Abstract
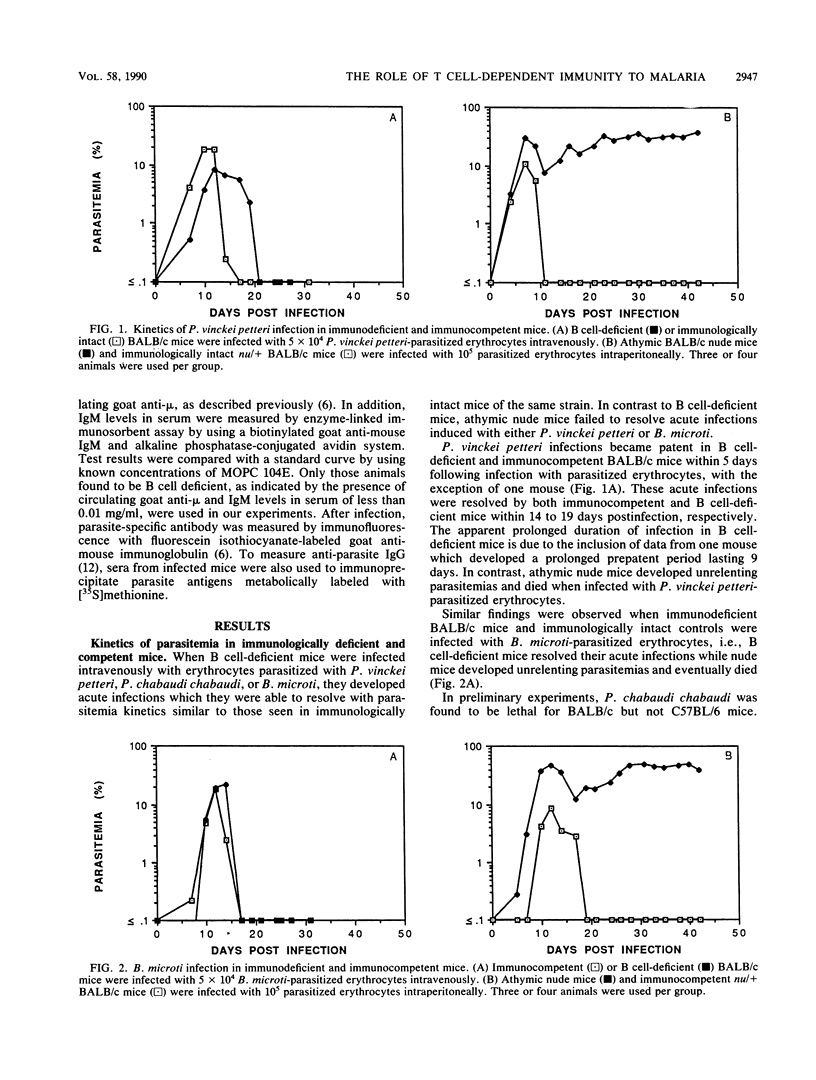
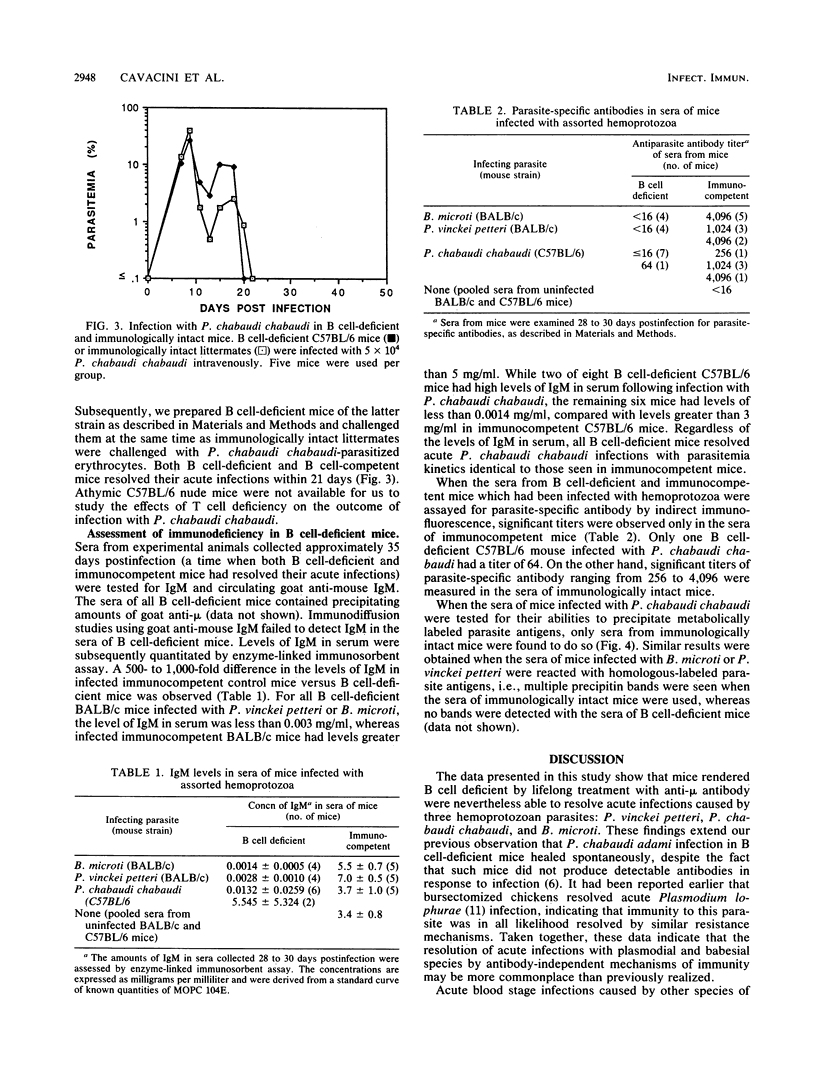
While it is generally accepted that acute blood stage malarial infections are resolved through the actions of protective antibodies, we observed that resistance to acute infection with Plasmodium chabaudi adami was mediated by T cell-dependent cellular immune mechanisms independent of antibody. We now report that acute blood stage infections caused by three additional murine hemoprotozoan parasites, Plasmodium vinckei petteri, Plasmodium chabaudi chabaudi, and Babesia microti, appear to be controlled by similar T cell-dependent mechanisms of immunity. Mice rendered B cell deficient by lifelong treatment with goat anti-mouse immunoglobulin M (IgM) had IgM levels in serum of less than 0.6 micrograms/ml and contained precipitating amounts of goat anti-mouse IgM. When these B cell-deficient mice were infected with blood stage P. vinckei petteri, P. chabaudi chabaudi, or B. microti, they resolved their infections with kinetics similar to those seen in immunologically intact mice. Infected B cell-deficient mice did not produce antiparasite antibodies. As assayed by immunofluorescence, significant titers of parasite-specific antibody were present only in the sera of infected immunocompetent mice. In addition, only sera from infected immunocompetent mice immunoprecipitated metabolically labeled parasite antigens. In contrast to B cell-deficient mice, athymic nude mice failed to resolve acute P. vinckei petteri or B. microti infections. These data suggest that antibody-independent, T cell-mediated immune mechanisms play a more significant role in resisting acute blood stage infections caused by hemoprotozoa than was recognized previously.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Brake D. A., Long C. A., Weidanz W. P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned T cell line. J Immunol. 1988 Mar 15;140(6):1989–1993. [PubMed] [Google Scholar]
- Brake D. A., Weidanz W. P., Long C. A. Antigen-specific, interleukin 2-propagated T lymphocytes confer resistance to a murine malarial parasite, Plasmodium chabaudi adami. J Immunol. 1986 Jul 1;137(1):347–352. [PubMed] [Google Scholar]
- Cavacini L. A., Guidotti M., Parke L. A., Melancon-Kaplan J., Weidanz W. P. Reassessment of the role of splenic leukocyte oxidative activity and macrophage activation in expression of immunity to malaria. Infect Immun. 1989 Dec;57(12):3677–3682. doi: 10.1128/iai.57.12.3677-3682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavacini L. A., Long C. A., Weidanz W. P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986 Jun;52(3):637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Jepsen S., Valerius N. H. Polymorphonuclear leucocytes defective in oxidative metabolism inhibit in vitro growth of Plasmodium falciparum. Evidence against an oxygen-dependent mechanism. Scand J Immunol. 1984 Jul;20(1):93–96. doi: 10.1111/j.1365-3083.1984.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Langford C. J., Anders R. F., Kemp D. J., Saul A., Fardoulys C., Geysen M., Sheppard M. A protective monoclonal antibody recognizes a linear epitope in the precursor to the major merozoite antigens of Plasmodium chabaudi adami. Proc Natl Acad Sci U S A. 1989 May;86(10):3768–3772. doi: 10.1073/pnas.86.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker B. M., Breitenbach R. P., Farmer J. N. The role of the bursa Fabricius, spleen and thymus in the control of a Plasmodium lophurae infection in the chicken. J Immunol. 1966 Nov;97(5):594–599. [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Parke L. M., Long C. A., Weidanz W. P. A method for freeing murine plasmodia of contaminating lactate dehydrogenase elevating virus. J Parasitol. 1986 Dec;72(6):956–958. [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]