Abstract
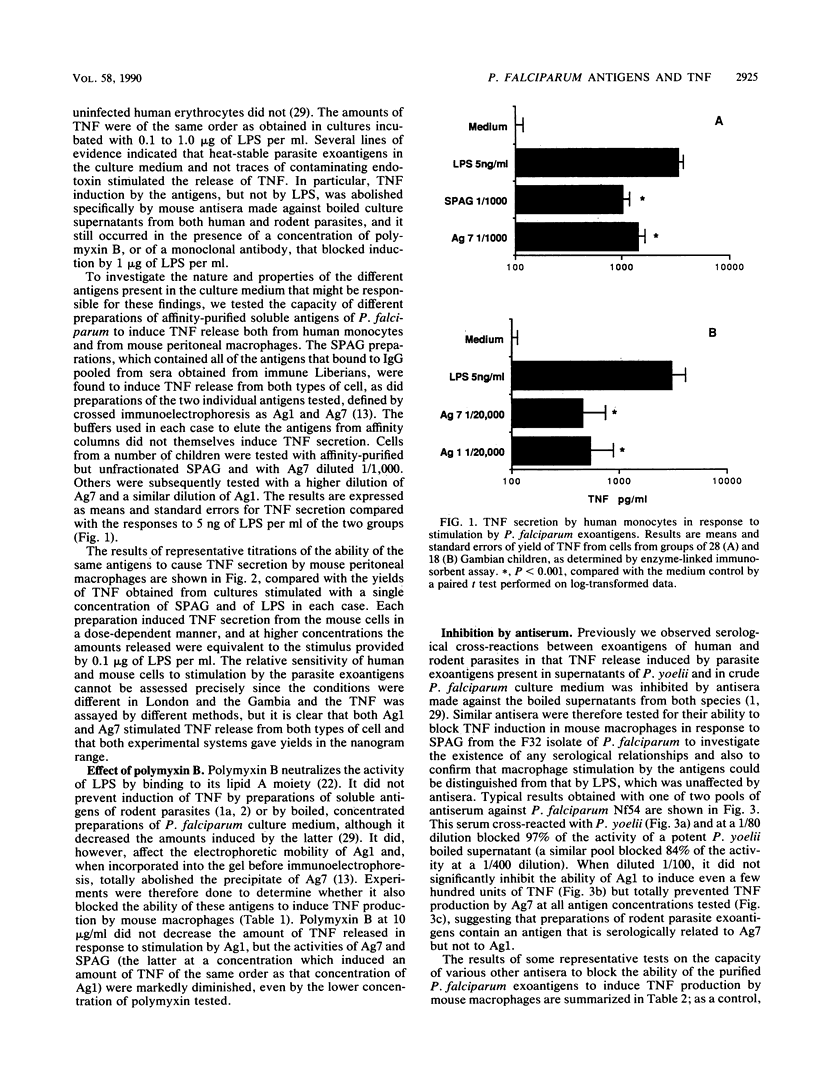
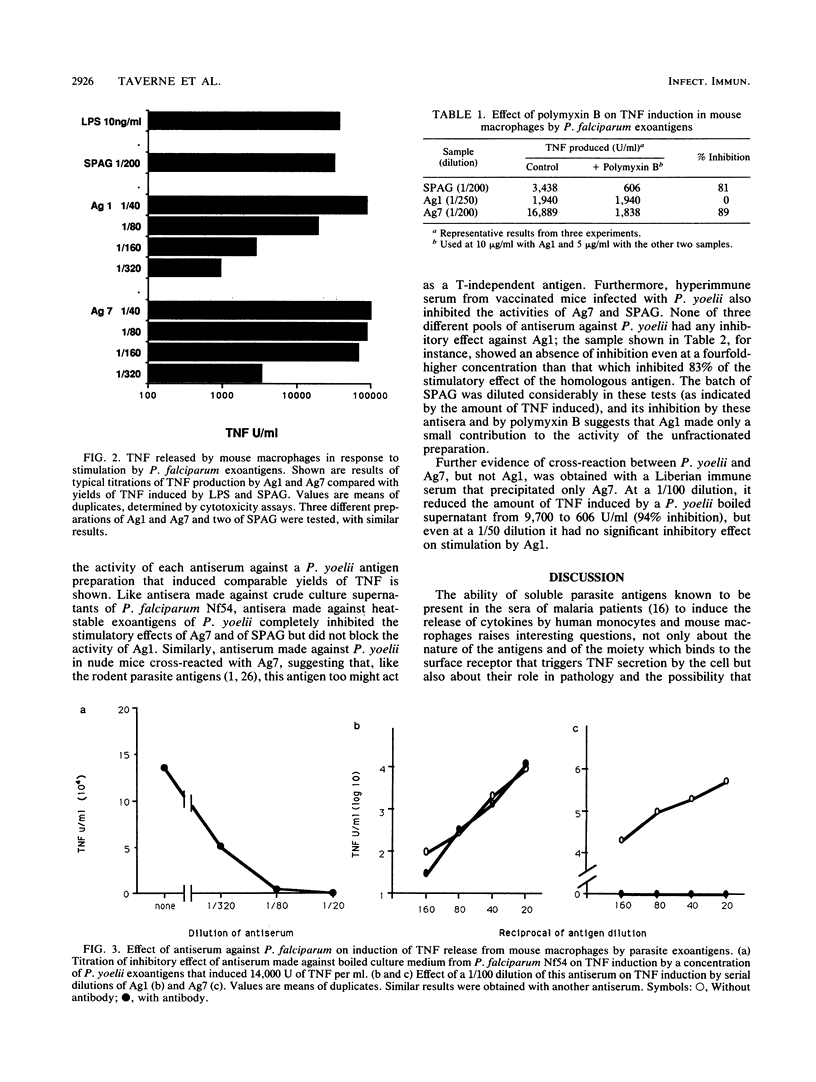
The production of cytokines such as tumor necrosis factor (TNF) may contribute to the pathology of malaria. We showed previously that crude preparations of heat-stable exoantigens from parasite cultures induce the release of TNF in vitro and in vivo. When separated from the culture medium by affinity chromatography, in which immune immunoglobulin G was used as ligand, the mixture of exoantigens of Plasmodium falciparum retained the capacity to induce the secretion of TNF, both by human monocytes from Gambian children and by mouse macrophages. Two individual antigens, Ag1 and Ag7, further purified by affinity chromatography and identified by crossed immunoelectrophoresis, also stimulated TNF production by both types of cell but differed in other functional properties. Thus, the activity of Ag7, but not that of Ag1, was inhibited by polymyxin B, and antisera made against boiled exoantigens of the rodent parasite Plasmodium yoelii which blocked the ability of these antigens to induce the production of TNF also inhibited the activity of Ag7 without affecting Ag1. Since the prevalence of antibody against Ag7 in sera from children in endemic areas appears to correlate with the development of immunity against the manifestations of the disease, this antigen may be one cause of pathology, perhaps through its ability to induce the production of TNF. Its serological relationship with rodent exoantigens suggests that it might be a candidate for an anti-disease vaccine which has the advantage that its active moiety is not subject to significant antigen polymorphism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Berendt A. R., Simmons D. L., Tansey J., Newbold C. I., Marsh K. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum. Nature. 1989 Sep 7;341(6237):57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Cell-mediated immunity in protection and pathology of malaria. Parasitol Today. 1987 Oct;3(10):300–305. doi: 10.1016/0169-4758(87)90187-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Delplace P., Fortier B., Tronchin G., Dubremetz J. F., Vernes A. Localization, biosynthesis, processing and isolation of a major 126 kDa antigen of the parasitophorous vacuole of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Apr;23(3):193–201. doi: 10.1016/0166-6851(87)90026-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983 Dec;54(3):609–616. [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Baek L., Jepsen S. Demonstration of soluble Plasmodium falciparum antigens reactive with Limulus amoebocyte lysate and polymyxin B. Parasite Immunol. 1988 Nov;10(6):593–606. doi: 10.1111/j.1365-3024.1988.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Jepsen S., Agger R. Inhibitory monoclonal antibodies to soluble Plasmodium falciparum antigens. Parasitol Res. 1987;73(6):518–523. doi: 10.1007/BF00535326. [DOI] [PubMed] [Google Scholar]
- Jakobsen P. H., Theander T. G., Jensen J. B., Mølbak K., Jepsen S. Soluble Plasmodium falciparum antigens contain carbohydrate moieties important for immune reactivity. J Clin Microbiol. 1987 Nov;25(11):2075–2079. doi: 10.1128/jcm.25.11.2075-2079.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen S., Andersen B. J. Immunoadsorbent isolation of antigens from the culture medium of in vitro cultivated Plasmodium falciparum. Acta Pathol Microbiol Scand C. 1981 Apr;89(2):99–103. doi: 10.1111/j.1699-0463.1981.tb02671.x. [DOI] [PubMed] [Google Scholar]
- Jepsen S., Axelsen N. H. Antigens and antibodies in Plasmodium falciparum malaria studied by immunoelectrophoretic methods. Acta Pathol Microbiol Scand C. 1980 Oct;88(5):263–270. doi: 10.1111/j.1699-0463.1980.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Barbe G., Marsh K., Ambroise-Thomas P. Tumor necrosis factor production by human macrophages stimulated in vitro by Plasmodium falciparum. Infect Immun. 1990 Jan;58(1):214–216. doi: 10.1128/iai.58.1.214-216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Vaccination of mice against malaria with soluble antigens. I. The effect of detergent, route of injection, and adjuvant. Parasite Immunol. 1986 Sep;8(5):409–414. doi: 10.1111/j.1365-3024.1986.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Andersson G., Otoo L. N., Jepsen S., Greenwood B. M. Cellular immune responses to Plasmodium falciparum antigens in Gambian children during and after an acute attack of falciparum malaria. Clin Exp Immunol. 1988 Jul;73(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- Riley E. M., Jepsen S., Andersson G., Otoo L. N., Greenwood B. M. Cell-mediated immune responses to Plasmodium falciparum antigens in adult Gambians. Clin Exp Immunol. 1988 Mar;71(3):377–382. [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Playfair J. H. Induction of TNF in vitro as a model for the identification of toxic malaria antigens. Lymphokine Res. 1989 Fall;8(3):317–322. [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Sarkar D. A., Meager A., Rook G. A., Playfair J. H. Human and murine macrophages produce TNF in response to soluble antigens of Plasmodium falciparum. Parasite Immunol. 1990 Jan;12(1):33–43. doi: 10.1111/j.1365-3024.1990.tb00934.x. [DOI] [PubMed] [Google Scholar]


