Abstract
Nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase (np-Ga3PDHase) is a cytosolic unconventional glycolytic enzyme of plant cells regulated by phosphorylation in heterotrophic tissues. After interaction with 14-3-3 proteins, the phosphorylated enzyme becomes less active and more sensitive to regulation by adenylates and inorganic pyrophosphate. Here, we acknowledge that in wheat (Triticum aestivum), np-Ga3PDHase is specifically phosphorylated by the SnRK (SNF1-related) protein kinase family. Interestingly, only the kinase present in heterotrophic tissues (endosperm and shoots, but not in leaves) was found active. The specific SnRK partially purified from endosperm exhibited a requirement for Mg2+ or Mn2+ (being Ca2+ independent), having a molecular mass of approximately 200 kD. The kinase also phosphorylated standard peptides SAMS, AMARA, and SP46, as well as endogenous sucrose synthase, results suggesting that it could be a member of the SnRK1 subfamily. Concurrently, the partially purified wheat SnRK was recognized by antibodies raised against a peptide conserved between SnRK1s from sorghum (Sorghum bicolor) and maize (Zea mays) developing seeds. The wheat kinase was allosterically inhibited by ribose-5-phosphate and, to a lesser extent, by fructose-1,6-bisphosphate and 3-phosphoglycerate, while glucose-6-phosphate (the main effector of spinach [Spinacia oleracea] leaves, SnRK1) and trehalose-6-phosphate produced little or no effect. Results support a distinctive allosteric regulation of SnRK1 present in photosynthetic or heterotrophic plant tissues. After in silico analysis, we constructed two np-Ga3PDHase mutants, S404A and S447A, identifying serine-404 as the target of phosphorylation. Results suggest that both np-Ga3PDHase and the specific kinase could be under control, critically affecting the metabolic scenario involving carbohydrates and reducing power partition and storage in heterotrophic plant cells.
Nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase (np-Ga3PDHase; EC 1.2.1.9) catalyzes the irreversible oxidation of glyceraldehyde-3-phosphate (Ga3P) to 3-phosphoglycerate (3PGA), specifically using NADP+ to generate NADPH (Arnon et al., 1954; Iglesias, 1990; Habenicht, 1997). This enzyme is found in the cytosol of cells from higher plants (Arnon et al., 1954; Pupillo and Faggiani, 1979; Iglesias and Losada, 1988; Mateos and Serrano, 1992; Gao and Loescher, 2000; Gómez Casati et al., 2000) and green algae (Iglesias et al., 1987; Mateos and Serrano, 1992), and it is also present in some specialized bacteria (Brown and Wittenberger, 1971; Boyd et al., 1995; Brunner and Hensel, 2001; Iddar et al., 2005). In the different organisms, the enzyme is coded by a single gene (Bustos and Iglesias, 2002; Rius et al., 2006) that gives rise to a tetrameric functional protein (approximately 200 kD) structurally related to the aldehyde dehydrogenase superfamily (Habenicht, 1997). An early report (Kelly and Gibbs, 1973) highlighted the occurrence, in photosynthetic cells, of a shuttle system exporting photosynthetically generated NADPH from the chloroplast to the cytosol, after the association of np-Ga3PDHase with the plastidic phosphorylating NADP-dependent Ga3PDHaseA/B (EC 1.2.1.13) and the triose-P/inorganic orthophosphate (Pi) translocator of the chloroplast envelope. In plants accumulating acyclic polyols (such as celery [Apium graveolens]), the enzyme is involved in the supply of NADPH necessary for synthesis of reduced sugars (Rumpho et al., 1983; Gao and Loescher, 2000). On the other hand, in nongreen cells, np-Ga3PDHase could take part in an alternative pathway in cytosolic glycolysis, also involving inorganic pyrophosphate (PPi)-dependent phosphofructokinase and phosphoenolpyruvate (PEP) phosphatase (Plaxton, 1996; Givan, 1999). The latter route can produce NADPH after coupling anabolism with glycolysis (Habenicht, 1997).
The occurrence of np-Ga3PDHase in the cytosol of plant cells establishes an alternative for the Ga3P oxidation step during glycolysis. As a result, Ga3P can be metabolized to 3PGA either by the coupling of phosphorylating NAD-dependent Ga3PDHase (EC 1.2.1.12) and 3PGA kinase (EC 2.7.2.3) or by np-Ga3PDHase (Iglesias, 1990; Plaxton, 1996). This branch point, providing NADH and ATP or NADPH (but not ATP), is expected to be regulated in order to effectively modulate cellular production of energetic and reductive power according to transitory cell requirements (Iglesias, 1990; Plaxton, 1996). In agreement with such a view, it has been reported that in nonphotosynthetic plant cells, np-Ga3PDHase is a target for posttranslational phosphorylation (Bustos and Iglesias, 2002); afterward, the enzyme interacts with 14-3-3 regulatory proteins (Bustos and Iglesias, 2003). Kinetic consequence of the latter is an enzyme with lower activity and enhanced sensitivity to regulation by adenylates and PPi. Furthermore, the complex formed by phosphorylated np-Ga3PDHase and 14-3-3 is disrupted by Mg2+. Therefore, phosphorylation plus the interaction with 14-3-3 proteins determine a divalent cation-dependent regulatory mechanism to inhibit np-Ga3PDHase activity at high energy value (Bustos and Iglesias, 2003). This regulation is the rationale behind the cytosolic metabolism of plant cells where PPi is accumulated at significant levels, thus determining, together with ATP, intracellular energy contents (Plaxton, 1996; Sttit, 1998).
Despite the above picture, where plant np-Ga3PDHase is a critical target for regulation of carbon metabolism, the mechanism for posttranslational regulation of the enzyme is poorly understood. For example, the kinase responsible for the enzyme phosphorylation and the amino acid residue specifically modified in the process are both unknown. In this work, we report studies performed on highly purified wheat (Triticum aestivum) np-Ga3PDHase obtained recombinantly (Piattoni et al., 2010). We found that the enzyme is specifically phosphorylated by SNF1-related protein kinase (SnRK1) from nonphotosynthetic (endosperm and shoots) wheat tissues, though absent in photosynthetic (leaf) ones. Furthermore, generation of site-directed mutants allowed us to identify Ser-404 as the amino acid residue being phosphorylated in np-Ga3PDHase.
RESULTS
Phosphorylation of np-Ga3PDHase in Wheat Endosperm Specifically Depends on a Protein Kinase of the SnRK Family
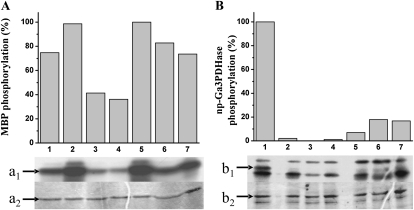
It has been reported that np-Ga3PDHase is present in a phosphorylated state in plant heterotrophic tissues (endosperm and shoot; Bustos and Iglesias, 2002). To identify the kinase involved in such a posttranslational modification, we performed in vitro phosphorylation studies by incubating the pure recombinant np-Ga3PDHase (Piattoni et al., 2010) with a wheat endosperm extract (as the kinase source) in the presence of [32P]γ-ATP under several conditions, each one optimal for different plant protein kinases already characterized (Stone and Walker, 1995; Champion et al., 2004; Rudrabhatla et al., 2006). Phosphorylation was detected by the incorporation of radioactivity into the target enzyme by SDS-PAGE resolution of the incubation mixtures followed by autoradiography, as illustrated in Figure 1. As a control of the presence of the different plant protein kinases in the wheat endosperm extract, we used the universal substrate myelin basic protein (MBP), which was phosphorylated to a certain degree under all the assays conditions (Fig. 1A). Interestingly, Figure 1B illustrates that np-Ga3PDHase was appreciably phosphorylated only under conditions specific for SnRK, Ca2+-independent protein kinases. Results thus support a high specificity respect to the protein kinase family involved in the phosphorylation of np-Ga3PDHase in wheat endosperm. Essentially identical results were obtained when working with extracts from wheat shoots (data not shown).
Figure 1.
Phosphorylation of MBP and np-Ga3PDHase by wheat endosperm extracts under optimal conditions for different plant protein kinase families. A, Schematic representation of MBP phosphorylation (percentage from maximum) by different plant protein kinase with data taken from [32P]γ-ATP incorporation into proteins after autoradiography (a1) of SDS-PAGE gel stained with Coomassie Brilliant Blue (a2). B, Schematic representation of np-Ga3PDHase phosphorylation (percentage from maximum) by different plant protein kinases, as calculated from [32P]γ-ATP incorporation revealed by autoradiography (b1) of SDS-PAGE gel stained with Coomassie Brilliant Blue (b2). Numbers indicate optimal phosphorylation conditions (for details, see “Materials and Methods”): 1, Ca2+-independent SNF1-related protein kinase (WPK4); 2, SOS2; 3, GSK3; 4, MAPK; 5, CKII; 6, Tsl; 7, CDPK. In schematic representations, values were calculated from autoradiography using LabImage Version 2.7.0 (free edition). Arrows indicate the respective migration of MBP or np-Ga3PDHase. All data are means from five repetitions and reproducible with differences below ±10%.
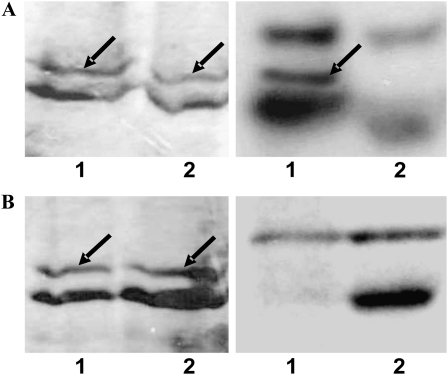
Figure 2 shows that the specificity for phosphorylation of np-Ga3PDHase is not only at the level of the protein kinase but that it also depends on the tissue. Thus, phosphorylation of the recombinant enzyme observed after incubation with endosperm extracts under optimal conditions for SnRK (Fig. 2A) was not observed with leaf extracts treated under identical conditions (Fig. 2B). The latter indicates the absence of a very specific protein kinase in the leaf extract, as controls performed with MBP showed that all the kinase families were active (data not shown), although none of them were able to phosphorylate the recombinant np-Ga3PDHase. This result strongly agrees with preceding works characterizing np-Ga3PDHase from wheat and finding the enzyme in a phosphorylated state in endosperm but not in leaves (Bustos and Iglesias, 2002, 2003). A further degree of specificity is observed in Figure 2, as it depicts the highly related Ca2+-dependent salt overly sensitive-2 protein kinase (SOS2) as unable to modify the enzyme (Fig. 2A, lane 2). Therefore, data support the definite involvement of a Ca2+-independent SNF1-related protein kinase in phosphorylation of np-Ga3PDHase.
Figure 2.
Tissue specificity for np-Ga3PDHase phosphorylation. A, Phosphorylation of np-Ga3PDHase by wheat endosperm extract. B, Phosphorylation of np-Ga3PDHase by wheat leaves extract. 1, Optimal phosphorylation conditions for WPK4. 2, Optimal phosphorylation conditions for SOS2. Left panels, Coomassie Brilliant Blue staining; right panels, [32P]γ-ATP incorporation in autoradiography of SDS-PAGE. Arrows indicate np-Ga3PDHase.
Partial Purification and Characterization of Wheat Endosperm SnRK Protein Kinase
Extracts from several plants contain protein kinases able to phosphorylate the synthetic peptide HMRSAMSGLHLVKRR (namely, SAMS), which was designed as a specific substrate for mammalian AMPK (Davies et al., 1989). One of these protein kinases, purified from cauliflower (Brassica oleracea; Mackintosh et al., 1992), was shown to have properties quite similar to those of mammalian AMPK, in terms of both specificity for peptide substrates (Weekes et al., 1993; Dale et al., 1995) and regulation by autophosphorylation (Mackintosh et al., 1992). Moreover, McMichael et al. (1995a) and Douglas et al. (1997) resolved three kinase activities from spinach (Spinacia oleracea) leaf extracts by anion-exchange chromatography. A detailed analysis indicated that the third peak (PKIII), corresponding to a Ca2+-independent enzyme, should be classified as a member of the SnRK1 family. Such a kinase was able to phosphorylate Suc-P synthase (SPSase) and nitrate reductase (McMichael et al., 1995a) in addition to the SAMS peptide (Sugden et al., 1999b). Protocols described in the above studies were broadly used to purify various plant calcium-dependent protein kinase (CDPK) and SnRK1 protein kinases, which are involved in phosphorylation of different plant enzymes, such as pyruvate kinase (Tang et al., 2003), trehalose-P synthase 5 (Harthill et al., 2006), and SPSase (Toroser and Huber, 1997). In addition, the purified kinases were useful to characterize allosteric regulation of SnRK1 kinases by Glc-6-P (Glc6P; Toroser et al., 2000) and trehalose-6-P (Tre6P; Zhang et al., 2009).
Based on the above experience, and using the highly purified recombinant np-Ga3PDHase as a specific substrate, we partially purified a SnRK kinase from wheat endosperm. As detailed in “Materials and Methods,” we basically worked with the protocol described by Toroser et al. (2000). Total proteins of a wheat endosperm extract were precipitated between 3% and 20% (w/v) polyethylene glycol 8000 (PEG8000) to obtain a concentrated crude extract devoid of low-Mr components. Proteins were further resolved on a 2-mL Resource-Q column, a method that effectively eliminates the majority of contaminating protein from the PKIII fraction previously isolated from spinach leaves (Toroser et al., 2000). Kinases from wheat endosperm were extracted in the presence of the phosphatase inhibitors NaF, EDTA, and EGTA. Active fractions obtained after ionic chromatography were pooled, supplemented with dithiothreitol (DTT; 10 mm) plus glycerol (to reach 10% [v/v]), and conserved at −80°C. Under these conditions, the sample remained active, conserving 100% SnRK activity for at least 3 months. Following this method, the SnRK from wheat endosperm was approximately 18-fold purified, as the specific activity (determined by measuring the incorporation of radioactivity from [32P]γ-ATP into np-Ga3PDHase; see details in “Materials and Methods”) increases from 11 ± 1 pmol min−1 mg−1 in the crude extract to 200 ± 21 pmol min−1 mg−1 after purification.
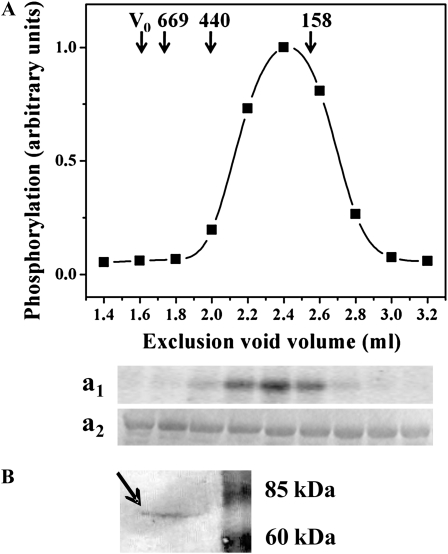
In plants, the SnRK family has diverged and expanded into three subfamilies (Halford and Hey, 2009): SnRK1, SnRK2, and SnRK3. SnRK1 is closely related to the metabolic regulators of yeast (SNF1) and mammals (AMPK), whereas SnRK2 and SnRK3 have diverged further. Moreover, the SnRK1 family of cereals can still be subdivided into two groups, SnRK1a and SnRK1b, on the basis of amino acid sequence similarity and expression patterns. SnRK1a is expressed throughout the plant and is intimately connected to SnRK1 from dicotyledonous plants, while SnRK1b is expressed at the highest levels in the seed and is only present in monocotyledonous plants (Halford et al., 2003). Results shown in Figure 3 give information about the identity of the SnRK partially purified from wheat endosperm. Figure 3A illustrates that gel filtration on Superdex 200 HR column revealed a molecular mass of approximately 200 kD for the partially purified wheat kinase, a value close to the size previously reported for SnRK characterized from spinach leaves (Sugden et al., 1999b). Further evaluation on the identity of the SnRK purified from wheat endosperm was reached by western-blot analysis using specific antibodies raised against a peptide conserved between sorghum (Sorghum bicolor) and maize (Zea mays) SnRK1 from developing seeds (Jain et al., 2008). These antibodies recognized a polypeptide in the partially purified SnRK from wheat endosperm sample (Fig. 3B) substantiating the presence of a SnRK1, though with a mass of approximately 70 kD, a size slightly higher than that reported for plant SnRK1 (approximately 60 kD). Also, results support that at least one SnRK from wheat endosperm contains a region with the peptide sequence specific for the antibodies recognition, information that was not clearly established in databases from wheat available at this time.
Figure 3.
Molecular mass determination of wheat endosperm SnRK by gel filtration and immunodetection with anti-SnRK1b antibodies. A, Schematic representation of wheat endosperm SnRK activity (with np-Ga3PDHase as substrate) eluted from Superdex 200 gel filtration column. Elution volumes of marker proteins are indicated by arrows with molecular mass in kilodaltons. [32P]γ-ATP incorporation was revealed by autoradiography (a1) of SDS-PAGE gel stained with Coomassie Brilliant Blue (a2). B, Western blotting of partially purified SnRK from wheat endosperm with antibodies raised against a peptide conserved between sorghum and maize SnRK1. BenchMark prestained molecular mass markers (Invitrogen) were used. Experiments were reproducible in three independent repetitions.
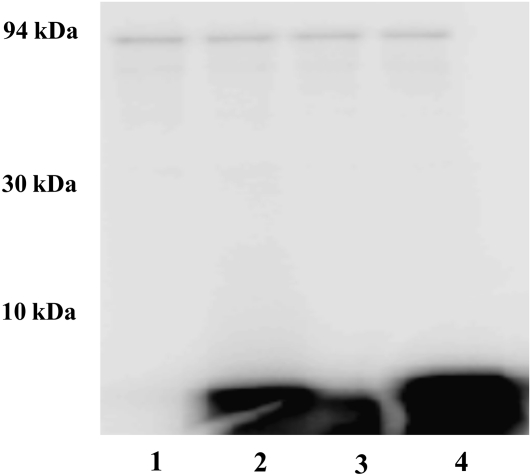
Synthetic peptides such as SAMS and AMARA have been established as characteristic substrates for the SNF1 family of protein kinases, which includes plant SnRK1 (Davies et al., 1989; Weekes et al., 1993; Dale et al., 1995). Figure 4 shows that this is also the case for the wheat endosperm kinase under study, as it was able to phosphorylate SAMS (lane 2) and AMARA (lane 3), as well as SP46 (lane 4), a peptide previously used in the characterization of spinach leaves SnRK1 (Toroser et al., 2000). Figure 4 also illustrates about phosphorylation of a protein band of approximately 90 kD (see lanes 1–4 in Fig. 4) that is present in the sample of the partially purified kinase. By matrix-assisted laser-desorption ionization tandem time of flight (MALDI-TOF/TOF) analysis, such a protein was identified as Suc synthase (SuSy) 2, a homotetrameric enzyme already shown phosphorylated on Ser-15 by SnRK1 in plants (Hardin et al., 2003). Thus, SuSy 2 constitutes an endogenous wheat endosperm enzyme that is substrate of the partially purified protein kinase under the experimental conditions used for assay SnRK activity.
Figure 4.
Phosphorylation of SAMS, AMARA, and SP46 peptides by partially purified SnRK. 32P incorporation copurifying SuSy without further additions (lane 1), SAMS (lane 2), AMARA (lane 3), and SP46 (lane 4) peptides, revealed by autoradiography of Tricine-SDS-PAGE electrophoresis carried out on precast 4% to 20% polyacrylamide gradient gels. Molecular mass markers (in kilodaltons) are indicated on the left.
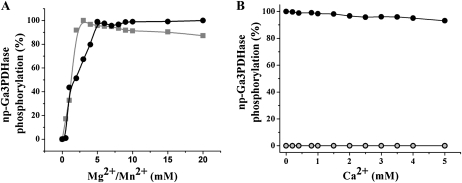
Kinases, like other phosphotransferases, require a divalent cation to coordinate the phosphate groups of the nucleotide triphosphate substrate (Gong et al., 2002). To further characterize the wheat endosperm SnRK1, we determined the in vitro divalent cation preference of the partially purified enzyme (Fig. 5). Figure 5A shows that phosphorylation of np-Ga3PDHase by the kinase exhibited a compelling requirement for the presence of Mg2+ or Mn2+, with maximal activity observed at cation concentrations higher than 2.5 mm (Mn2+) or 5 mm (Mg2+). Also, the kinase activity was insensitive to the presence of up to 5 mm Ca2+ (Fig. 5B); thus, the process was strictly Ca2+ independent, as expected for an SnRK1 protein kinase (McMichael et al., 1995b; Toroser et al., 2000).
Figure 5.
Divalent cation dependence of the wheat endosperm SnRK1 activity. Phosphorylation of np-Ga3PDHase by the SnRK1 partially purified from wheat endosperm was performed at various concentrations of Mg2+ (as MgCl2, gray squares) or Mn2+ (as MnCl2, black circles; A) and Ca2+ (as CaCl2) without Mg2+ (gray circles) or with 5 mm Mg2+ (black circles; B). Values of percentage of phosphorylation were calculated from autoradiography using LabImage Version 2.7.0 (free edition). Curves were made by triplicate and reproducible in ±10%.
Allosteric Regulatory Properties of Wheat Endosperm SnRK1 Protein Kinase
In several cases, protein kinases are regulated enzymes. In the SNF1 family (SNF1/AMPK/SnRK1), regulation was broadly studied for AMPK and SNF1 (Hardie, 1999b; Polge and Thomas, 2007; Halford and Hey, 2009), but information available for SnRK1 is scarce. In mammals, AMPK is involved in sensing the cellular energy level (Polge and Thomas, 2007; Halford and Hey, 2009), whereas in yeast, the SNF1 gene is required for derepression of genes that are repressed by Glc, and the SNF1 complex is rapidly activated on Glc removal by a mechanism involving phosphorylation by an upstream kinase (Hardie, 1999a). Despite precise physiological functions and regulation of SnRK1 in plant growth and development remaining elusive, during the last few years, significant information has been revealed. Thus, the differential transcriptional regulation of genes of SnRK1 complex by abscisic acid and gibberellins has been established (Bradford et al., 2003), while two SnRK1 isoforms (AKIN10 and AKIN11) present in Arabidopsis (Arabidopsis thaliana) were found differentially regulated under phosphate starvation (Fragoso et al., 2009). On the other hand, spinach leaf SnRK1 is activated by phosphorylation of a Thr residue by an upstream protein kinase, a process reverted by protein phosphatases, through a mechanism regulated by AMP (Sugden et al., 1999a). Also, allosteric regulation of SnRK1 protein kinases has been reported. SnRK1 spinach leaves are inhibited by Glc6P (Toroser et al., 2000), while micromolar concentrations of Tre6P inhibit the kinase activity in Arabidopsis seedling extracts and other young plant material but not in mature leaves (Zhang et al., 2009). Inhibition of SnRK1 by Tre6P occurs at a site distinct and separable from its catalytic site via a yet unknown intermediary factor absent in mature leaves.
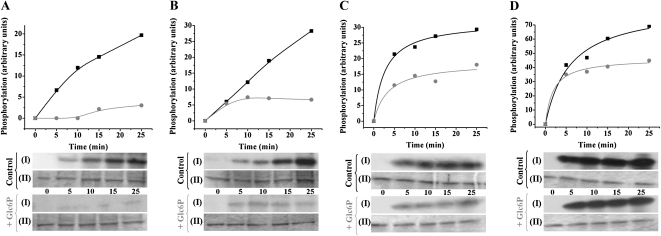
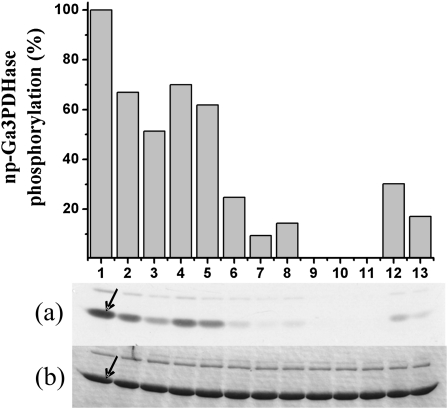
To analyze for allosteric regulatory properties of the SnRK1 partially purified from wheat endosperm, we first performed phosphorylation assays for np-Ga3PDHase and MBP in the presence or the absence of Glc6P at two different Mg2+ concentrations (2.5 and 10 mm) as previously described (Toroser et al., 2000). Figure 6 shows how phosphorylation of np-Ga3PDHase (Fig. 6, A and B) and MBP (Fig. 6, C and D) diminished in the presence of 10 mm Glc6P at both Mg2+ concentrations, more markedly at the lower cation level. This result obtained in vitro supports negative allosteric regulation of wheat endosperm SnRK1 by Glc6P, as it was described for the enzyme from spinach leaf (Toroser et al., 2000). In addition, we evaluated other metabolites (at 10 mm levels and with 10 mm Mg2+) as probable allosteric regulators. As shown in Table I, Rib-5-P (Rib5P), Fru1,6bisP, 3PGA, and ADPGlc produced important (>50%) inhibition of wheat endosperm SnRK1, whereas other metabolites had lower (Fru-6-P [Fru6P], Man-6-P [Man6P], Pi, UDPGlc, Glc-1-P [Glc1P], and Glc6P) or no (PEP, Glc, and Suc) effect. A further evaluation (Fig. 7) of the principal effectors (those inhibiting >50% in Table I, and Glc6P) at near physiological conditions (2.5 and 5 mm metabolite, with 2.5 mm Mg2+) showed that ADPGlc and Glc6P caused a modest inhibition but Rib5P, Fru1,6bisP, and 3PGA still maintained an inhibition >50% under the same conditions, thus suggesting these three metabolites are key allosteric regulators of SnRK1. Furthermore, Fru-2,6-bisP (Fru2,6bisP) and Tre6P were also evaluated (up to 0.2 mm concentration) as probable effectors, though these did not affect SnRK1 activity at near physiological concentrations. The insensitivity of the partially purified SnRK1 toward Tre6P, in comparison to the allosteric regulator Rib5P, is detailed in Supplemental Figure S2.
Figure 6.
Inhibition of wheat endosperm SnRK1 by 10 mm Glc6P. Effect of Glc6P on SnRK1 activity assayed as phosphorylation of np-Ga3PDHase in the presence of 2.5 mm (A) or 10 mm (B) Mg2+ or as phosphorylation of MBP in the presence of 2.5 mm (C) or 10 mm (D) Mg2+. Top plots are schematic representations of kinase activity assayed under optimal phosphorylation conditions for Ca2+-independent SnRK1 in the absence (black squares) or presence (gray circles) of 10 mm Glc6P; values were calculated from autoradiography with LabImage Version 2.7.0 (free edition). [32P]γ-ATP incorporation in autoradiography (I) of SDS-PAGE stained with Coomassie Brilliant Blue (II). Activity was assay at different incubation times (0, 5, 10, 15, and 25 min). Values were reproducible within ±10% variations in at least three independent experiments.
Table I. Effect of several metabolites on wheat endosperm SnRK1.
Kinase activity was assayed in the presence of 10 mm Mg2+ with the addition of different metabolites, as specified. Values are means from three different determinations reproducible within ±10%.
| Metabolite (10 mm) | Relative Phosphorylation of np-Ga3PDHase |
| % | |
| None | 100 |
| UDPGlc | 59 |
| ADPGlc | 42 |
| Glc6P | 63 |
| Glc1P | 77 |
| Man6P | 60 |
| Fru6P | 62 |
| Fru1,6bisP | 26 |
| 3PGA | 38 |
| Rib5P | 4 |
| Pi | 65 |
| PEP | 100 |
| Glc | 100 |
| Suc | 100 |
Figure 7.
Effect of different metabolites on wheat endosperm SnRK1 activity. Assays of np-Ga3PDHase phosphorylation were performed in the presence of 2.5 mm Mg2+ and with addition of none (1), 2.5 mm ADPGlc (2), 5 mm ADPGlc (3), 2.5 mm Glc6P (4), 5 mm Glc6P (5), 2.5 mm Fru1,6bisP (6), 5 mm Fru1,6bisP (7), 0.5 mm Rib5P (8), 1.0 mm Rib5P (9), 2.5 mm Rib5P (10), 5 mm Rib5P (11), 2.5 mm 3PGA (12), or 5 mm 3PGA (13). The top plot was calculated from autoradiography (a) of SDS-PAGE gel stained with Coomassie Brilliant Blue (b). All data were reproduced (within ±10%) in three independent experiments.
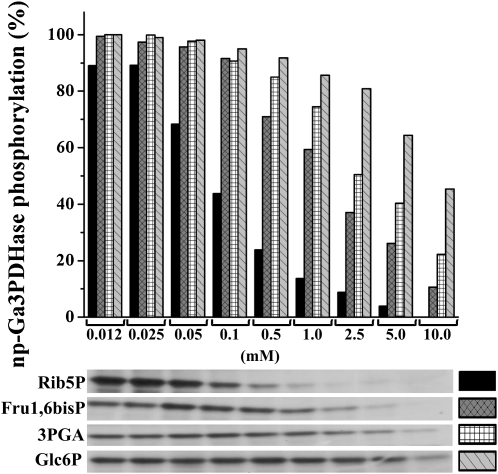
Figure 8 illustrates a more detailed analysis of the capacity of Rib5P, Fru1,6bisP, and 3PGA to inhibit phosphorylation of np-Ga3PDHase by the SnRK1 partially purified from wheat endosperm. Rib5P behaved as the more effective inhibitor, since at 0.1 mm the metabolite caused about 50% inhibition, whereas Fru1,6bisP and 3PGA produced the same effect at concentrations more than 1 order of magnitude higher. Figure 8 also shows the effect of Glc6P, which required 10 mm concentrations or higher. These results support distinctive regulatory properties of the wheat endosperm SnRK1 when compared with those reported for the kinase from plant leaf (Toroser et al., 2000). Thus, at a concentration of 10 mm, Glc6P produced the greatest inhibition on spinach leaf SnRK1; Fru1,6bisP, Man6P, and Glc1P caused some inhibition, but Rib5P and 3PGA had essentially no effect. Contrarily, in this work, we found that the kinase from endosperm is mainly inhibited by Rib5P, followed by Fru1,6bisP and 3PGA with relatively low affinity, and by Glc6P with a very low affinity. This comparison suggests that SnRK1 is allosterically regulated in a differential form respect to the autotrophic or heterotrophic characteristics of the plant tissue.
Figure 8.
Effect of Rib5P, Fru1,6bisP, 3PGA, and Glc6P on wheat endosperm SnRK1 activity. The principal effectors were evaluated at different concentrations by analyzing phosphorylation of np-Ga3PDHase in the presence of 5 mm Mg2+. Top, Schematic representation; bottom, autoradiographs. One hundred percent corresponds to the respective phosphorylation obtained in the absence of effector in each case. Values are the mean figures resulting from three different determinations reproducible within ±10%.
Phosphorylation of np-Ga3PDHase Occurs at Ser-404
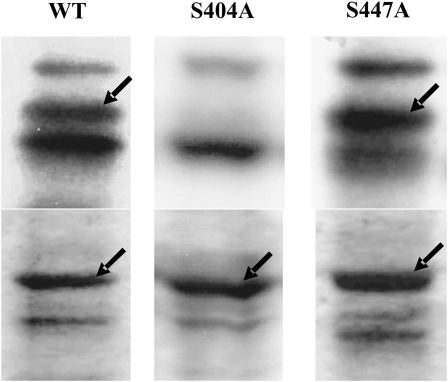
Protein phosphorylation generally occurs on Ser, Thr, or Tyr residues exposed to the solvent, accessible to interact with the kinase, and having particular amino acid sequences in their surrounding (Boyer and Krebs, 1986). To explore about the specific residue that is the target for phosphorylation in np-Ga3PDHase, we constructed site-directed (in Ser residues) mutant enzymes. After the information obtained from a molecular modeling study (Bustos and Iglesias, 2005), residues Ser-404 and Ser-447 were identified in the wheat np-Ga3PDHase as the most probable sites for phosphorylation and subsequent binding of 14-3-3 proteins. On such a basis, we constructed and expressed S404A and S447A mutant enzymes, which were found to be fully active, both having near 2-fold higher specific activity and similar affinity for substrates than the wild-type enzyme (Table II). Phosphorylation assays with wheat endosperm extracts indicated that the S447A mutant was phosphorylated by SnRK as occurred with the wild-type enzyme, but the S404A mutant was recalcitrant to the action of the kinase (Fig. 9). Furthermore, none of the mutant enzymes was modified by other kinases present in the endosperm (data not shown). Results are indicative of Ser-404 being the phosphorylation site in np-Ga3PDHase, thus validating one of the domains alleged for modification by molecular modeling.
Table II. Kinetic parameters for the wild type and site-directed np-Ga3PDHase mutants.
Initial velocities were measured at variable concentrations of NADP+ (0.001–0.5 mm) or Ga3P (0.01–5 mm). NADP+ was fixed at 0.11 mm, and Ga3P was maintained at 1.2 mm when the respective cosubstrate was variable. Kinetic parameters were obtained from saturation plots as specified in “Materials and Methods.”
| np-Ga3PDHase | Wild Type | S404A | S447A |
| NADP+ | |||
| S0.5 (μm) | 10.8 ± 0.4 | 12.0 ± 0.6 | 14.1 ± 0.3 |
| Vmax (U/mg) | 22 ± 1 | 57 ± 5 | 46 ± 3 |
| nH | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Ga3P | |||
| S0.5 (μm) | 116 ± 6 | 134 ± 8 | 136 ± 5 |
| Vmax (U/mg) | 24 ± 2 | 50 ± 4 | 47 ± 3 |
| nH | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
Figure 9.
Identification of the Ser residue phosphorylated in np-Ga3PDHase. Phosphorylation reactions were performed using wheat endosperm extract as kinase resource in specific phosphorylation conditions for Ca2+-independent SNF1-related protein kinase. Top panels, [32P]γ-ATP incorporation; bottom panels, Coomassie Brilliant Blue staining of the SDS-PAGE. Radioactivity was detected by autoradiography. Arrows indicate the wild type (WT), S404A, and S447A np-Ga3PDHases.
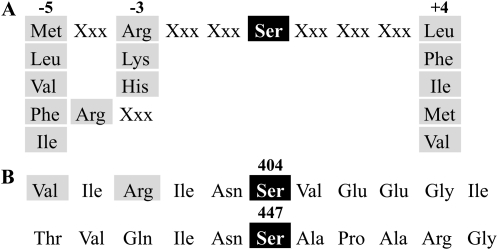
Protein kinases exhibit a moderately high degree of specificity for the sequence surrounding the target phosphorylatable amino acid in their substrates. Phosphorylation domains in various proteins have been elucidated by amino acid sequences alignment as well as by the use of synthetic peptides serving as alternative substrates for different protein kinases (Boyer and Krebs, 1986). Figure 10 details a recognition motif established as typical of SnRK1 action (Weekes et al., 1993) in comparison with putative sites for phosphorylation found in np-Ga3PDHase. The recognition motif comprises the target Ser residue (SnRK1 can also phosphorylate Thr but much less efficiently than Ser) surrounded by hydrophobic residues at positions −5 and +4, plus at least one basic residue that could be at position −3 or −4 (Fig. 10A; Halford et al., 2003). As shown in Figure 10B, a comparison of amino acid sequences surrounding Ser-404 and Ser-447 in wheat np-Ga3PDHase indicates that the former region matches better than that of Ser-447 with a motif of SnRK1 action. Specifically, Ser-404 is surrounded by a Val (position −5) and an Arg (position −3), clearly different from Thr and Gln residues neighboring Ser-447 at the same respective positions. Also, a Gly is found at position +4 of Ser-404, which is much closer to the hydrophobic group identified in the recognition motif than the basic Arg residue that surrounds Ser-447. Thus, the sequence analysis of putative phosphorylation domains in wheat np-Ga3PDHase is in agreement with the above-mentioned results with site-directed mutant enzymes.
Figure 10.
Alignment of amino acid sequences surrounding Ser residues phosphorylated by SnRK1 in different proteins. A, Consensus sequence for phosphorylation by SnRK1 (Halford et al., 2003). B, Amino acids surrounding Ser-404 and Ser-447 on np-Ga3PDHase, the two probable phosphorylation sites determined by molecular modeling (Bustos and Iglesias, 2005). Residues required for recognition are highlighted in gray.
DISCUSSION AND CONCLUDING REMARKS
SnRK1s are plant protein kinases with a catalytic domain similar to that of SNF1 (Suc nonfermenting-1) kinase of yeast and AMPK (AMP-activated protein kinase) of animals (Halford et al., 2003). SNF1/AMPK/SnRK1 are considered to be crucial elements of transcriptional, metabolic, and developmental regulation in response to stress (Polge and Thomas, 2007). In plants, SnRK1s were found to play key roles as major integrators of energy signaling and growth (Baena-González et al., 2007). Various enzymes of the carbon and nitrogen metabolism in plants are substrates of SnRK1, as in, for example, SPSase (Huber and Huber, 1992, 1996; Halford et al., 2003), nitrate reductase (Huber et al., 1992, 1994; Kaiser and Huber, 2001), 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Sugden et al., 1999b), trehalose-P synthase 5 (Harthill et al., 2006), and the bifunctional enzyme 6-phosphofructo-2-kinase/Fru-2,6-bisphosphatase responsible for determining levels of the signaling metabolite Fru2,6bisP (Kulma et al., 2004). From results detailed in this work, np-Ga3PDHase constitutes a new target for regulation governed by SnRK1, which matches correctly with a key functional relevance of the enzyme in carbon metabolism and partitioning in the cytosol of plant cells. The kinase was partially purified and identified as a SnRK1, having a strict dependence of Mg2+ (or Mn2+) but being independent of Ca2+ for activity or regulation.
In its native state, the protein kinase from wheat endosperm exhibited a molecular mass corresponding to plant SnRK1 enzymes, whereas by western blot from SDS-PAGE, it was identified as a protein band having a peptide also found in SnRK1 from sorghum and maize seeds. In our hands, such a protein exhibited a molecular mass of approximately 10 kD higher than that reported for other plant SnRK1s. Data available from wheat, including EST collection and also two clones reporting SnRK1 full sequence (see BT009004.1 wheat clone wdk2c.pk018.c16:fis, full insert mRNA sequence, and AK333437.1 wheat cDNA, clone WT006_H14, cv Chinese Spring) support a high similarity of the wheat protein kinase with that from other plants. Thus, an explanation for the slightly dissimilar size we found is not straightforward, and we speculate that it could be related to specific conditions used in SDS-PAGE or with different degrees of SnRK1 phosphorylation (Sugden et al., 1999a; Crozet et al., 2010) that could affect its electrophoretic mobility. It has been proposed that the SnRK family in plants has diverged and expanded into three subfamilies: SnRK1, SnRK2, and SnRK3 (Halford and Hey, 2009); but an all inclusive picture to clearly differentiate the different subgroups is far from being complete. Results in this work strongly support that the kinase phosphorylating np-Ga3PDHase in wheat endosperm and shoots is highly related with plant SnRK1. So far, because this enzyme exhibited some distinctive characteristics, a more refined cataloguing remains uncertain and requires further studies on the plant protein kinases.
Interestingly, we found that posttranslational modification of np-Ga3PDHase by SnRK1 takes place in endosperm, but it does not occur in the leaf. This differential regulation in the nonphotosynthetic tissue agrees with previous reports related to the occurrence of the target enzyme in a phosphorylated form in wheat endosperm and shoots (Bustos and Iglesias, 2002, 2003). Although the differential regulation by protein kinases in the different kind of plant tissues has not yet been well defined, other cytosolic enzymes related to the glycolytic pathway were found to be regulated by phosphorylation in heterotrophic tissues. Thus, cytosolic pyruvate kinase was reported to be phosphorylated, subsequently ubiquitinated, and degraded by the proteasome in developing soybean seeds (Tang et al., 2003), while PEP carboxylase was found to be regulated by phosphorylation in endosperm of developing castor oil seeds (Tripodi et al., 2005). It was also determined that Ser-404 is specifically phosphorylated in wheat np-Ga3PDHase, with the residue localized in a domain matching the minimal phosphorylation motif (φ-x-basic-x-x-S/T) of SnRK1 action (Weekes et al., 1993; Huang and Huber, 2001; Halford et al., 2003; Hardin et al., 2003). However, the structural characteristics of the phosphorylation domain suppose a site suboptimal for phosphorylation by a typical SnRK1, from which some relationship of the wheat kinase with the SnRK2 subfamily cannot be completely discarded, with this remaining as an open question for future studies. Furthermore, the domain containing Ser-404 site is predicted as a putative motif for interaction with 14-3-3 proteins after phosphorylation (Bustos and Iglesias, 2005). It is remarkable that the specific motif in the enzyme is a differential target recognized by SnRK1 present in wheat endosperm but not for the same type of kinase from the leaf.
Under the current view, it can be proposed that in heterotrophic plant cells, a specific SnRK1 modifies np-Ga3PDHase and afterward the phosphorylated enzyme interacts with 14-3-3 regulatory proteins, forming a complex disrupted by Mg2+ (Bustos and Iglesias, 2003). When forming the complex with 14-3-3, np-Ga3PDHase is less active and more sensitive to regulation by adenylates and PPi. Thus, SnRK1 ultimately exerts a regulation that is the rationale behind the cytosolic scenario of higher plant cells, where little or no soluble levels of PPi, together with the presence of PPi-dependent phosphofructokinase (catalyzing a reversible reaction and mainly found in heterotrophic plant cells) result in a significant accumulation of PPi (Plaxton, 1996; Sttit, 1998). Thus, not only ATP but also PPi levels are related with energy content in plant tissues (Sttit, 1998). Increases in ATP and/or PPi constitute a signal for high energy content in the cytosol of nonphotosynthetic cells that, in combination with low levels of divalent cations, would produce a fine regulation of np-Ga3PDHase bound to 14-3-3 leading to a marked inhibition of its activity (Bustos and Iglesias, 2003). Regulation would affect carbon metabolism but markedly would use triose-P to produce ATP and/or NADPH in the cytosol.
A further level of carbon metabolism regulation in the cytosol of heterotrophic plant cells can be inferred from the sensitivity to metabolites found for the SnRK1 that phosphorylates np-Ga3PDHase. In fact, SnRK1 partially purified from wheat endosperm exhibited specificity toward metabolites exerting its inhibition. Rib5P was a potent allosteric inhibitor, acting at micromolar concentrations, followed by 3PGA and Fru1,6bisP that inhibited in the millimolar range, whereas the endosperm kinase exhibited low affinity to Glc6P. The latter is remarkably different with the properties of SnRK1 from spinach leaves, which has Glc6P as the main allosteric inhibitor, followed by Fru1,6bisP, Man6P, and Glc1P as minor effectors, but being essentially insensitive to Rib5P and 3PGA (Toroser et al., 2000). This comparison suggests that SnRK1 could be allosterically regulated by different metabolites according to the trophic characteristics (auto- or heterotrophic) and/or the metabolic state of the plant tissue. Moreover, we found that the partially purified SnRK1 was insensitive to regulation by Tre6P, a result somehow contradictory with a recent article (Martínez-Barajas et al., 2011), indicating that the sugar signal inhibits SnRK1 in wheat grains. However, the authors report that the degree of the effect by Tre6P is different depending on the substrate used for the protein kinase and very dynamic during grain development. Thus, the conflicting results could be explained considering that we used wheat endosperm harvested 15 to 20 DPA, while Martínez-Barajas et al. (2011) showed a relatively high level of SnRK1 inhibition by Tre6P at 7 DPA (pregrain filling), which significantly decreased later, during the grain-filling period. Besides, the possibility of distinctive regulatory properties for the SnRK1 specifically phosphorylating np-Ga3PDHase that we characterize in this study should not be ruled out.
SNF1-related kinases conserved in all eukaryotes (SNF1/AMPK/SnRK1) exist as heterotrimeric complexes with a catalytic α-subunit and noncatalytic β- and γ-subunits, which appear to have roles in regulation and targeting of the complex (Hardie, 1999a). Until now, it is not well understood which of the subunits is involved in the allosteric regulation of the complex, but it has been suggested that it is noncompetitive with respect to ATP and that it occurs in a site distinct and physically separable from the catalytic site (Zhang et al., 2009). Regulation of SnRK1 also occurs at transcriptional level. It was reported that expression of genes coding for SnRK1 in sorghum and maize changes during seed development, reaching the higher level of expression during the sugar-to-starch metabolic transition of endosperm sink tissues (Jain et al., 2008). As a whole, the different allosteric properties of the protein kinase could be a consequence of the occurrence of distinctive enzymes and/or the expression of specific subunits being part of the functional protein.
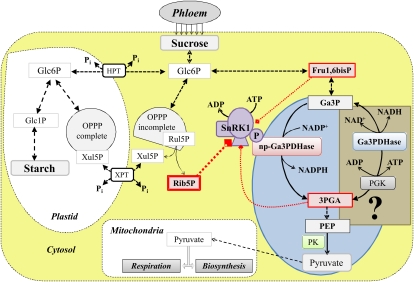
Regulation of np-Ga3PDHase by SnRK1 followed by interaction with 14-3-3 proteins has a foundation in the metabolic context of the heterotrophic cell of wheat endosperm (Bustos and Iglesias, 2002, 2003). In good agreement with the latter, Figure 11 schematizes an integrative view of the functional role of the specific SnRK1 involved in the process and the relationships established after its regulatory properties. The picture depicted by Figure 11 strongly agrees with the functions previously ascribed to SnRK1, mainly coordinating energy signaling in plants (Baena-González et al., 2007). These authors demonstrated that SnRK1 is activated in response to low carbon supply after catabolic and anabolic pathways are expressed and repressed, respectively. In such a scenario, SnRK1 would phosphorylate np-Ga3PDHase, thus lowering the production of NADPH required for reductive biosynthesis. Inhibition of the kinase by Fru1,6bisP and 3PGA may be functional because they are two main intermediates in the metabolic routes occurring in the cytosol of wheat endosperm cells. Also, the high sensitivity of SnRK1 toward Rib5P could be of importance, since the inhibitor is a key end product of the oxidative pentose-P pathway (OPPP), which occurs in an incomplete and a complete version in the cytosol and the plastid, respectively. Remarkably, the OPPP generates NADPH for synthetic reactions and provides precursors for nucleotide synthesis after the inhibition exerted by Rib5P fits very well with the above-described involvement of SnRK1 in repressing anabolism. Moreover, both forms of the OPPP are connected by a specific xylulose-5-P translocator of the plastid envelope (Fig. 11; Arias et al., 2011).
Figure 11.
Scheme representing the metabolic scenario in wheat endosperm involving np-Ga3PDHase and SnRK1. Suc transported through the phloem enters the heterotrophic cell to provide carbon and energy requirements. Glc6P, positioned in the middle, is a key point of carbon partitioning in wheat endosperm metabolism. Glc6P can be transported inside the plastid to fuel carbon reserve synthesis (mainly starch) or alternatively the OPPP metabolism. Alternatively, Glc6P can be metabolized in the cytosol by oxidation through the glycolytic pathway or an incomplete OPPP. Glycolysis cans supply energy and building blocks for anabolic routes as well as to fuel carbon for respiration inside the mitochondria. In the cytosolic pathway, triose-P partitioning is a key metabolic step where Ga3P can be used to produce ATP or NADPH. HPT, Hexose-P translocator of the plastid envelope; XPT, xylulose-5-P translocator of the plastid envelope; PGK, 3PGA kinase; Ga3PDHase, phosphorylating Ga3P dehydrogenase; PK, pyruvate kinase; SnRK1, SNF-1 related protein kinase 1.
Seed development is genetically programmed and is correlated with metabolite changes. The work accumulated after several years has shown that changes occurring during the different developmental stages involve a complex signaling network determined by sugars (Glc and Suc), the hormone abscisic acid, and SnRK1 kinases (Weber et al., 2005). This complex metabolic network could constitute an adaptive metabolic response to changing carbon sources and availability of energy and oxygen. The maintenance of subcellular compartmentalization during several developing phases is critical for the highly organized and controlled metabolic events, allowing redirection of carbon building blocks from catabolic (as glycolytic flux) to anabolic (as synthesis of reserve compounds) pathways. From previous data, plus the results detailed here, it is tempting to speculate that phosphorylation of np-Ga3PDHase by SnRK1 in wheat endosperm would occur during the stage of carbon reserve accumulation in developing wheat seeds. In such a period, the main fate for hexose-P, coming from SuSy-mediated Suc degradation, could be its transport into the plastid to fuel carbon reserve synthesis (mainly starch; Fig. 11). During this period, SnRK1 protein kinase could reach maximal activity levels, thus playing a key role in regulation and coordination of carbon metabolism. Active SnRK1 would phosphorylate np-Ga3PDHase, thus blocking oxidation of Ga3P to produce NADPH (see light-blue oval in Fig. 11), after which the triose-P could be conducted toward the synthesis of Glc6P and further to starch. Currently, it is not clear if the alternative pathway metabolizing Ga3P to produce NADH and ATP is under control (see light-brown rectangle in Fig. 11), which could complement the tracking of metabolites toward synthetic rather than to degrading routes. This work contributes to support the idea that triose-P use is a regulatory point in carbon metabolism of plant cells cytosol, and it affords results on the molecular mechanisms involved in such a regulation.
MATERIALS AND METHODS
Chemicals
Rabbit muscle aldolase, ATP, NADP+, Fru6P, Fru1,6bisP, Fru2,6bisP, 3PGA, Glc6P, Glc1P, Man6P, ADPGlc, UDPGlc, PEP, Glc, Suc, Rib5P, and Tre6P were purchased from Sigma-Aldrich. All other reagents were of the highest quality available.
Plant Material
Plant material was provided essentially as in previous studies (Bustos and Iglesias, 2002, 2003). Wheat (Triticum aestivum) seeds were collected from the field when they had reached 15 to 20 DPA, frozen immediately in liquid nitrogen, and stored at −80°C until required for experimentation. Leaves were obtained from wheat seeds grown at room temperature (22–25°C) in plastic boxes on two layers of filter paper soaked with sterile water in darkness to a shoot size of 2 to 3 cm. Then, approximately 1 cm of soil was added and the plants were grown in a natural light period to a leaf size of about 30 cm. At this time, leaves were collected, frozen immediately in liquid nitrogen, and stored at −80°C until required for experimentation. Shoots were developed from seeds for 2 d incubation in the dark in 5 mm Pi (pH 7.0)-buffered medium and used fresh.
Kinase Extraction and Recombinant np-Ga3PDHase Purification
Protein kinases extracts were prepared from frozen immature seeds separated from the ear or from frozen leaves; each tissue was ground to a fine powder in a liquid nitrogen-frozen mortar and pestle. Following that, kinase extraction buffer (50 mm HEPES-KOH, pH 7.5, 1 mm EDTA, 1 mm EGTA, 25 mm NaF, 0.1% [v/v] Triton X-100, 20% [v/v] glycerol, 10 mm MgCl2, 5 mm thiourea, 2 mm DTT, 2 mm PMSF, 5 mm malate, and 1% [w/v] polyvinyl polypyrrolidone) was used to extract total proteins (Turner et al., 2005). Total protein homogenates were centrifuged at 15,000g for 15 min at 4°C, and supernatants were either immediately used or stored at −80°C until required for experimentation.
Recombinant wheat np-Ga3PDHase was obtained from Escherichia coli BL21-CodonPlus(DE3)-RIL cells transformed with [pRSETB/TagapN], and the enzyme was highly purified by metal affinity chromatography (Hi-Trap Chelating HP; GE Healthcare) as previously described (Piattoni et al., 2010).
Site-Directed Mutagenesis
The QuikChange (Stratagene) site-directed mutagenesis method was used to introduce the S404A and S447A mutations in the TagapN gene. Two complementary primers, with the desired mutation in the middle of the sequence, were used for each mutant construction, using 10 ng of the [pRSETB/TagapN] plasmid as the template: fowS404A (5′-GTGATCAGGATCAACGCGGTTGAGGAAGGCATC-3′), revS404A (5′-GATGCCTTCCTCAACCGCGTTGATCCTGATCAC-3′), fowS447A (5′-CTGTGCAGATCAACGCCGCCCCGGCTCGAGG-3′), and revS447A (5′-CCTCGAGCCGGGGCGGCGTTGATCTGCACAG-3′).
PCR conditions consisted of 16 cycles at 95°C for 1 min, 55°C for 1 min, and 72°C for 10 min. E. coli Top 10 F’ (Invitrogen) cells were used for plasmids propagation and mutant selection. Desired mutations and the entire sequences of the np-Ga3PDHases were verified by double-stranded DNA sequencing. Mutant plasmids with the correct sequences were used for expression and purification as was described for the wild-type np-Ga3PDHase.
Protein Measurements
Total protein concentration was determined by the modified Bradford assay (Bradford, 1976) using bovine serum albumin as a standard.
np-Ga3PDHase Activity Assay and Kinetics Studies
Enzyme activity assay was performed as previously described (Gómez Casati et al., 2000). Reaction mixture (50 μL) contained (unless otherwise specified) 50 mm Tricine-NaOH, pH 8.5, 0.11 mm NADP+, 0.5 units of aldolase (rabbit muscle), 2.4 mm Fru1,6bisP, and an adequate quantity of enzyme. The reaction was started with the addition of Fru1,6bisP. NADPH generation was monitored spectrophotometrically at 30°C and 340 nm. One unit (U) is defined as the amount of enzyme that catalyzes the formation of 1 μmol NADPH per minute under the specified assay conditions.
Saturation curves were performed by assaying the respective enzyme activity at saturating level of the fixed substrate and different concentrations of the variable substrate. S0.5 values and Hill coefficients (nH) for NADP+ and d-Ga3P were obtained fitting the experimental data to the generalized Hill equation by a nonlinear least-squares regression kinetics computer program (Brooks, 1992). All kinetic parameters are the mean of at least three determinations and are reproducible within at least ±10%.
Phosphorylation Assay
Phosphorylation of the proteins was achieved by incubating 1 μg of recombinant np-Ga3PDHase wild type, S404A, S447A, or MBP (Sigma-Aldrich) under optimal phosphorylation conditions for different plant protein kinases. Specific phosphorylation conditions were as follows: Ca2+-independent SNF1-related protein kinase (WPK4; 20 mm Tris-HCl, pH 8.0, 1 mm EDTA, 0.1 mm PMSF, 20 mm MgCl2, and 100 μm ATP at 25°C; Ikeda et al., 1999); SOS2 (20 mm Tris-HCl, pH 7.2, 5 mm MgCl2, 0.5 mm CaCl2, 2 mm DTT, and 10 μm ATP at 30°C; Gong et al., 2002); Glycogen synthase kinase 3 (GSK3; 20 mm HEPES-KOH, pH 7.4, 15 mm MgCl2, 5 mm EGTA, 1 mm DTT, and 10 μm ATP at 25°C; Jonak et al., 2000); MAPK (25 mm Tris-MES, pH 7.5, 12 mm MgCl2, 2 mm EGTA, 1 mm DTT, and 25 μm ATP at 30°C; Lalle et al., 2005); Casein Kinase II (CKII; 10 mm Tris-HCl, pH 7.4, 50 mm KCl, 10 mm MgCl2, and 100 μm ATP at 30°C; Jeong et al., 2004); TOUSLED nuclear protein kinase (Tsl; 50 mm HEPES-KOH, pH 7.6, 150 mm NaCl, 10 mm MgCl2, 2 mm MnCl2, and 100 μm ATP at 25°C; Roe et al., 1997); CDPK (25 mm Tris-HCl, pH 7.5, 0.5 mm DTT, 10 mm MgCl2, 0.1 mm CaCl2, and 50 μm ATP at 30°C; Zhang et al., 2005).
Unless otherwise specified, enzymatic reactions were performed in a final volume of 20 μL with 2 μCi of [32P]γ-ATP (Perkin-Elmer) and were initiated by adding wheat endosperm or leaves extract as kinase resource. Alternatively, partially purified SnRK1 from wheat endosperm was used as kinase. After reaction, resolution of the protein mixtures was reached by protein electrophoresis under denatured conditions, carried out on discontinuous 12% polyacrylamide gels (SDS-PAGE) as described previously (Laemmli, 1970). For synthetic SAMS, AMARA (Ana Spec), and SP46 (Genebiotech) peptide phosphorylation assays, resolution of the sample mixtures was reached using Tricine-SDS-PAGE conditions (Schägger, 2006) carried out on precast 4% to 20% polyacrylamide gradient gels (Invitrogen).
For detection of radioactivity incorporation, the gels were stained with Coomassie Brilliant Blue R-250, dried, and autoradiographed on x-ray films (Kodak) at −80°C for 16 h. As an alternative to x-ray films, radioactivity incorporation was detected by storing phosphor screen (GE Healthcare) exposure and scanning with Typhoon system (GE Healthcare).
Wheat Endosperm SNF1-Related Protein Kinase Partial Purification
Partial purification of wheat endosperm SNF1-related protein kinase was performed as previously described (Toroser et al., 2000). Frozen wheat endosperms were ground in a chilled mortar. Twenty-five grams fresh weight was extracted in 100 mL of extraction buffer containing 50 mm MOPS-NaOH, pH 7.5, 2 mm EGTA, 2 mm EDTA, 5 mm DTT, 0.5 mm PMSF, 25 mm NaF, and 0.1% (v/v) Triton X-100. The homogenates were centrifuged at 10,000g for 15 min. To the supernatant, PEG8000 was added from a 50% (w/v) solution to give an initial concentration of 3% (w/v). After stirring for 10 min, the solution was centrifuged at 10,000g for 10 min and the pellet discarded. The supernatant was then adjusted to 20% (w/v) PEG8000 and stirred for 15 min. The precipitated protein pellet was harvested by centrifugation at 10,000g for 15 min and solubilized (0.5 mL/g tissue used) in buffer containing 50 mm MOPS-NaOH, pH 7.5, 2 mm EGTA, 2 mm EDTA, 5 mm Na4P2O7, 5 mm NaF, and 2.5 mm DTT. The solution was finally clarified (by centrifugation at 10,000g for 10 min) and the supernatant applied to a 2-mL Q-Sepharose column (Amersham Pharmacia Biotech). The column was washed with 20 bed volumes of buffer A (50 mm MOPS-NaOH, pH 7.5, and 1 mm DTT). The bound proteins were eluted with a 70-mL linear gradient from 0 to 500 mm NaCl in buffer A. Fractions were collected and assayed for protein kinase activity as outlined above using the purified np-Ga3PDHase as a substrate. Following purification, active fractions were supplemented with 10 mm DTT and stored at −80°C and used within a few days after preparation.
SnRK-Specific Activity Determination
Specific activity of wheat endosperm SnRK was measured by following the incorporation of radioactivity from [32P]γ-ATP on np-Ga3PDHase. Phosphorylation reactions (20 μL) under saturating conditions were performed in 20 mm Tris-HCl, pH 8.0, 1 mm EDTA, 0.1 mm PMSF, 10 mm MgCl2, and 100 μm ATP (containing 2 μCi [32P]γ-ATP per reaction) at 25°C. Supplemental Figure S1 shows experiments developed to establish quantitative conditions for measuring initial velocities of the kinase under study. Thus, phosphorylation assays were run at variable quantities of the partially purified kinase or its protein substrate np-Ga3PDHase (Supplemental Fig. S1A). Once we selected 0.4 μg of partially purified SnRK and a saturating amount (4 μg) of np-Ga3PDHase, we performed a time course of phosphorylation to determine lineal reaction conditions (Supplemental Fig. S1B). After each reaction, protein mixtures were resolved by SDS-PAGE. For quantification of 32P incorporation on np-Ga3PDHase, the gels were stained with Coomassie Brilliant Blue R-250 and dried; afterward, the np-Ga3PDHase bands were sliced, disposed in 2-mL Eppendorf tubes, added with 1 mL of scintillation cocktail OPTIPHASE HISAFE 3 (Perkin-Elmer), and measured for radioactivity in a scintillation counter Triathler LSC (HIDEX). Lineal conditions determined from the time course in Supplemental Figure S1B were used for kinetic studies of the partially purified wheat SnRK1.
Western Blotting, Gel Filtration, and MALDI-TOF/TOF Analysis
Western blotting was achieved by transferring the SDS-PAGE gel to nitrocellulose membranes using a semidry blot system (Bio-Rad). Immunodetection was carried out according to Jain et al. (2008) using the SnRK1 antiserum raised against synthetic peptide GTRNYVPGSSDPHSSGLRPYY (spanning a region conserved between sorghum [Sorghum bicolor] and maize [Zea mays] SnRK1 proteins), which was generously provided by Prem S. Chourey (University of Florida, Gainesville, FL). Primary antibodies were incubated with the membrane during 16 h at 4°C under agitation. For detection, horseradish-linked antirabbit IgG secondary antibody and 3,30-diaminobenzidine (Sigma-Aldrich) were used.
Gel filtration chromatography on Superdex 200 HR was attained in a Tricorn 5/200 column using AKTA Explorer equipment (GE-Healthcare). Column calibration was performed using the specific kit from GE-Healthcare containing the following protein standards: thyroglobulin (669 kD), ferritin (440 kD), aldolase (158 kD), conalbumin (75 kD), and ovoalbumin (44 kD). The column void volume was determined using Blue Dextran loading solution (Promega).
For MALDI-TOF/TOF studies, the corresponding protein band was sliced from the SDS-PAGE gel stained with Coomassie Brilliant Blue and sent to the Pasteur Institute in Montevideo (Uruguay) for processing tandem mass spectrometry analysis.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BT009004.1 (wdk2c.pk018.c16:fis) and AK333437.1 (WT006_H14, cv Chinese Spring).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Determination of wheat endosperm SnRK specific activity.
Supplemental Figure S2. Effect of Rib5P and Tre6P on wheat endosperm SnRK1 activity.
Supplementary Material
Acknowledgments
We thank the courtesy of Dr. Prem S. Chourey (U.S. Department of Agriculture Research Service and University of Florida, Gainesville, FL), who provided antiserum-recognizing peptide regions present in plant SnRK1. C.V.P. is a postdoctoral fellow from Consejo Nacional de Investigaciones Científicas y Técnicas, and D.M.B., S.A.G., and A.A.I. are members of the investigator career from the same institution.
References
- Arias DG, Piattoni CV, Guerrero SA, Iglesias AA. (2011) Biochemical mechanisms for the maintenance of oxidative stress under control in plants. Pessarakli M, , Handbook of Plant and Crop Stress, Ed 3 Taylor and Francis Group, Boca Raton, FL, pp 157–190 [Google Scholar]
- Arnon DI, Rosenberg LL, Whatley FR. (1954) A new glyceraldehyde phosphate dehydrogenase from photosynthetic tissues. Nature 173: 1132–1134 [PubMed] [Google Scholar]
- Baena-González E, Rolland F, Thevelein JM, Sheen J. (2007) A central integrator of transcription networks in plant stress and energy signalling. Nature 448: 938–942 [DOI] [PubMed] [Google Scholar]
- Boyd DA, Cvitkovitch DG, Hamilton IR. (1995) Sequence, expression, and function of the gene for the nonphosphorylating, NADP-dependent glyceraldehyde-3-phosphate dehydrogenase of Streptococcus mutans. J Bacteriol 177: 2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer PD, Krebs EG.editors (1986) The Enzymes: Control by Phosphorylation, Ed 3, Vol 17 Academic Press, Orlando, FL [Google Scholar]
- Bradford KJ, Downie AB, Gee OH, Alvarado V, Yang H, Dahal P. (2003) Abscisic acid and gibberellin differentially regulate expression of genes of the SNF1-related kinase complex in tomato seeds. Plant Physiol 132: 1560–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brooks SPJ. (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13: 906–911 [PubMed] [Google Scholar]
- Brown AT, Wittenberger CL. (1971) The occurrence of multiple glyceraldehyde-3-phosphate dehydrogenases in cariogenic streptococci. Biochem Biophys Res Commun 43: 217–224 [DOI] [PubMed] [Google Scholar]
- Brunner NA, Hensel R. (2001) Nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase from Thermoproteus tenax. Methods Enzymol 331: 117–131 [DOI] [PubMed] [Google Scholar]
- Bustos DM, Iglesias AA. (2002) Non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase is post-translationally phosphorylated in heterotrophic cells of wheat (Triticum aestivum). FEBS Lett 530: 169–173 [DOI] [PubMed] [Google Scholar]
- Bustos DM, Iglesias AA. (2003) Phosphorylated non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from heterotrophic cells of wheat interacts with 14-3-3 proteins. Plant Physiol 133: 2081–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos DM, Iglesias AA. (2005) A model for the interaction between plant GAPN and 14-3-3zeta using protein-protein docking calculations, electrostatic potentials and kinetics. J Mol Graph Model 23: 490–502 [DOI] [PubMed] [Google Scholar]
- Champion A, Kreis M, Mockaitis K, Picaud A, Henry Y. (2004) Arabidopsis kinome: after the casting. Funct Integr Genomics 4: 163–187 [DOI] [PubMed] [Google Scholar]
- Crozet P, Jammes F, Valot B, Ambard-Bretteville F, Nessler S, Hodges M, Vidal J, Thomas M. (2010) Cross-phosphorylation between Arabidopsis thaliana sucrose nonfermenting 1-related protein kinase 1 (AtSnRK1) and its activating kinase (AtSnAK) determines their catalytic activities. J Biol Chem 285: 12071–12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale S, Arró M, Becerra B, Morrice NG, Boronat A, Hardie DG, Ferrer A. (1995) Bacterial expression of the catalytic domain of 3-hydroxy-3-methylglutaryl-CoA reductase (isoform HMGR1) from Arabidopsis thaliana, and its inactivation by phosphorylation at Ser577 by Brassica oleracea 3-hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem 233: 506–513 [DOI] [PubMed] [Google Scholar]
- Davies SP, Carling D, Hardie DG. (1989) Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem 186: 123–128 [DOI] [PubMed] [Google Scholar]
- Douglas P, Pigaglio E, Ferrer A, Halfords NG, MacKintosh C. (1997) Three spinach leaf nitrate reductase-3-hydroxy-3-methylglutaryl-CoA reductase kinases that are required by reversible phosphorylation and/or Ca2+ ions. Biochem J 325: 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso S, Espindola L, Páez-Valencia J, Gamboa A, Camacho Y, Martínez-Barajas E, Coello P. (2009) SnRK1 isoforms AKIN10 and AKIN11 are differentially regulated in Arabidopsis plants under phosphate starvation. Plant Physiol 149: 1906–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Loescher WH. (2000) NADPH supply and mannitol biosynthesis. Characterization, cloning, and regulation of the non-reversible glyceraldehyde-3-phosphate dehydrogenase in celery leaves. Plant Physiol 124: 321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givan CV. (1999) Evolving concepts in plant glycolysis: two centuries of progress. Biol Rev Camb Philos Soc 74: 277–309 [Google Scholar]
- Gómez Casati DF, Sesma JI, Iglesias AA. (2000) Structural and kinetic characterization of NADP-dependent, non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from celery leaves. Plant Sci 154: 107–115 [DOI] [PubMed] [Google Scholar]
- Gong D, Guo Y, Jagendorf AT, Zhu JK. (2002) Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance. Plant Physiol 130: 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A. (1997) The non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase: biochemistry, structure, occurrence and evolution. Biol Chem 378: 1413–1419 [PubMed] [Google Scholar]
- Halford NG, Hey S, Jhurreea D, Laurie S, McKibbin RS, Paul M, Zhang Y. (2003) Metabolic signalling and carbon partitioning: role of Snf1-related (SnRK1) protein kinase. J Exp Bot 54: 467–475 [DOI] [PubMed] [Google Scholar]
- Halford NG, Hey SJ. (2009) Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419: 247–259 [DOI] [PubMed] [Google Scholar]
- Hardie DG. (1999a) Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol 50: 97–131 [DOI] [PubMed] [Google Scholar]
- Hardie DG. (1999b) Roles of the AMP-activated/SNF1 protein kinase family in the response to cellular stress. Biochem Soc Symp 64: 13–27 [PubMed] [Google Scholar]
- Hardin SC, Tang GQ, Scholz A, Holtgraewe D, Winter H, Huber SC. (2003) Phosphorylation of sucrose synthase at serine 170: occurrence and possible role as a signal for proteolysis. Plant J 35: 588–603 [DOI] [PubMed] [Google Scholar]
- Harthill JE, Meek SE, Morrice N, Peggie MW, Borch J, Wong BH, Mackintosh C. (2006) Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J 47: 211–223 [DOI] [PubMed] [Google Scholar]
- Huang J-Z, Huber SC. (2001) Phosphorylation of synthetic peptides by a CDPK and plant SNF1-related protein kinase. Influence of proline and basic amino acid residues at selected positions. Plant Cell Physiol 42: 1079–1087 [DOI] [PubMed] [Google Scholar]
- Huber JL, Huber SC. (1992) Site-specific serine phosphorylation of spinach leaf sucrose-phosphate synthase. Biochem J 283: 877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JL, Huber SC, Campbell WH, Redinbaugh MG. (1992) Reversible light/dark modulation of spinach leaf nitrate reductase activity involves protein phosphorylation. Arch Biochem Biophys 296: 58–65 [DOI] [PubMed] [Google Scholar]
- Huber JL, Redinbaugh MG, Huber SC, Campbell WH. (1994) Regulation of maize leaf nitrate reductase activity involves both gene expression and protein phosphorylation. Plant Physiol 106: 1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Huber JL. (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47: 431–444 [DOI] [PubMed] [Google Scholar]
- Iddar A, Valverde F, Assobhei O, Serrano A, Soukri A. (2005) Widespread occurrence of non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase among gram-positive bacteria. Int Microbiol 8: 251–258 [PubMed] [Google Scholar]
- Iglesias AA. (1990) On the metabolism of triose-phosphates in photosynthetic cells. Their involvement on the traffic of ATP and NADPH. Biochem Educ 18: 1–4 [Google Scholar]
- Iglesias AA, Losada M. (1988) Purification and kinetic and structural properties of spinach leaf NADP-dependent nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys 260: 830–840 [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Serrano A, Guerrero MG, Losada M. (1987) Purification and properties of NADP-dependent glyceraldehyde-3-phosphate dehydrogenase from green alga Chlamydomonas reinhardtii. Biochim Biophys Acta 925: 1–10 [Google Scholar]
- Ikeda Y, Koizumi N, Kusano T, Sano H. (1999) Sucrose and cytokinin modulation of WPK4, a gene encoding a SNF1-related protein kinase from wheat. Plant Physiol 121: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Li QB, Chourey PS. (2008) Cloning and expression analyses of sucrose non-fermenting-1-related kinase 1 (SnRK1b) gene during development of sorghum and maize endosperm and its implicated role in sugar-to-starch metabolic transition. Physiol Plant 134: 161–173 [DOI] [PubMed] [Google Scholar]
- Jeong SY, Peffer N, Meier I. (2004) Phosphorylation by protein kinase CKII modulates the DNA-binding activity of a chloroplast nucleoid-associated protein. Planta 219: 298–302 [DOI] [PubMed] [Google Scholar]
- Jonak C, Beisteiner D, Beyerly J, Hirt H. (2000) Wound-induced expression and activation of WIG, a novel glycogen synthase kinase 3. Plant Cell 12: 1467–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser WM, Huber SC. (2001) Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. J Exp Bot 52: 1981–1989 [DOI] [PubMed] [Google Scholar]
- Kelly GJ, Gibbs M. (1973) A mechanism for the indirect transfer of photosynthetically reduced nicotinamide adenine dinucleotide phosphate from chloroplasts to the cytoplasm. Plant Physiol 52: 674–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulma A, Villadsen D, Campbell DG, Meek SE, Harthill JE, Nielsen TH, MacKintosh C. (2004) Phosphorylation and 14-3-3 binding of Arabidopsis 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. Plant J 37: 654–667 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lalle M, Visconti S, Marra M, Camoni L, Velasco R, Aducci P. (2005) ZmMPK6, a novel maize MAP kinase that interacts with 14-3-3 proteins. Plant Mol Biol 59: 713–722 [DOI] [PubMed] [Google Scholar]
- Mackintosh RW, Davies SP, Clarke PR, Weekes J, Gillespie JG, Gibb BJ, Hardie DG. (1992) Evidence for a protein kinase cascade in higher plants. 3-Hydroxy-3-methylglutaryl-CoA reductase kinase. Eur J Biochem 209: 923–931 [DOI] [PubMed] [Google Scholar]
- Martínez-Barajas E, Delatte T, Schluepmann H, de Jong GJ, Somsen GW, Nunes C, Primavesi LF, Coello P, Mitchell RAC, Paul MJ. (2011) Wheat grain development is characterized by remarkable trehalose 6-phosphate accumulation pre-grain filling: tissue distribution and relationship to SNF1-related protein kinase1 activity. Plant Physiol 156: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos MI, Serrano A. (1992) Occurrence of phosphorylating and non-phosphorylating NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases in photosynthetic organisms. Plant Sci 84: 163–170 [Google Scholar]
- McMichael RW, Jr, Bachmann M, Huber SC. (1995a) Spinach leaf sucrose-phosphate synthase and nitrate reductase are phosphorylated/inactivated by multiple protein kinases in vitro. Plant Physiol 108: 1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael RW, Jr, Kochansky J, Klein RR, Huber SC. (1995b) Characterization of the substrate specificity of sucrose-phosphate synthase protein kinase. Arch Biochem Biophys 321: 71–75 [DOI] [PubMed] [Google Scholar]
- Piattoni CV, Rius SP, Gomez-Casati DF, Guerrero SA, Iglesias AA. (2010) Heterologous expression of non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from Triticum aestivum and Arabidopsis thaliana. Biochimie 92: 909–913 [DOI] [PubMed] [Google Scholar]
- Plaxton WC. (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47: 185–214 [DOI] [PubMed] [Google Scholar]
- Polge C, Thomas M. (2007) SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci 12: 20–28 [DOI] [PubMed] [Google Scholar]
- Pupillo P, Faggiani R. (1979) Subunit structure of three glyceraldehyde 3-phosphate dehydrogenases of some flowering plants. Arch Biochem Biophys 194: 581–592 [DOI] [PubMed] [Google Scholar]
- Rius SP, Casati P, Iglesias AA, Gomez-Casati DF. (2006) Characterization of an Arabidopsis thaliana mutant lacking a cytosolic non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase. Plant Mol Biol 61: 945–957 [DOI] [PubMed] [Google Scholar]
- Roe JL, Durfee T, Zupan JR, Repetti PP, McLean BG, Zambryski PC. (1997) TOUSLED is a nuclear serine/threonine protein kinase that requires a coiled-coil region for oligomerization and catalytic activity. J Biol Chem 272: 5838–5845 [DOI] [PubMed] [Google Scholar]
- Rudrabhatla P, Reddy MM, Rajasekharan R. (2006) Genome-wide analysis and experimentation of plant serine/threonine/tyrosine-specific protein kinases. Plant Mol Biol 60: 293–319 [DOI] [PubMed] [Google Scholar]
- Rumpho ME, Edwards GE, Loescher WH. (1983) A pathway for photosynthetic carbon flow to mannitol in celery leaves: activity and localization of key enzymes. Plant Physiol 73: 869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H. (2006) Tricine-SDS-PAGE. Nat Protoc 1: 16–22 [DOI] [PubMed] [Google Scholar]
- Stone JM, Walker JC. (1995) Plant protein kinase families and signal transduction. Plant Physiol 108: 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sttit M. (1998) Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta 111: 167–175 [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. (1999a) Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5′-AMP. Plant J 19: 433–439 [DOI] [PubMed] [Google Scholar]
- Sugden C, Donaghy PG, Halford NG, Hardie DG. (1999b) Two SNF1-related protein kinases from spinach leaf phosphorylate and inactivate 3-hydroxy-3-methylglutaryl-coenzyme A reductase, nitrate reductase, and sucrose phosphate synthase in vitro. Plant Physiol 120: 257–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GQ, Hardin SC, Dewey R, Huber SC. (2003) A novel C-terminal proteolytic processing of cytosolic pyruvate kinase, its phosphorylation and degradation by the proteasome in developing soybean seeds. Plant J 34: 77–93 [DOI] [PubMed] [Google Scholar]
- Toroser D, Huber SC. (1997) Protein phosphorylation as a mechanism for osmotic-stress activation of sucrose-phosphate synthase in spinach leaves. Plant Physiol 114: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toroser D, Plaut Z, Huber SC. (2000) Regulation of a plant SNF1-related protein kinase by glucose-6-phosphate. Plant Physiol 123: 403–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripodi KE, Turner WL, Gennidakis S, Plaxton WC. (2005) In vivo regulatory phosphorylation of novel phosphoenolpyruvate carboxylase isoforms in endosperm of developing castor oil seeds. Plant Physiol 139: 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WL, Knowles VL, Plaxton WC. (2005) Cytosolic pyruvate kinase: subunit composition, activity, and amount in developing castor and soybean seeds, and biochemical characterization of the purified castor seed enzyme. Planta 222: 1051–1062 [DOI] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. (2005) Molecular physiology of legume seed development. Annu Rev Plant Biol 56: 253–279 [DOI] [PubMed] [Google Scholar]
- Weekes J, Ball KL, Caudwell FB, Hardie DG. (1993) Specificity determinants for the AMP-activated protein kinase and its plant homologue analysed using synthetic peptides. FEBS Lett 334: 335–339 [DOI] [PubMed] [Google Scholar]
- Zhang M, Liang S, Lu YT. (2005) Cloning and functional characterization of NtCPK4, a new tobacco calcium-dependent protein kinase. Biochim Biophys Acta 1729: 174–185 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149: 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.