Abstract
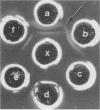
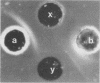
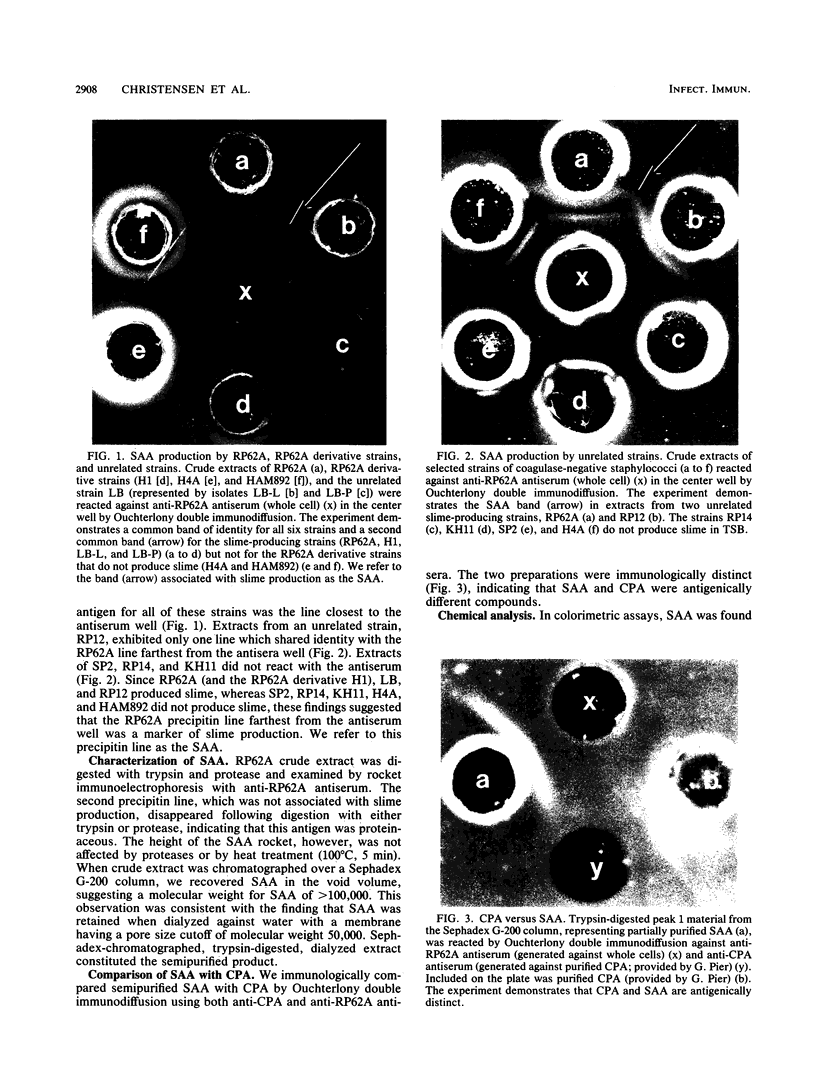
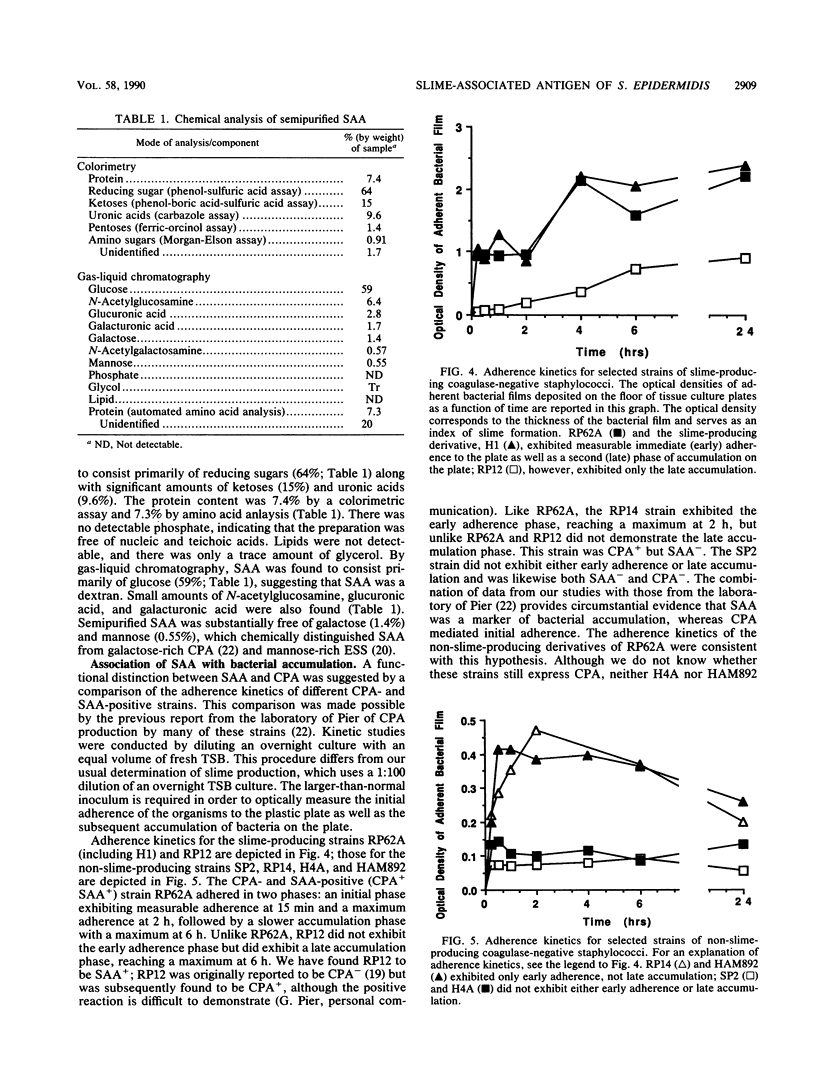
The pathogenic Staphylococcus epidermidis strain RP62A (ATCC 35984) adheres to smooth surfaces by forming a tenacious bacterial film known as slime. The mechanism of slime production is not known; however, workers in the laboratory of G. Pier (Harvard Medical School, Boston, Mass.) have isolated from RP62A a galactose-rich capsular polysaccharide adhesin (CPA) which mediates the attachment of the organism to smooth surfaces. We have obtained two daughter strains from RP62A that no longer produce slime. One daughter strain, H4A, was obtained by selection for a spontaneous variant; the other strain, HAM892, was obtained by treating growing cultures of RP62A with acriflavin. Using an antiserum generated against whole cells of RP62A, we have examined lysozyme-lysostaphin digests of RP62A, H4A, and HAM892 by double immunodiffusion. The two strains that no longer produced slime no longer produced a particular antigen, which we refer to as the slime-associated antigen (SAA). SAA was also produced by unrelated strains of slime-producing S. epidermidis. SAA was heat and protease stable, had a molecular weight of greater than 50,000, and could be partially purified by chromatographing trypsin-digested material over a Sephadex G-200 column. Chemical analysis of partially purified SAA by gas-liquid chromatography found SAA to be glucose rich (59%) and galactose poor (1.4%). This analysis chemically distinguished SAA from CPA. When tested together by double immunodiffusion with anti-RP62A and anti-CPA antisera, partially purified SAA did not cross-react with CPA. Kinetic studies suggested that SAA is a marker for surface accumulation whereas CPA mediates initial adherence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Barker L. P., Christensen G. D., Parisi J. T., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis in infection of transvenous endocardial pacemaker electrodes. J Clin Microbiol. 1990 Apr;28(4):676–679. doi: 10.1128/jcm.28.4.676-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour L. M., Smalley D. L., Kraus A. P., Jr, Lamoreaux W. J., Christensen G. D. Comparison of microbiologic characteristics of pathogenic and saprophytic coagulase-negative staphylococci from patients on continuous ambulatory peritoneal dialysis. Diagn Microbiol Infect Dis. 1986 Sep;5(3):197–205. doi: 10.1016/0732-8893(86)90002-7. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Bisno A. L., Parisi J. T., McLaughlin B., Hester M. G., Luther R. W. Nosocomial septicemia due to multiply antibiotic-resistant Staphylococcus epidermidis. Ann Intern Med. 1982 Jan;96(1):1–10. doi: 10.7326/0003-4819-96-1-1. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Diaz-Mitoma F., Harding G. K., Hoban D. J., Roberts R. S., Low D. E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987 Oct;156(4):555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- Franson T. R., Sheth N. K., Rose H. D., Sohnle P. G. Scanning electron microscopy of bacteria adherent to intravascular catheters. J Clin Microbiol. 1984 Sep;20(3):500–505. doi: 10.1128/jcm.20.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Mawhinney T. P., Feather M. S., Barbero G. J., Martinez J. R. The rapid, quantitative determination of neutral sugars (as aldononitrile acetates) and amino sugars (as O-methyloxime acetates) in glycoproteins by gas--liquid chromatography. Anal Biochem. 1980 Jan 1;101(1):112–117. doi: 10.1016/0003-2697(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Olson M. E., Ruseska I., Costerton J. W. Colonization of n-butyl-2-cyanoacrylate tissue adhesive by Staphylococcus epidermidis. J Biomed Mater Res. 1988 Jun;22(6):485–495. doi: 10.1002/jbm.820220605. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Staat R. H., Langley S. D., Doyle R. J. Streptococcus mutans adherence: presumptive evidence for protein-mediated attachment followed by glucan-dependent cellular accumulation. Infect Immun. 1980 Feb;27(2):675–681. doi: 10.1128/iai.27.2.675-681.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]