSNO-treated group.
Figure 4.
Evaluation of mucus metaplasia by periodic acid-Schiff (PAS) staining. (A) Paraffin-embedded lungs were stained using PAS reagent to visualize mucus-producing cells (stained in pink) in the airways, and were scored (B) for the extent of reactivity, as described in Materials and Methods. *P < 0.05 compared with corresponding Alum/OVA control group using ANOVA (n = 6).
TABLE 1.
QUANTITATIVE REAL-TIME PCR ANALYSIS OF MARKERS OF MUCUS METAPLASIA AND NITRIC OXIDE SYNTHASE–2
| Gene (RQ) | Alum/OVA PBS | Alum/OVA GSNO | OVA/OVA PBS | OVA/OVA GSNO |
|---|---|---|---|---|
| IL-13 | 1.0 ± 0.42 | 1.89 ± 0.32 | 30.45 ± 5.30* | 24.0 ± 5.80* |
| MUC5ac | 1.0 ± 0.28 | 2.12 ± 1.03 | 48.98 ± 7.31* | 64.75 ± 17.00* |
| GOB5 | 1.0 ± 0.54 | 5.72 ± 4.64 | 767.0 ± 113.0* | 778.0 ± 68.0* |
| NOS2 | 1.0 ± 0.33 | 1.46 ± 0.15 | 2.06 ± 0.43 | 3.21 ± 0.96 |
Definition of abbreviations: Alum, aluminum hydroxide; GSNO, S-nitrosoglutathione; OVA, ovalbumin; RQ, relative quantity.
Quantitative real-time PCR was performed on cDNA synthesized from RNA isolated from whole mouse lung and evaluated for the expression levels of markers of allergic airway inflammation or mucus metaplasia.
P < 0.05 compared to the corresponding Alum/OVA control group using ANOVA (n = 8).
Taken together, these results indicate that GSNO administration, although capable of inhibiting NF-κB activation, did not result in significant changes in allergic airway inflammation or mucus metaplasia when administered immediately before aeroallergen challenge in a model of allergic airway disease. In fact, some markers of inflammation (Table E3) or mucus metaplasia (Figure 4) might be enhanced because of possible effects of GSNO on other signaling pathways.
Activation of HIF-1 during Allergic Airway Inflammation: Modulation by GSNO
Because inhibition of NF-κB by GSNO was not associated with significant changes in allergic airway inflammation in OVA/OVA mice, we considered that administration of GSNO might also affect other signaling pathways that are important in regulating inflammation. One pathway that has recently been implicated in allergic inflammation, and is subject to regulation by NO or S-nitrosothiols at various levels (17), involves the activation of the heterodimeric transcription factor, HIF-1. The precise role of HIF-1 activation in airway inflammation is unclear, and may be both proinflammatory and protective (28, 29). To determine HIF-1 activation during allergic airway inflammation, lung nuclear extracts were analyzed using an HIF-1 activity assay (Figure 5A), and HIF-1α stabilization was determined in whole-lung protein lysates by Western blot (Figure 5B). As shown, HIF-1 was markedly activated in OVA/OVA animals compared with Alum/OVA control animals, in agreement with recent reports (30, 31). The changes in overall lung HIF-1α protein levels and HIF-1 DNA binding activity in OVA/OVA mice were not associated with significant changes in HIF-1α mRNA, as determined by real-time PCR (Figure 5C), suggesting HIF-1 activation by a post-transcriptional mechanism. For comparison, analysis of HIF-2α mRNA actually revealed a twofold decrease in OVA/OVA mice compared with Alum/OVA control animals (Table 2). In spite of enhanced HIF-1 activity in the lungs of OVA/OVA mice, analysis of several selected HIF-1–related genes did not reveal significant increases in mRNA expression in lung tissues from OVA/OVA mice compared with Alum/OVA mice (Table 2). Curiously, mRNA levels of vascular endothelial growth factor (VEGF) or CD73 were actually found to be suppressed in OVA/OVA mice compared with Alum/OVA control animals (Table 2).
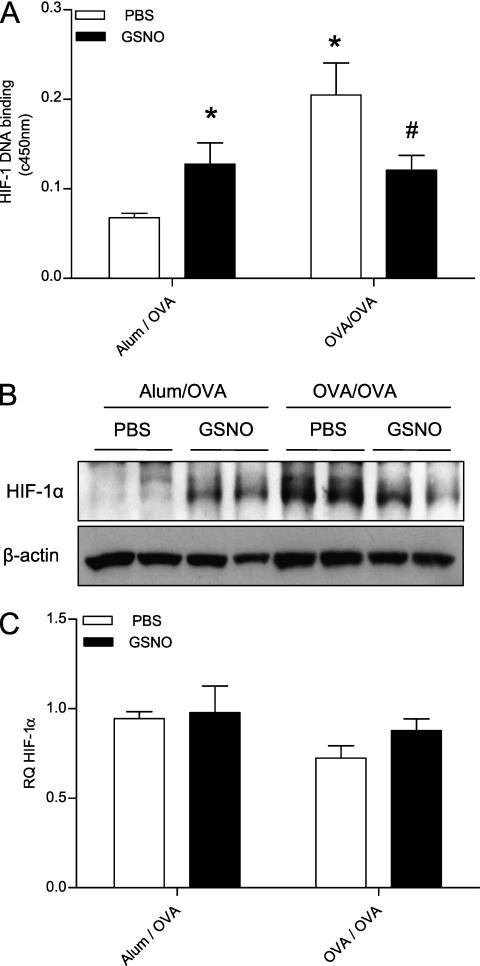
Figure 5.
The transcription factor, hypoxia-inducible factor (HIF)–1, is activated in the OVA model of allergic airway disease, and is modified by administration of GSNO. (A) Mice were treated as described in Materials and Methods, and HIF-1 DNA binding was evaluated in nuclear extracts. (B) HIF-1α protein levels were analyzed by Western analysis in whole lung protein lysates. (C) Expression levels of the HIF-1α transcript were determined by quantitative real-time PCR from cDNA generated from whole mouse lung RNA. *P < 0.05 compared with Alum/OVA PBS group; #P < 0.05 compared with OVA/OVA PBS group using ANOVA (n = 8).
TABLE 2.
QUANTITATIVE REAL-TIME PCR ANALYSIS OF HYPOXIA-INDUCIBLE GENES
| Gene (RQ) | Alum/OVA PBS | Alum/OVA GSNO | OVA/OVA PBS | OVA/OVA GSNO |
|---|---|---|---|---|
| PAI-1 | 1.0 ± 0.13 | 0.77 ± 0.16 | 0.66 ± 0.1 | 0.90 ± 0.13 |
| LOX | 1.0 ± 0.09 | 0.88 ± 0.14 | 1.17 ± 0.08 | 1.47 ± 0.23 |
| VEGF | 1.0 ± 0.04 | 0.65 ± 0.08* | 0.42 ± 0.02* | 0.35 ± 0.04* |
| CD-73 | 1.0 ± 0.07 | 1.27 ± 0.19 | 0.59 ± 0.09* | 0.69 ± 0.1* |
| HIF-2α | 1.0 ± 0.07 | 1.17 ± 0.22 | 0.51 ± 0.11* | 0.40 ± 0.08* |
Definition of abbreviations: Alum, aluminum hydroxide; GSNO, S-nitrosoglutathione; OVA, ovalbumin; RQ, relative quantity.
Quantitative real-time PCR was performed on cDNA synthesized from whole mouse lung RNA and evaluated for the expression levels of genes regulated by hypoxia-inducible factor–1.
P < 0.05 compared to Alum/OVA PBS group using ANOVA (n = 8).
Consistent with previous observations that S-nitrosothiols are capable of activating the HIF-1 pathway (17), administration of GSNO to Alum/OVA mice resulted in enhanced HIF-1 DNA binding activity (Figure 5A) and increased HIF-1α protein accumulation in lung tissues (Figure 5B). However, similar GSNO administration in OVA/OVA mice actually attenuated HIF-1 DNA binding activity and HIF-1α protein to levels comparable to those seen in Alum/OVA GSNO mice (Figure 5). Analysis of the effects of GSNO administration on lung mRNA levels of various HIF-regulated genes again did not reveal significant differences (Table 2). Collectively, these findings indicate that GSNO can enhance HIF-1 activation in naive mice, but can also suppress HIF-1 activation by hypoxia or other mechanisms during allergic inflammation.
Role of NOS2 in Activation of NF-κB, HIF-1, and Allergic Airway Inflammation
Although NOS2 induction is a common feature of allergic airway inflammation, its precise contribution to the overall pathophysiology is still controversial (3, 7, 32). Although NOS2 has been positively linked to allergic airway inflammation in some studies (3, 33), NOS2 induction can also control inflammatory signaling by feedback inhibition of NF-κB signaling through S-nitrosylation (9, 26). To test this possibility, we performed additional experiments using NOS2-deficient mice in comparison to wild-type (WT) C57BL/6J control animals. Although OVA sensitization and challenge resulted in an approximately twofold increase in NOS2 mRNA in WT mice (Table 1), no NOS2 mRNA was detected in lung tissues from NOS2−/− mice (Figure E1). Levels of BAL nitrite, a measure of lung NO production, were increased in OVA/OVA mice compared with Alum/OVA control animals in both WT and NOS2−/− mice (Figure E1), suggesting additional sources for enhanced NO production in response to OVA challenge. To determine whether NOS2 deficiency impacted NF-κB activation in response to OVA challenge, we evaluated NF-κB activity in lung nuclear extracts. Indeed, as shown in Figure 6A, NF-κB p65 DNA binding was significantly increased in OVA/OVA groups in both strains of mice compared with Alum/OVA control animals. However, lung NF-κB DNA binding was slightly higher in NOS2−/− OVA/OVA mice compared with WT OVA/OVA mice (P = 0.073), consistent with a potential role of NOS2 in feedback inhibition of NF-κB activation. Accordingly, nuclear levels of p65 protein, as assessed by Western blot analysis, were also slightly increased in lungs from OVA/OVA NOS2−/− compared with WT mice (Figure 6B). Analysis of S-nitrosylated NF-κB p65 in lung tissues again revealed elevated levels of p65 SNO in OVA/OVA mice, but this was not significantly reduced in NOS2−/− mice (Figures 6C and 6D). To determine a potential role for NOS2-derived NO in HIF-1 activation during allergic inflammation, we compared OVA-induced HIF-1 activity (Figure 6E) and HIF-1α protein levels (Figure 6F) in lung tissues from WT and NOS2−/− mice. As shown, HIF-1α protein accumulation and HIF-1 activation in OVA/OVA mice was similar in both strains, indicating that HIF-1 activation during allergic inflammation occurred by a mechanism independent of NOS2.
Figure 6.
Effect of nitric oxide synthase (NOS)–2 deficiency on NF-κB and HIF-1 activity in response to OVA challenge. C57BL/6J and NOS2−/− mice were sensitized and challenged with OVA, as described in the Materials and Methods. Whole-lung nuclear extracts were analyzed by NF-κB p65 DNA binding assay (A) and Western blot (B). *P < 0.05 compared with the corresponding Alum/OVA control group using ANOVA (n = 5). (C) SNO-p65 levels were analyzed in whole lung tissue 48 hours after the latest OVA challenge by the biotin switch technique, and the SNO-p65 to total p65 ratio was determined by densitometric analysis (D). *P < 0.05 compared with the corresponding Alum/OVA control group using ANOVA (n = 5). (E) HIF-1 DNA binding activity was evaluated in nuclear extracts from whole lung and (F) HIF-1α protein levels in whole lung lysates were analyzed by Western analysis. *P < 0.05 compared with corresponding Alum/OVA control group using ANOVA (n = 5).
We next investigated the consequences of NOS2 deficiency on markers of allergic inflammation and mucus metaplasia, and, as shown in Figure 7A, increases in total BAL cell counts in OVA/OVA groups were similar in both WT and NOS2−/− mice, although modest differences in cell differentials were seen (Figure 7B), with significantly higher neutrophil counts in NOS2−/− mice compared with WT mice (Figure 7C). Analysis of other markers of allergic airway inflammation (H&E, BAL protein, serum levels of OVA-specific IgE and IgG) did not reveal significant differences between the two strains (Figure E2). Induction of IL-13 or mucus metaplasia markers, MUC5AC and GOB5, in OVA/OVA mice were comparable between NOS2−/− and WT animals (Table 3), although both the basal (Alum/OVA) and OVA-induced (OVA/OVA) expression of these genes appeared to be slightly, but not significantly, higher in NOS2−/− mice compared with WT mice. Similarly, evaluation of PAS-stained tissue sections from OVA/OVA NOS2−/− mice also showed trends toward slightly enhanced mucus metaplasia compared with OVA/OVA B6 mice (Figures 7D and 7E; P = 0.10).
Figure 7.
Effect of NOS2-deficiency on OVA-induced allergic airway inflammation and markers of mucus metaplasia. C57BL/6J and NOS2−/− mice were sensitized and challenged with OVA, as described in Materials and Methods. Total (A), differential (B), and neutrophil (C) cell counts were assessed from the BAL. *P < 0.05, compared with corresponding Alum/OVA control group using ANOVA (n = 5); #P < 0.05 compared with the wild-type (C57BL/6J) OVA/OVA group using ANOVA (n = 5). Reactivity of PAS was evaluated (D) and scored (E) in paraffin-embedded lung sections to visualize mucus-producing cells in the airways of OVA-sensitized (or control) and -challenged, wild-type and NOS2−/− mice. *P < 0.05 compared with corresponding Alum/OVA control group using ANOVA (n = 5).
TABLE 3.
QUANTITATIVE REAL-TIME PCR ANALYSIS OF IL-13 AND MUCUS METAPLASIA MARKERS, MUC5AC AND GOB5
| Gene (RQ) | B6 Alum/OVA | NOS2−/− Alum/OVA | B6 OVA/OVA | NOS2−/− OVA/OVA |
|---|---|---|---|---|
| IL-13 | 1.0 ± 0.42 | 2.49 ± 0.67 | 10.0 ± 1.37* | 18.0 ± 13.9* |
| MUC5ac | 1.0 ± 0.23 | 2.91 ± 0.15 | 81.13 ± 47.64* | 110.03 ± 23.60* |
| GOB5 | 1.0 ± 0.54 | 0.91 ± 0.26 | 878.78 ± 342.68* | 1699.0 ± 433.74* |
Definition of abbreviations: Alum, aluminum hydroxide; NOS2, nitric oxide synthase–2; OVA, ovalbumin; RQ, relative quantity.
Quantitative real-time PCR was performed on cDNA synthesized from whole mouse lung RNA and evaluated for the expression levels of IL-13 or the mucus metaplasia markers, MUC5ac and GOB5.
P < 0.05 compared to corresponding Alum/OVA group using ANOVA (n = 5).
Collectively, these results indicate that NOS2 might play a role in regulating NF-κB during OVA-induced allergic airway inflammation, and could thereby control some features of inflammation, such as neutrophil levels and mucus metaplasia. However, NOS2 deficiency did not significantly alter the major features of the allergic inflammation (IgG production, eosinophilia, or overall markers of airway inflammation and injury).
DISCUSSION
Several studies in animal models have implicated NF-κB as an important contributor to various phenotypes of allergic asthma, including airways eosinophilia and mucus hyperplasia (34–36). Previous studies demonstrated that the inflammatory mediator, NO, can suppress activation of this transcription factor through S-nitrosylation of redox-sensitive components of this signaling pathway (9, 10, 26, 37), and that airway NO metabolism may be disrupted during allergic asthma, resulting in reduced SNO levels (11, 12, 38). Therefore, we proposed that supplementation of airway SNO might be capable of suppressing NF-κB activation during allergic airway inflammation and reducing eosinophilic inflammation or mucus production. Our present study indeed demonstrates that airway administration of GSNO in sensitized mice is capable of suppressing NF-κB activation in association with enhanced S-nitrosylation of NF-κB p65. Multiple proteins within the NF-κB pathway have been identified as targets for S-nitrosylation, including NF-κB p50 and p65 (26) and IKK (10). Our analyses revealed that GSNO administration resulted in increased levels of p65 SNO, although we did not observe detectable S-nitrosylation of IKKβ. Our findings are consistent with those of Marshall and coworkers (15), who recently reported that inhalation of ethyl nitrite enhances airway SNO levels and minimizes acute airway inflammation and injury in response to aerosolized LPS, in association with increased S-nitrosylation of NF-κB p65 in mouse lung tissue.
In spite of the ability of GSNO to suppress NF-κB DNA binding, analysis of various markers of allergic airway inflammation and mucus hyperplasia did not reveal significant effects of GSNO administration in our present studies. Increased GSNO metabolism in aeroallergen-challenged animals has been attributed to increased activity of GSNOR (39, 40), and genetic deletion of this enzyme was found to restore concentrations of airway GSNO and SNO protein levels, and protected from airway hyperresponsivity, but had negligible effects on acute lung inflammation (40). The bronchodilatory activity of GSNO has also been implicated in human asthma, with increased GSNOR activity being inversely correlated with parameters associated with hyperresponsiveness in patients with asthma (12). Although airway GSNO may have prominent roles as a bronchodilator, which was not specifically addressed in the present study, these findings and our present results suggest that it has more limited capacity to suppress allergic airway inflammation.
The lack of effect of GSNO on allergic airway inflammation and mucus metaplasia, in spite of inhibition of NF-κB signaling, may also be due to the fact that GSNO can affect other redox-sensitive signaling pathways in addition to NF-κB, as is illustrated by our PCR array data. Variable effects on these different pathways may have differential and, as yet, undetermined roles in this complex model. Potential inhibitory effects of GSNO administration on NF-κB may, therefore, be obscured by additional alterations in other biological pathways, which could either suppress or promote allergic inflammation. For example, the inhibitory effects of GSNO on matrix metalloproteinase−7, an epithelial protease involved in regulating epithelial repair and transepithelial leukocyte trafficking (41), might promote epithelial injury and inflammation.
Activation of HIF-1, by localized tissue hypoxia, or by various inflammatory mediators, including NO, is emerging as an important feature of inflammation and innate immunity, and may also contribute to allergic airway inflammation (30, 31, 42). Indeed, our studies confirm that overall lung HIF-1 activity is increased in response to allergen challenge of OVA-sensitized mice, and this is significantly affected by instillation of GSNO. Very little is known regarding the general involvement of HIF-1 in lung inflammation or injury, and the role of HIF-1 in the (patho)physiology of allergic airway disease is unclear. Increases in immunoreactivity of HIF-1α and HIF-2α, as well as VEGF in biopsy specimens of patients with asthma, and positive correlation between HIF-1 and VEGF and airways eosinophilia within these subjects, suggest a contribution of HIF-1 to allergic inflammation (30). Studies with partially deficient HIF-1α (+/−) mice have suggested a contribution of HIF-1 in OVA-induced airways eosinophilia (42). However, the overall involvement of HIF-1 in allergic inflammation should be addressed in more detailed studies on the kinetics and cellular localization of HIF-1 activation, which is beyond the scope of the present study. For example, HIF-1 activation in phagocytes promotes their survival, phagocytic function, and production of proinflammatory mediators (43, 44), whereas activation of HIF-1 in epithelia has been associated with regulation of epithelial integrity and permeability (29, 45), as well as remodeling (28). In addition, HIF-1 activation may also contribute to mucin gene expression (46). Based on these various considerations, the ability of GSNO to inhibit HIF-1 activation during allergic inflammation might suggest an anti-inflammatory role, similar to that of other anti-inflammatory compounds that were shown to suppress both NF-κB and HIF-1 (30, 47). However, direct activation of HIF-1 by GSNO (as seen in naive mice) might also promote inflammation, remodeling, and/or mucus metaplasia.
The opposing effects of GSNO on HIF-1 DNA binding under these different conditions are not necessarily conflicting, because effects of NO or S-nitrosothiols on HIF-1 activation are known to be highly context dependent (17). During normoxia, GSNO has been reported to promote HIF-1α stabilization, due to S-nitrosylation of Cys533 within the oxygen-dependent degradation domain, to prevent HIF-1α degradation (48) or S-nitrosylation of Cys800, which supports interaction with the coactivator p300 protein (49), or by increased HIF-1α accumulation and activity by Akt signaling (50). In contrast, NO and S-nitrosothiols, such as GSNO, are also capable of inhibiting HIF-1 activity during hypoxia by inhibition of HIF-1 DNA binding and/or by restoration of prolyl hydroxylase enzyme activity (51, 52). Another potential mechanism by which GSNO might have suppressed HIF-1 activity in OVA/OVA mice may be through inhibition of NF-κB, which positively regulates HIF-1α mRNA expression in response to various stimuli (53, 54). Although we did not observe significant differences in HIF-1α mRNA levels between the various groups at the time of our analysis, we cannot rule out a contribution of transient NF-κB–mediated HIF-1α mRNA expression on HIF-1 activation during allergic airway inflammation.
Our analysis of S-nitrosylation of NF-κB p65 indicated significant increases in p65 SNO in allergen-challenged animals compared with control animals, which appears to contrast the notion that SNO levels may be suppressed during airway inflammation (15, 40). This finding also suggests that endogenous NO production (e.g., by induction of NOS2) may mediate NF-κB S-nitrosylation as a feedback mechanism. Analyses in OVA-challenged NOS2−/− mice indeed indicated slightly enhanced NF-κB activity, and slightly suppressed p65 SNO levels (Figure 6), but substantial p65 SNO was still observed in NOS2−/− mice. This suggests the contribution of other sources of NO or S-nitrosothiols, rather than NOS2, which was also illustrated by the relative lack of effect of NOS2 deficiency in OVA-induced increase in BAL nitrite levels (Figure E1). In addition, airway acidification, a known feature of asthma (55), could conceivable promote airway protein S-nitrosylation, independent of NOS activity.
Consistent with some earlier studies (4, 7), other markers of allergic airway inflammation were not significantly altered in NOS2−/− mice compared with WT mice. Moreover, several studies report a contributing role of NOS2 in allergic inflammation (3, 33), which would appear to be unrelated to its inhibitory effects on NF-κB. Different observations, with respect to the role of NOS2 in allergic airway inflammation, are likely related to differences in the animal strain or model used, as well as variations in efficacy and specificity of NOS2 inhibitors (56). In contrast to markers of allergic inflammation and eosinophilia, airway neutrophils and some markers of mucus hyperplasia appear to be enhanced in NOS2−/− mice compared with WT mice, suggesting a protective role of NOS2 in these latter responses.
Because NO is known to promote HIF-1α stabilization and activation under normoxic conditions (17, 57), we postulated that NOS2 induction might contribute to the observed activation of HIF-1 during allergic inflammation. However, HIF-1 activation was similar in both NOS2−/− and WT allergic animals; hence, HIF-1 activation is most likely due to other factors, such as localized tissue hypoxia that accompanies active inflammation (58), or transcriptional or post-transcriptional induction of HIF-1α by proinflammatory cytokines, such as TNF-α and IL-1β (54, 59, 60). Based on a lack of observed increase in HIF-1α or HIF-2α mRNA levels during OVA-induced inflammation, we suspect that localized hypoxia may be the primary mechanism of HIF-1α stabilization and activation during allergic inflammation. Indeed, several features of allergic airway disease, including inflammation, airway obstruction, or alveolar damage, could all contribute to compromised oxygenation allowing for stabilization of HIF-1α. Nevertheless, we cannot rule out potential transient mechanisms of HIF-1 mRNA induction at early stages of allergic inflammation, which may have subsided at the time of tissue collection for our analyses.
In summary, our studies demonstrate that in vivo instillation of GSNO can modulate activation of the transcription factors, NF-κB and HIF-1, during allergic airway inflammation, but this is not necessarily accompanied by significant changes in several features of allergic airway inflammation. NF-κB activation is important in the development of allergic airway inflammation, but its role appears to be mouse strain–dependent, and epithelial NF-κB was recently found to be less critical in C57BL/6 mice, which were used in the present study (34). In addition, potential effects of GSNO on NF-κB may be obscured by additional effects on other important signaling pathways, such as HIF-1, that are also crucial in mediating functional outcomes, such as inflammation, mucus metaplasia, and remodeling. Hence, our studies indicate that supplementation of airway S-nitrosothiols may not necessarily be useful in mitigating allergic airway inflammation or mucus metaplasia. However, it may promote bronchodilation and minimize airways hyperresponsiveness, and its therapeutic potential may be limited due to effects on additional signaling pathways, such as HIF-1, with as yet unknown functional consequences.
Supplementary Material
Acknowledgments
The authors thank Jennifer L. Ather and Scott W. Aesif for their assistance with scoring hematoxylin and eosin– and periodic acid-Schiff–stained tissue sections, and the University of Vermont DNA core facility for performing the SuperArray analysis.
This work was supported by National Institutes of Health grants HL074295, HL068865, HL085646, and T32ES007122.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0035OC on August 27, 2010
Author Disclosure: Y.J.-H. received four sponsored grants from the National Institutes of Health (NIH), one for $10,001–$50,000 and three for more than $100,001 each; A.v.d.V. has a patent pending for the detection of nitrosylated proteins, and has received sponsored grants from Flight Attendant Medical Research Institute and NIH for more than $100,001 each; R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; D.I.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; N.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Bove PF, van der Vliet A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Rad Biol Med 2006;41:515–527. [DOI] [PubMed] [Google Scholar]
- 2.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 1994;149:538–551. [DOI] [PubMed] [Google Scholar]
- 3.Xiong Y, Karupiah G, Hogan SP, Foster PS, Ramsay AJ. Inhibition of allergic airway inflammation in mice lacking nitric oxide synthase. J Immunol 1999;162:445–452. [PubMed] [Google Scholar]
- 4.Feder LS, Stelts D, Chapman RW, Manfra D, Crawley Y, Jones H, Minnicozzi M, Fernandez X, Paster T, Egan RW, et al. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am J Respir Cell Mol Biol 1997;17:436–442. [DOI] [PubMed] [Google Scholar]
- 5.Raychaudhuri B, Dweik R, Connors MJ, Buhrow L, Malur A, Drazba J, Arroliga AC, Erzurum SC, Kavuru MS, Thomassen MJ. Nitric oxide blocks nuclear factor-B activation in alveolar macrophages. Am J Respir Cell Mol Biol 1999;21:311–316. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet A. Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L198–L209. [DOI] [PubMed] [Google Scholar]
- 7.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, et al. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med 1999;189:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-κB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall HE, Stamler JS. Inhibition of NF-κB by S-nitrosylation. Biochemistry 2001;40:1688–1693. [DOI] [PubMed] [Google Scholar]
- 10.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory κB kinase through S-nitrosylation. Proc Natl Acad Sci USA 2004;101:8841–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston B, Sears S, Woods J, Hunt J, Ponaman M, McMahon T, Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet 1998;351:1317–1319. [DOI] [PubMed] [Google Scholar]
- 12.Que LG, Yang Z, Stamler JS, Lugogo NL, Kraft M. S-nitrosoglutathione reductase: an important regulator in human asthma. Am J Respir Crit Care Med 2009;180:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson G, Benjamin N. Potential therapeutic uses for S-nitrosothiols. Clin Sci (Lond) 2002;102:99–105. [PubMed] [Google Scholar]
- 14.Snyder AH, McPherson ME, Hunt JF, Johnson M, Stamler JS, Gaston B. Acute effects of aerosolized S-nitrosoglutathione in cystic fibrosis. Am J Respir Crit Care Med 2002;165:922–926. [DOI] [PubMed] [Google Scholar]
- 15.Marshall HE, Potts EN, Kelleher ZT, Stamler JS, Foster WM, Auten RL. Protection from lipopolysaccharide-induced lung injury by augmentation of airway S-nitrosothiols. Am J Respir Crit Care Med 2009;180:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 16.Lee SY, Kwon S, Kim KH, Moon HS, Song JS, Park SH, Kim YK. Expression of vascular endothelial growth factor and hypoxia-inducible factor in the airway of asthmatic patients. Ann Allergy Asthma Immunol 2006;97:794–799. [DOI] [PubMed] [Google Scholar]
- 17.Brüne B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res 2007;75:275–282. [DOI] [PubMed] [Google Scholar]
- 18.Ckless K, Lampert A, Reiss J, Kasahara D, Poynter ME, Irvin CG, Lundblad LK, Norton R, van der Vliet A, Janssen-Heininger YM. Inhibition of arginase activity enhances inflammation in mice with allergic airway disease, in association with increases in protein S-nitrosylation and tyrosine nitration. J Immunol 2008;181:4255–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poynter ME, Irvin CG, Janssen-Heininger YM. A prominent role for airway epithelial NF-κB activation in lipopolysaccharide-induced airway inflammation. J Immunol 2003;170:6257–6265. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara DI, Poynter ME, Othman Z, Hemenway D, van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol 2008;181:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 2001;3:193–197. [DOI] [PubMed] [Google Scholar]
- 22.Forrester MT, Foster MW, Stamler JS. Assessment and application of the biotin switch technique for examining protein S-nitrosylation under conditions of pharmacologically induced oxidative stress. J Biol Chem 2007;282:13977–13983. [DOI] [PubMed] [Google Scholar]
- 23.Tsikas D, Denker K, Frolich JC. Artifactual-free analysis of S-nitrosoglutathione and S-nitroglutathione by neutral-pH, anion-pairing, high-performance liquid chromatography: study on peroxynitrite-mediated S-nitration of glutathione to S-nitroglutathione under physiological conditions. J Chromatogr A 2001;915:107–116. [DOI] [PubMed] [Google Scholar]
- 24.Donovan CE, Mark DA, He HZ, Liou HC, Kobzik L, Wang Y, De Sanctis GT, Perkins DL, Finn PW. NF-κB/rel transcription factors: C-rel promotes airway hyperresponsiveness and allergic pulmonary inflammation. J Immunol 1999;163:6827–6833. [PubMed] [Google Scholar]
- 25.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor-κB in the induction of eosinophilia in allergic airway inflammation. J Exp Med 1998;188:1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelleher ZT, Matsumoto A, Stamler JS, Marshall HE. NOS2 regulation of NF-κB by S-nitrosylation of p65. J Biol Chem 2007;282:30667–30672. [DOI] [PubMed] [Google Scholar]
- 27.Long AJ, Sypek JP, Askew R, Fish SC, Mason LE, Williams CM, Goldman SJ. Gob-5 contributes to goblet cell hyperplasia and modulates pulmonary tissue inflammation. Am J Respir Cell Mol Biol 2006;35:357–365. [DOI] [PubMed] [Google Scholar]
- 28.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 2007;117:3810–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia–inducible factor-1 is protective in murine experimental colitis. J Clin Invest 2004;114:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KS, Kim SR, Park SJ, Park HS, Min KH, Jin SM, Lee MK, Kim UH, Lee YC. Peroxisome proliferator activated receptor-γ modulates reactive oxygen species generation and activation of nuclear factor-κB and hypoxia-inducible factor-1α in allergic airway disease of mice. J Allergy Clin Immunol 2006;118:120–127. [DOI] [PubMed] [Google Scholar]
- 31.Huerta-Yepez S, Baay-Guzman GJ, Garcia-Zepeda R, Hernandez-Pando R, Vega MI, Gonzalez-Bonilla C, Bonavida B. 2-Methoxyestradiol (2-ME) reduces the airway inflammation and remodeling in an experimental mouse model. Clin Immunol 2008;129:313–324. [DOI] [PubMed] [Google Scholar]
- 32.Hjoberg J, Shore S, Kobzik L, Okinaga S, Hallock A, Vallone J, Subramaniam V, De Sanctis GT, Elias JA, Drazen JM, et al. Expression of nitric oxide synthase-2 in the lungs decreases airway resistance and responsiveness. J Appl Physiol 2004;97:249–259. [DOI] [PubMed] [Google Scholar]
- 33.Trifilieff A, Fujitani Y, Mentz F, Dugas B, Fuentes M, Bertrand C. Inducible nitric oxide synthase inhibitors suppress airway inflammation in mice through down-regulation of chemokine expression. J Immunol 2000;165:1526–1533. [DOI] [PubMed] [Google Scholar]
- 34.Alcorn JF, Ckless K, Brown AL, Guala AS, Kolls JK, Poynter ME, Irvin CG, Van Der Vliet A, Janssen-Heininger YM. Strain-dependent activation of NF-κB in the airway epithelium and its role in allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol 2010;298:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by κB kinase β–dependent genes in airway epithelium. Proc Natl Acad Sci USA 2005;102:17723–17728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. Nf-κB activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.delaTorre A, Schroeder RA, Bartlett ST, Kuo PC. Differential effects of nitric oxide–mediated S-nitrosylation on p50 and c-Jun DNA binding. Surgery 1998;124:137–141; discussion 141–132. [PubMed] [Google Scholar]
- 38.Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. No chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 2001;98:2622–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001;410:490–494. [DOI] [PubMed] [Google Scholar]
- 40.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 42.Guo J, Lu W, Shimoda LA, Semenza GL, Georas SN. Enhanced interferon-γ gene expression in T cells and reduced ovalbumin-dependent lung eosinophilia in hypoxia-inducible factor-1α–deficient mice. Int Arch Allergy Immunol 2009;149:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1α expression regulates the bactericidal capacity of phagocytes. J Clin Invest 2005;115:1806–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J Exp Med 2005;201:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor–dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol 2009;10:195–202. [DOI] [PubMed] [Google Scholar]
- 46.Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med 2009;15:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Han HJ, Lee YR, et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-κB and hypoxia-inducible factor-1α. Exp Mol Med 2007;39:756–768. [DOI] [PubMed] [Google Scholar]
- 48.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, Li CY. Regulation of HIF-1α stability through S-nitrosylation. Mol Cell 2007;26:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasinska IM, Sumbayev VV. S-nitrosation of cys-800 of HIF-1α protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett 2003;549:105–109. [DOI] [PubMed] [Google Scholar]
- 50.Carver DJ, Gaston B, Deronde K, Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol 2007;37:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor-1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci USA 1998;95:7368–7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF-1α. Science 2003;302:1975–1978. [DOI] [PubMed] [Google Scholar]
- 53.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. Nf-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008;453:807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem J 2008;412:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol 2004;113:610–619. [DOI] [PubMed] [Google Scholar]
- 56.Hesslinger C, Strub A, Boer R, Ulrich WR, Lehner MD, Braun C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem Soc Trans 2009;37:886–891. [DOI] [PubMed] [Google Scholar]
- 57.Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor−1α. J Biol Chem 2008;283:17919–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med 2007;85:1317–1324. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Schmid T, Brune B. Tumor necrosis factor-α causes accumulation of a ubiquitinated form of hypoxia inducible factor-1α through a nuclear factor-κB–dependent pathway. Mol Biol Cell 2003;14:2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1β and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res 2005;65:4690–4697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.