Abstract
Cytosolic/nuclear molecular chaperones of the heat shock protein families HSP90 and HSC70 are conserved and essential proteins in eukaryotes. These proteins have essentially been implicated in the innate immunity and abiotic stress tolerance in higher plants. Here, we demonstrate that both chaperones are recruited in Arabidopsis (Arabidopsis thaliana) for stomatal closure induced by several environmental signals. Plants overexpressing HSC70-1 or with reduced HSP90.2 activity are compromised in the dark-, CO2-, flagellin 22 peptide-, and abscisic acid (ABA)-induced stomatal closure. HSC70-1 and HSP90 proteins are needed to establish basal expression levels of several ABA-responsive genes, suggesting that these chaperones might also be involved in ABA signaling events. Plants overexpressing HSC70-1 or with reduced HSP90.2 activity are hypersensitive to ABA in seed germination assays, suggesting that several chaperone complexes with distinct substrates might tune tissue-specific responses to ABA and the other biotic and abiotic stimuli studied. This study demonstrates that the HSC70/HSP90 machinery is important for stomatal closure and serves essential functions in plants to integrate signals from their biotic and abiotic environments.
The control of water vapor and carbon dioxide exchange between the mesophyll and the atmosphere is essential for plant growth and adaptation to varying environmental conditions and is mediated by modulating the aperture of pores at the leaf surface called stomata (Hetherington and Woodward, 2003). Those structures composed of two guard cells respond continuously to environmental signals such as light, CO2 concentration, and the plant hormone abscisic acid (ABA). While strong light and low CO2 concentrations favor stomatal aperture and thereby carbon fixation through photosynthesis, it also causes important water losses by transpiration. Thus, water deficit conditions will inhibit stomatal aperture and prevent excessive plant dehydration. ABA plays a central role in physiological processes, including the adaptation of vegetative tissues to water stresses as well as in seed maturation and dormancy. ABA will promote on the one hand a rapid stomatal closure that is mediated by solute efflux in the guard cells and on the other hand specific transcriptional responses for long-term adaptation to drought and dehydration tolerance in vegetative tissues and seeds. In seeds, ABA establishes dormancy and inhibits early seedling development and greening. Forward and reverse genetic analyses have led to the identification of many components that cover the ABA metabolism (Finkelstein et al., 2002; Nambara and Marion-Poll, 2005) and ABA signal transduction (Israelsson et al., 2006; Shinozaki and Yamaguchi-Shinozaki, 2007). Several intracellular ABA receptors such as PYR/PYL/RCAR protein family have recently been identified (Ma et al., 2009; Park et al., 2009; Santiago et al., 2009) and allowed the recapitulation of early ABA signaling events within a larger molecular complex composed of PYL/protein phosphatase 2C/SnRK2 (Suc nonfermenting related kinases2) at the atomic resolution (Melcher et al., 2009; Miyazono et al., 2009) as well as in vitro (Fujii et al., 2009). Upon ABA binding to PYL proteins, ABI1/ABI2/HAB1 protein phosphatase 2C activities are inhibited, resulting in a modified phosphorylation of SnRK2.2/3/6 and in the activation of SnRK2.2/3/6 kinase activities. This signaling module is essential for stomatal closure in response to ABA, for transcriptional responses to ABA, for drought tolerance, for seed dormancy, and for ABA-dependent inhibition of seedling development.
Besides controlling gas exchange, stomata also constitute natural entry sites for numerous foliar pathogens (Melotto et al., 2008). The first layer of the plant innate immune system is probably the stomatal closure upon perception of conserved pathogen-associated molecular patterns (PAMPs) since many open stomata mutants show enhanced susceptibility to pathogens (Melotto et al., 2006). For instance, the Pseudomonas syringae flagellin peptide flg22 induces rapid stomatal closure in wild-type Arabidopsis (Arabidopsis thaliana) but not in the SnRK2.6 mutant ost1 (Melotto et al., 2006). Thus, PAMP- and ABA-triggered stomatal closure use common signaling components in guard cells. As an attempt to evade this immune response, pathogens have evolved strategies to bypass/corrupt this signaling cascade (Emi et al., 2001; Melotto et al., 2006; Gudesblat et al., 2009).
Molecular chaperones are key components of innate immunity in mammals (Ting et al., 2008) and plants (Shirasu, 2009). These conserved proteins (usually heat shock proteins [HSPs]) are globally essential and define a balance of protein folding, assembly, and degradation in physiological as well as stress conditions (Wegele et al., 2004; Bukau et al., 2006). On the one hand, DnaK/HSP70 (70 kD) chaperones from prokaryotes/eukaryotes mediate ATP-dependent chaperoning of nascent polypeptides, protein addressing, and degradation by somewhat promiscuous interactions to solvent-exposed hydrophobic residues (Erbse et al., 2004; Wegele et al., 2004). On the other hand, HSP90 ATPases are much more selective in their recognition specificity, are essentially involved in protein maturation, and play essential functions in regulating numerous physiological responses (Young et al., 2001; Wegele et al., 2004). In Arabidopsis, there are 14 HSP70 (also named HSC70; heat shock cognate) genes, five of which (HSC70-1 to -5) encode functionally redundant and essential proteins localized in the cytosol and nuclei (Lin et al., 2001; Sung et al., 2001; Noël et al., 2007). There are seven HSP90 genes in Arabidopsis, four of which encode mostly redundant and essential proteins predicted to be cytosolic/nuclear (Krishna and Gloor, 2001; Hubert et al., 2009). Little is known about HSC70 and HSP90 physiological functions in plants since their essential roles during early embryogenesis have hampered their genetic analyses. The use of HSC70-1 overexpression (which results in general up-regulation of other HSC70 gene expression levels) and particular point mutant alleles of HSP90.2 were of particular interest. These genetic resources were instrumental to perform most of the functional analysis along with two mechanistically related inhibitors of HSP90 ATPase activity, such as geldanamycin and radicicol (Queitsch et al., 2002; Hubert et al., 2003; Sung and Guy, 2003; Takahashi et al., 2003; Noël et al., 2007; Cazalé et al., 2009). Besides the contribution of chaperones to plant innate immunity, HSP90s have been implicated in buffering genetic variation (Queitsch et al., 2002) and drought stress tolerance (Song et al., 2009) while HSC70s are important for meristem function and tolerance to heat shock, heavy metals, γ-rays, and salt (Noël et al., 2007; Cazalé et al., 2009). HSC70 and HSP90 are regulated by a complex network of cochaperones that modulate their enzymatic activities directly or spatially coordinate their functions. For instance, plant SGT1 (suppressor of G2/M transition allele of skp1), a conserved eukaryotic protein, presumably acts as a scaffold to bridge HSC70/HSP90 functions (Catlett and Kaplan, 2006) and is important for SCF E3 ubiquitin ligase-dependent signaling (Kitagawa et al., 1999; Gray et al., 2003), plant innate immunity, and heat shock tolerance (Austin et al., 2002; Noël et al., 2007; Uppalapati et al., 2011). In Arabidopsis, SGT1a and SGT1b encode two SGT1 proteins that are functionally redundant and globally essential (Austin et al., 2002; Takahashi et al., 2003). Because SGT1a is much less expressed than SGT1b in healthy tissues, the loss of SGT1a did not yield any mutant phenotypes but SGT1a overexpression complements all known sgt1b mutant phenotypes (Austin et al., 2002; Gray et al., 2003; Azevedo et al., 2006; Noël et al., 2007). In contrast to SGT1a and SGT1b that are important for auxin and jasmonic acid phytohormones signaling (Gray et al., 2003), plant HSP90s and HSC70s do not seem to participate in these phytohormone signaling cascades (Cazalé et al., 2009).
In this study, we show that the HSC70/HSP90 machinery is required for stomatal closure and modulates transcriptional and physiological responses to ABA. In addition, our results intimately implicate ABA into plant immunity and the contribution of the SGT1/HSC70/HSP90 proteins to the different layers of plant immunity should be carefully reinvestigated in the light of their newly identified functions in stomata.
RESULTS
Modulation of Whole-Plant Water Losses in Response to Environmental Conditions Is Compromised by HSC70-1 and HSP90.2 Deregulation
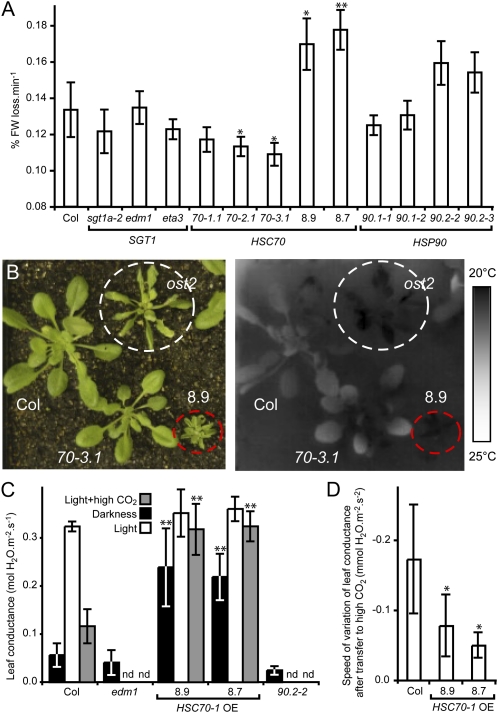
We analyzed the kinetic of water loss in darkness for individual mutants affecting the HSC70/SGT1/HSP90 molecular chaperone complex (Fig. 1): edm1 (sgt1b deletion mutant); eta3 (point mutation in SGT1b affecting HSC70-1 binding); HSC70, SGT1a, and HSP90.1 T-DNA insertion mutants, HSC70-1 overexpressing (OE) lines (8.7-, 7-fold OE; 8.9-, 4-fold OE), hsp90.2-2, and hsp90.2-3 (dominant negative mutations in HSP90.2). Compared to wild-type Columbia-0 (Col-0) plants and sgt1, hsc70, and hsp90.1 mutants, the rate of fresh weight loss for detached rosettes was significantly increased in HSC70-1 OE plants and hsp90.2 mutants (Fig. 1A). These observations were confirmed using measurements of leaf surface temperatures by infrared imaging on intact plants. Leaf surface temperature partially depends on evaporative cooling by transpiration (Merlot et al., 2002). In darkness, the leaves of HSC70-1 OE and hsp90.2 mutant plants were on average 1°C cooler than the other plant genotypes (Fig. 1B; Supplemental Fig. S1). As a more direct measurement of water loss, continuous recording of the conductance of attached leaves for the two HSC70-1 OE lines and the edm1 and hsp90.2-2 mutants was performed (Fig. 1, C and D). Leaf conductance for the HSC70-1 OE lines in darkness was already 4-fold higher than for wild-type or edm1 and hsp90.2-2 plants. Responses to dark/light transition and high CO2 concentrations were strongly hampered both in amplitude (Fig. 1C) and rate of variation (Fig. 1D) as compared to wild-type responses. Thus, dehydration experiments, infrared imaging, and leaf conductance analyses on whole plants showed that a deregulation of HSC70-1 and HSP90.2 functions alters physiological responses to darkness and high CO2 concentrations. Because stomatal densities and morphologies were not significantly different in all the lines studied (Fig. 2A; Supplemental Fig. S2A), our observations suggest that the deregulation of HSC70-1 and HSP90.2 causes an aberrant stomatal response to these two stimuli.
Figure 1.
Effect of HSC70 and HSP90 deregulation on plant water losses in response to environmental conditions. A, Rate of fresh weight (FW) loss in darkness of 3- to 4-week-old detached rosettes from different Arabidopsis genotypes was measured after 195 min. Four plants per genotype were used and the experiment performed in triplicate. B, Bright-field (left) and thermal imaging (right) of 5-week-old plants: wild-type (Col), hsc70-3.1 (70-3.1), and ost2-2D (ost2) mutants and an HSC70-1 OE line (8.9). C and D, Leaf conductance was measured on attached leaves from 6-week-old plants at 22°C in darkness or light and ambient (400 μL L−1) or high (2,000 μL L−1) CO2. C, Leaf conductance values were taken at the equilibrium while its speed of variation (D) was measured over a 10-min window following the change of condition. Three independent experiments were performed on at least three different plants. Error bar indicate standard deviations. * and **, Significant differences compared to the wild type (Student’s t test, P < 0.05 and P < 0.01, respectively). nd, Not determined.
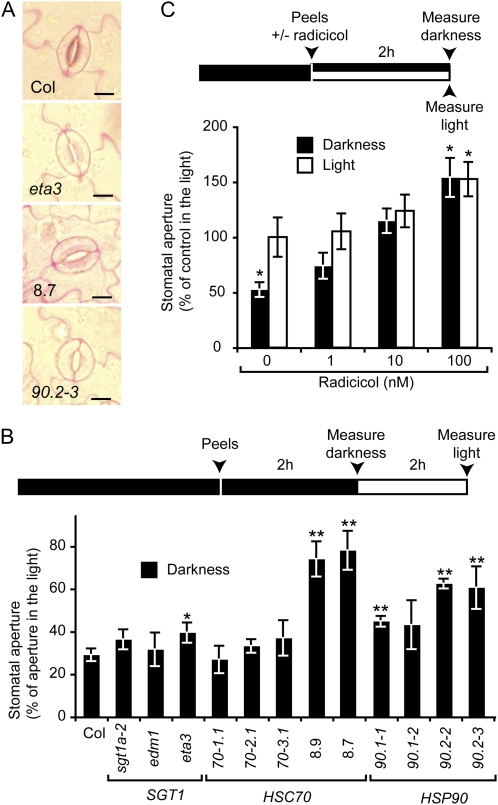
Figure 2.
Stomatal closure in response to darkness but not morphology is compromised by HSC70-1 and HSP90 deregulation. A, Representative stomata observed on epidermal peels incubated in darkness and stained with ruthenium red. Bars = 10 μm. B, Stomatal apertures were measured on epidermal peels after 2 h in darkness and subsequently after a 2-h incubation under light conditions. Average stomatal apertures in darkness were expressed as a percentage of aperture compared to light conditions. Average aperture values (μm) under light are available in Supplemental Figure S2B. C, Average stomatal apertures in wild-type Col-0 epidermal peels expressed as percentage of control condition under light was measured and incubated for 2 h in light or darkness with 0 to 100 nm radicicol. Three independent measurements (n > 50) were performed per condition on at least three different plants. Experiments were repeated at least twice. Error bar indicate sds. * and **, Significant differences compared to the wild type (Student’s t test, P < 0.05 and P < 0.01, respectively). Chronology of dark (black box)/light (white box) conditions, preparation of peels from plant leaves, treatments with radicicol, and measurements of stomatal apertures are indicated above the corresponding experiments (B and C).
Dark-Induced Stomatal Closure Is Compromised by HSC70-1 Overexepression and Requires HSP90 ATPase Activity
Leaf epidermal peels, a classical model to study stomatal opening, were used to measure directly stomatal aperture following a 2-h incubation under light or in darkness. All the different genotypes exhibited similar stomatal apertures under light (Supplemental Fig. S2B). While wild-type plants and sgt1 and hsc70 mutants responded to darkness by closing their stomata to 30% of the aperture in light condition, stomata of the HSC70-1 OE and hsp90 mutants remained opened to 75% and 60%, respectively (Fig. 2B). In a pharmacological approach, radicicol, a specific inhibitor of HSP90 ATPase activity, was used to investigate the role of HSP90s in the stomatal closure: Stomatal apertures on wild-type epidermal peels were measured in response to radicicol in light and darkness conditions (Fig. 2C). Radicicol treatment (100 nm) enhanced stomatal aperture under light by 50%. Darkness-induced stomatal closure was partially and fully suppressed by 1 and 10 nm radicicol, respectively. Altogether these results show that HSC70-1 OE compromises dark-induced stomatal closure and that HSP90 ATPase activity is required for stomatal closure.
Stomata Overexpressing HSC70-1 or with Reduced HSP90 ATPase Activity Are Insensitive to Exogenous ABA
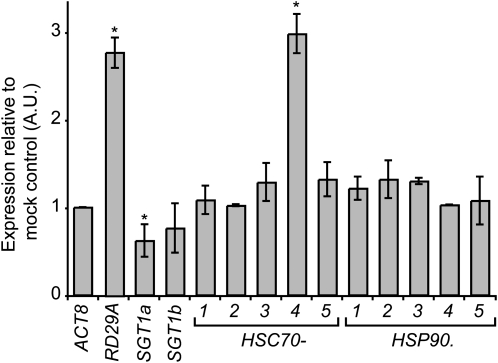
Because ABA triggers stomatal closure, we followed the effect of exogenous ABA application on expression of SGT1, HSC70, and HSP90 genes by quantitative reverse transcription (RT)-PCR (Fig. 3). As expected, the ABA treatment induced the expression of the ABA-responsive gene RD29A. The expression of HSC70-4 was increased 3-fold by the ABA treatment while no strong effect was observed on other HSC70 and HSP90 genes. On the other hand, a 30% reduction in SGT1a mRNA accumulation was observed after ABA treatment. These observations suggest that ABA directly or indirectly regulates the expression of specific HSC70/SGT1 genes.
Figure 3.
Effect of exogenous ABA treatment relative to mock control on SGT1, HSC70, and HSP90 transcripts accumulation. cDNAs were prepared from wild-type 10-d-old Col-0 seedlings 3 h after a 10 μm ABA or mock treatment and transcripts abundance measured by quantitative RT-PCR. The ABA-responsive gene RD29A was used as positive control while the Actin8 (ACT8) expression was used to normalize the transcript levels. Three independent RT-PCR experiments were performed on three independent biological samples. The bars represent mean values from three independent experiments and error bars indicate sds. *, Significant differences compared to the wild type (Student’s t test, P < 0.05).
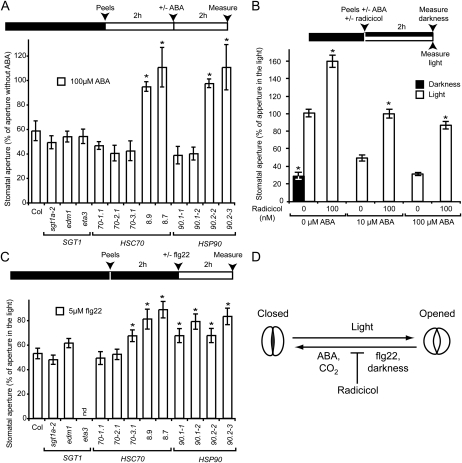
To test a possible involvement of the corresponding proteins in the ABA signaling and/or ABA-mediated stomatal closure, peels of the different genotypes were first incubated under light for 2 h to preopen stomata and then with 100 μm ABA. After 2h, the ABA-induced stomatal closure was measured (Fig. 4A). ABA promoted stomatal closure for wild-type plants as well as sgt1, hsc70, and hsp90.1 mutants. Interestingly, stomata of the HSC70-1 OE lines and hsp90.2 mutants were still fully opened. Furthermore, inhibition of HSP90 ATPase activity by 100 nm radicicol on wild-type epidermal peels was sufficient to attenuate ABA-dependent stomatal closure (Fig. 4B), indicating that HSP90 ATPase activity is partially epistatic on the ABA signal. The effects of radicicol on stomatal movements in response to environmental conditions are illustrated in Figure 4D. All together our results indicate that HSP90 activity stimulates stomatal closure while HSC70s would inhibit it.
Figure 4.
ABA and flg22 treatments cannot block light-induced stomatal opening in plants deregulated for HSC70-1 and HSP90 functions. A, Stomatal apertures were measured on epidermal peels incubated 2 h under light and then 2 additional h with or without 100 μm ABA. Average stomatal apertures in presence of ABA were expressed as a percentage of aperture without ABA. B, Average stomatal apertures expressed as percentage of aperture under light without ABA nor radicicol treatment was measured on Col-0 epidermal peels incubated for 2 h in light or darkness with 0 to 100 nm radicicol and 0 to 100 μm ABA. C, Stomatal apertures were measured on epidermal peels incubated in darkness for 2 h and then transferred under light for 2 h with or without 5 μm flg22. Average stomatal apertures in presence of flg22 were expressed as a percentage of aperture without flg22. Three independent measurements (n > 50) were performed per condition on at least three different plants. Experiments were repeated at least twice. Error bar indicate sds. *, Significant differences compared to the wild type (A and C) or the samples without radicicol treatment for each ABA condition (B; Student’s t test, P < 0.001). Chronology of dark (black box)/light (white box) conditions, preparation of peels from plant leaves, treatments with ABA/radicicol/flg22, and measurements of stomatal apertures are indicated above the corresponding experiments. D, Schematic representation of the regulation of stomatal opening/closure by biotic and abiotic factors. Radicicol that inhibits HSP90 ATPase activity acts as a general inhibitor of stomatal closure.
Stomata Overexpressing HSC70-1 or with Reduced HSP90 ATPase Activity Have a Reduced Sensitivity to flg22 Peptide Treatment
HSC70-1 OE has been shown to enhance susceptibility to virulent and avirulent Pseudomonas (e.g. Hubert et al., 2003; Noël et al., 2007) and HSP90 inactivation affects recognition of avirulent Pseudomonas (Hubert et al., 2003; Takahashi et al., 2003). Interestingly, the bacterial PAMP flagellin (or its 22-amino acid peptide flg22) triggers stomatal closure upon infection by a pathway that depends on signaling components shared with the ABA-dependent pathway such as SnRK2.6 (Mustilli et al., 2002; Yoshida et al., 2002; Melotto et al., 2006). To test whether this ancestral immune response might be also affected by HSC70-1 and HSP90 deregulation, we tested the responsiveness of stomata to flg22 treatment. Peels of the different genotypes were first incubated in darkness for 2 h and transferred under light for 2 h with or without 5 μm flg22 (Fig. 4C). flg22 treatment blocked light-induced stomatal opening for wild-type plants as well as sgt1, hsc70, and hsp90.1 mutants. Interestingly, stomata of the HSC70-1 OE lines and hsp90 mutants were significantly more opened under light despite the flg22 treatment. Therefore, HSC70-1 and HSP90s are important components of the flg22-dependent stomatal closure.
HSC70-1 and HSP90 Affect the Expression of Several ABA-Responsive Genes
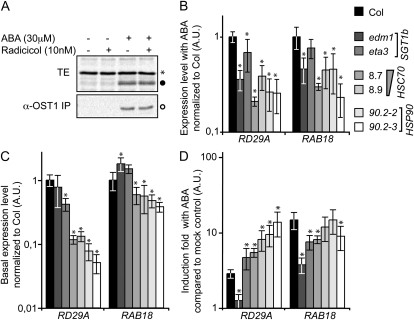
To study whether HSC70-1 and HSP90 components belong to the ABA/flg22 signaling cascade, different experiments were conducted. We first tested whether radicicol would inhibit the activation of the SnRK2s, including OST1 (SnRK2.6) that is required for ABA- and flg22-mediated stomatal closure (Mustilli et al., 2002; Yoshida et al., 2002; Melotto et al., 2006). A 2-h pretreatment of a Col-0 cell suspension culture with 10 nm radicicol followed by a 10-min treatment with 30 μm ABA did not affect total SnRK2 in-gel kinase activity, nor SnRK2.6 activity as determined by immunoprecipitation (Fig. 5A). Thus, HSP90 ATPase activity likely acts downstream or independently of ABA-mediated SnRK2 activation.
Figure 5.
Transcriptional responses to ABA in sgt1 and hsp90.2 DN mutants and HSC70-1 OE lines and SnRK activities. A, Autoradiogram of an in-gel kinase assay performed with total extracts (top section) and immunoprecipitated OST1 (bottom section) prepared from Col-0 cell suspension cultures preincubated for 2 h with 0 or 10 nm radicicol and subsequently treated with/without 30 μm ABA for 10 min before harvest. Asterisk, black, and white circle correspond essentially to MPK6, SnRK2.2/3, and SnRK2.6 (OST1) kinase activities. B to D, cDNA was prepared from 10-d-old seedlings 3 h after a 10 μm ABA or mock treatment and its abundance measured by quantitative RT-PCR. Relative cDNA abundance of the ABA-responsive genes RD29A and RAB18 was expressed relative to ABA-treated Col control (B), relative to Col mock control (C), and as induction fold relative to mock control within each genotype (D). Actin8 (ACT8) and ROC3 expression was used to normalize the transcript levels. Three independent RT-PCR experiments were performed on three independent biological samples. The bars represent mean values from three independent experiments and error bars indicate sds. *, Significant differences compared to the wild type (Student’s t test, P < 0.05).
We then studied the expression of two SnRK2-dependent genes RD29A and COR15A (Fujii and Zhu, 2009) along with other drought- and ABA-responsive genes (RD29B, RAB18, ABI1, and ABI2) by quantitative RT-PCR in the plants deregulated for SGT1, HSC70, and HSP90 functions with or without ABA treatment (Fig. 5, B and C; Supplemental Fig. S3). The level of the ABA-responsive transcripts is reduced after ABA treatment in lines deregulated in HSC70 and HSP90 functions (Fig. 5B; Supplemental Fig. S3A). For instance, the expression of RD29A and RAB18 is reduced 3-fold in hsp90.2 mutants and HSC70-1 OE lines while the eta3 mutation in SGT1b has no impact on expression levels of the studied genes. Interestingly, this effect essentially results from low basal levels of transcription in absence of exogenous ABA treatment in HSC70-1 OE lines and hsp90.2 mutants as compared to the wild type and the sgt1b mutants (Fig. 5C; Supplemental Fig. S3B) and the induction in response to ABA for those genes was similar or greater in the various lines when compared to wild type (Fig. 5D; Supplemental Fig. S3C). These basal expression levels are for example 10 and 100 times lower in hsp90.2 mutants than those measured in the wild-type plants for RD29A and COR15A, respectively. Importantly, the basal expression levels of genes that are not ABA regulated (PCS1, FAD8, SGT1b) is not modified in HSC70-1 OE lines and hsp90.2 mutants relative to wild type (Supplemental Fig. S3D). These results indicate that HSC70-1 and HSP90.2 are needed to establish to the basal level of transcription of ABA-responsive genes and suggest a role in the long-term adaptation to drought and dehydration tolerance in vegetative tissues and/or seeds.
SGT1b, HSC70-1, and HSP90 Deregulation Causes Hypersensitivity to ABA in Seed Germination Assays
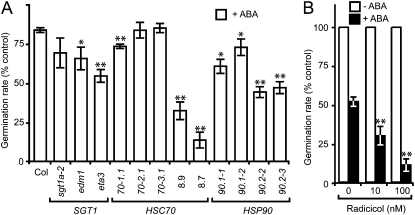
To test the biological importance of HSC70/SGT1/HSP90 in another ABA-dependent physiological response, we studied the inhibition of seed germination by exogenous ABA treatment. ABA (5 μm) caused approximately 20% inhibition of germination rate for the wild type and the sgt1a-2, hsc70-2.1, and hsc70-3.1 mutants relative to the condition without ABA (Fig. 6A). Surprisingly, all the other genotypes showed a stronger inhibition of germination rate, HSC70-1 OE and hsp90.2 mutations causing the most pronounced hypersensitivity to ABA compared to hsc70-1.1 and sgt1b mutants. Furthermore, while radicicol itself did not affect the germination of wild-type seeds, it strongly enhanced ABA action in inhibiting seed germination (Fig. 6B). These results indicate that HSP90 and SGT1b act as negative regulators in the ABA-mediated inhibition of seed germination and that HSC70 and HSP90 deregulation can affect differentially ABA-dependent responses in seeds compared to guard cells.
Figure 6.
SGT1b, HSC70-1, and HSP90 deregulation causes hypersensitivity to ABA in seed germination assays. A, Fresh seeds harvested simultaneously from different genotypes were sown on Murashige and Skoog/10 in absence or presence of 5 μm ABA. Radicule emergence (germination) was scored after 5 d. The average germination rate is expressed as a percentage of the germination rate in absence of ABA for each genotype tested. * and **, Significant differences compared to the wild type (Student’s t test, P < 0.05 and P < 0.01, respectively). B, Fresh wild-type Col-0 seeds were sown on Murashige and Skoog/10 in absence or presence of 3 μm ABA in combination with 0 to 100 nm radicicol and germination scored as in A. The average germination rate is expressed as a percentage of the germination rate in absence of ABA and radicicol. * and **, Significant differences compared to the no radicicol control (Student’s t test, P < 0.05 and P < 0.01, respectively). Approximately 50 seeds per condition were scored in triplicate samples. Error bars indicate sds. Experiments were performed at least twice.
DISCUSSION
In this study, we provide genetic and pharmacological evidence for the multiple functions of the HSC70/HSP90 molecular chaperone machinery in the fine-tuned regulation of stomatal aperture in response to various environmental conditions and of physiological responses to the ABA hormone in Arabidopsis.
HSC70/HSP90 Are Major Modulators of Stomatal Movement and Responses to ABA in Arabidopsis
The deregulation of cytosolic/nuclear HSC70/HSP90 functions caused very strong insensitivity to darkness, high CO2, flg22 peptide, and ABA in guard cells that might be explained by a general incapacity in closing stomata. These observations suggest that HSC70/HSP90 could function on one event involved in stomatal closure itself after convergence of those four signaling pathways. In addition to this defect that is not ABA specific, our study and two independent reports also suggest that HSP90s could be implicated in transcriptional responses to ABA for long-term adaptation to drought tolerance: The overexpression of HSP90.2 increased Arabidopsis sensitivity to drought stress (Song et al., 2009) and, transcriptome analysis of hsp90.2-2/hsp90.2-3 plants and HSP90 RNAi transgenics revealed that the ABA signaling pathway was among the most perturbed (Sangster et al., 2007).
One possible explanation for the late discovery of their involvement in ABA signal transduction could be that both gene families are essential and to some extent functionally redundant thus rendering their functional analysis genetically difficult in Arabidopsis (Sung and Guy, 2003; Noël et al., 2007; Hubert et al., 2009). On the one hand, single loss-of-function mutants in the different HSC70 and HSP90 genes did not cause any mutant phenotypes for the different physiological responses tested here and single hsc70 or hsp90 loss-of-function mutants displayed no or subtle mutant phenotypes (Hubert et al., 2003; Takahashi et al., 2003; Noël et al., 2007). On the other hand, the mutant screens performed on ABA insensitivity or aberrant guard cell movements did not reach saturation yet so that the identification of HSC70 OE plants or particular hsp90 alleles was unlikely. Only very saturated screens such as those performed by Hubert et al. (2003, 2009) identified unusual mutant alleles such as hsp90.2-2 and hsp90.2-3 that caused very pronounced defects in immunity and physiology when compared to hsp90.1 or hsp90.2 loss-of-function mutants. The hsp90.2-2 and hsp90.2-3 alleles code for proteins that do not have ATPase activity and are unable to dimerize or interact with SGT1b in vitro but are not null alleles of hsp90.2 (Hubert et al., 2003, 2009). The use of radicicol that inhibits ATP binding to HSP90s (Roe et al., 1999) confirmed independently that the ATPase activity of HSP90s is essential to mediate ABA signaling for the phenotypes tested here. Single loss-of-function mutants in genes encoding cytosolic/nuclear HSP90s and HSC70s had either no or only mild phenotypes in the different physiological responses investigated here. For instance, the hsc70-3.1 or hsp90.1 mutants showed some weakly altered responses to flg22, whole-plant water loss, or dark-induced stomatal closure (Figs. 1A, 2B, and 4C). To overcome genetic redundancy in the HSC70 gene family and mimic what usually happens during most biotic and abiotic stresses, we globally increased the expression of all cytosolic/nuclear isoforms of the HSP70 family by OE of the single HSC70-1 gene (Sung and Guy, 2003). While the molecular consequences of HSC70-1 OE remain elusive and may result in more complex consequences than a single gain of HSP70 activity, this approach has proven to be fruitful to dissect HSP70 functions in vivo in various model organisms including Arabidopsis (Sung and Guy, 2003; Noël et al., 2007; Cazalé et al., 2009; Dokladny et al., 2010). The specificity of such HSP90 and HSC70 deregulation could legitimately be questioned because, DnaK interacts on average every 36 amino acids in the Escherichia coli proteome (Rüdiger et al., 1997) and the yeast (Saccharomyces cerevisiae) HSP90 interacts physically or genetically with approximately 10% of yeast genes (Zhao et al., 2005). Yet, the hsp90.2-2 mutants have no reported developmental phenotypes (Hubert et al., 2003) while HSC70 OE lines show only a limited dwarfism (Sung and Guy, 2003). HSC70 OE plants are also fully fertile and most aspects of their physiology (auxin perception, phosphate uptake and signaling, photosynthetic efficiency) and development (flowering time, root architecture) are surprisingly normal considering that cytosolic/nuclear HSC70 are essential proteins (Sung and Guy, 2003; Noël et al., 2007; Cazalé et al., 2009). Furthermore, both chaperones contribute to very specific functions in signal transduction in vivo. For instance, in Arabidopsis, HSP90 compromised only Resistance (R) gene-specified immunity and HSC70-1 OE was shown to compromise specifically basal and R-gene-specified immunity but not nonhost resistance (Hubert et al., 2003; Takahashi et al., 2003; Noël et al., 2007). Similarly, we show that cytosolic/nuclear HSP90/HSC70-1 chaperones are differentially involved in the regulation of ABA-responsive gene expression compared to ABA-mediated inhibition of seed germination. These observations suggest that HSP90/HSC70-1 chaperones modulate specific and distinct signaling events important for the regulation of stomatal closure, seed germination, and transcriptional regulation of ABA-responsive genes in Arabidopsis.
Distinct Chaperone Clients Control Stomatal Closure, ABA Perception/Signaling in Seeds, and mRNA Accumulation of ABA- and Drought-Responsive Genes in Vegetative Tissues
The contrasted phenotypes conferred by the deregulation of cytosolic/nuclear HSC70/HSP90 functions are not incompatible with HSC70/HSP90 controlling the folding of a single target. For instance, the abo3 mutant is hypersensitive to ABA at the germination and seedling stages while partially insensitive to ABA at the stomatal level (Ren et al., 2010). Since abo3 partially phenocopies HSC70 OE lines and HSP90DN mutants, ABO3 is a candidate substrate for HSC70/HSP90. Yet, we favor the hypothesis where HSP90/HSC70-1 chaperones act on distinct tissue-specific substrates to mediate ABA-independent stomatal closure, to establish basal levels of ABA- and drought-induced gene transcripts in vegetative tissues, and to modulate ABA-dependent inhibition of seed germination. For instance, the general inability of stomata to close in response to multiple stimuli (high CO2, darkness, flg22, ABA) suggests that general processes involved in guard cell movements themselves are affected rather than specific signal perception of any of these stimuli. Our results also suggest the existence of a second chaperone substrate in vegetative tissues responsible for the maintenance of basal expression levels of ABA- and drought-responsive genes (Fig. 5). This substrate does not control the ABA responsiveness of those genes but HSC70/HSP90 deregulation would still result in an apparently weakened transcriptional response to ABA and drought conditions since sufficient transcripts levels would not be reached to mount wild-type physiological responses to ABA. In seedlings, HSP90 inhibition did not affect germination rate (Fig. 6B) but strongly sensitized seeds to exogenous ABA treatment. It remains unclear whether HSC70 and HSP90 effect is direct or indirect, but, if in the ABA signaling pathway, this effect would be situated downstream of SnRK2s, since ABA-dependent activation of SnRK2s (including OST1) was not compromised by inhibition of HSP90 activity (Fig. 5A).
The differential requirement of SGT1b for stomatal responses and germination tests also suggests that distinct substrates interacting with distinct chaperone/cochaperone complexes are involved to mediate these responses in the different tissues. Alternatively, the requirements for SGT1b in stomatal responses could be below a threshold where SGT1a might be able to compensate for the loss of SGT1b as observed for plant immunity (Azevedo et al., 2006) or an SGT1-independent HSC70/HSP90 complex using other cochaperone scaffolds might be involved. We thus tested the involvement of RAR1, an HSP90 cochaperone and SGT1 interactor in ABA-mediated inhibition of seed germination and control of leaf water losses. The rar1-21 null mutant was not affected in its water loss when analyzed by infrared thermal imaging, rosette dehydration experiments, or stomatal conductance in the dark nor in germination assays in presence of ABA (data not shown). In addition, HSP90.2-2 retains ability to interact with RAR1 in yeast two hybrid while HSP90.2-3 loses it. This suggests that RAR1 might not be directly involved in ABA signaling but a yet-to-be-identified cochaperone of HSC70/HSP90.
The HSC70/SGT1/HSP90 Complexes Link Stomatal Closure, ABA Signaling, and Immunity
The identification of ABA mutants by forward and reverse genetic screens with compromised innate immunity have recently highlighted the very contrasted and important effects of ABA on plant immune systems (de Torres-Zabala et al., 2007; Fan et al., 2009) though the precise molecular mechanisms involved await elucidation (for review, see Asselbergh et al., 2008). This hormone can either act as a positive or negative modulator of plant innate immunity depending on the pathosystem (e.g. Fan et al., 2009). As such, the sgt1b and hsp90.2 mutants that were first isolated in genetic screens for loss of innate immunity are a perfect example of the potential overlap between ABA signaling and immunity. The compromised immunity of those mutants was initially mainly explained in the light of SGT1 and HSP90 functions in the stabilization/activation of several R proteins (Shirasu and Schulze-Lefert, 2003) as observed by specific RPM1 destabilization in hsp90.2-2 and hsp90.2-3 mutants (Hubert et al., 2003). While these molecular observations hold true, the careful analysis of these phenotypes in the light of their new stomatal and ABA-dependent functions may help to evidence the precise contribution of these players in the different layers of innate immunity (Lipka et al., 2005). For instance, the stomata are often the first barrier that plant pathogens have to cross to gain access to the leaf intercellular spaces. Thus, several pathogens have evolved important virulence factors to bypass this first layer of innate immunity and prevent stomatal closure upon infection. Pseudomonas and Xanthomonas both inhibit PAMP-triggered stomatal closure by producing coronatine and the rpf/diffusible signal factor, respectively, thus facilitating bacterial penetration inside the leaf tissue (Melotto et al., 2006; Gudesblat et al., 2009). Not so surprisingly, the ABA-insensitive ost1-2 or PAMP-insensitive plants eds16-2 and nahG that stomata do not close normally showed enhanced susceptibility to a Pseudomonas coronatine-deficient mutant after dip inoculation but not infiltration (Melotto et al., 2006). In parallel, Pseudomonas virulence factors directly injected inside the plant cells such as AvrRpm1, AvrB, and AvrRpt2 target RIN4, an interactor of the proton pump AHA1 that is directly responsible for stomatal closure (Liu et al., 2009). This further highlights that prevention of stomatal closure upon infection is a key issue for bacterial pathogens (Melotto et al., 2008). Interestingly, sgt1b mutants were recently shown to be less sensitive to virulent Pseudomonas when spray inoculated and to coronatine that inhibits ABA-dependent stomatal closure (Uppalapati et al., 2011). Still, sgt1b mutations did not affect PAMP/flg22-triggered resistance (Zipfel et al., 2004), stomatal responses to ABA, high CO2, and darkness nor basal immunity when Pseudomonas were hand infiltrated into the leaf tissue (Holt et al., 2005). These observations suggest that SGT1b is involved in coronatine/jasmonic acid signaling rather than in general pathways leading to stomatal closure. As inferred from their insensitivity to ABA and flg22 treatments and their opened stomata, HSC70-1 OE lines should also have a compromised basal immunity. Such loss of basal immunity was observed when virulent Pseudomonas were syringe infiltrated into leaves thus bypassing the stomatal barrier (Noël et al., 2007). The breakdown of basal resistance against nonpathogenic Pseudomonas in the hsc70-1 mutant is more pronounced when bacteria are spray inoculated than infiltrated (Jelenska et al., 2010). This indicates that HSC70s serve a role in basal immunity before and after the stomatal barrier is crossed. These functions in innate immunity are further confirmed by the identification of the DnaJ domain virulence protein HopI1 used by Pseudomonas to modify cytosolic HSC70 ATPase activity and subcellular localization (Jelenska et al., 2007, 2010). For HSP90s, no defect in basal immunity was observed in hsp90.2-2/3 mutants when spray inoculated (Hubert et al., 2003) though on epidermal peels their stomata stay open in all conditions studied here including flg22 treatment. These surprising observations indicate that, besides the regulation of R-protein stabilization/activation in the incompatible interactions (Hubert et al., 2003, 2009), HSP90s might also exert an antagonist effect on another layer of the innate immune system. Interestingly, enhanced susceptibility to Pseudomonas is achieved by effector-mediated induction of ABA production and ABA-dependent suppression of SA-dependent defenses in Arabidopsis to which HSP90 could contribute (de Torres-Zabala et al., 2007, 2009).
Yet, the key question remains: What are the HSC70 and HSP90 clients? HSP90 and HSC70-1 deregulation has unraveled the physiological importance of some of their clients for stomatal closure, ABA signaling, and plant innate immunity that identification may have been masked by genetic redundancy in the past. Interestingly, HSP90s and HSP70s were shown to serve as a buffer for genetic variation in Drosophila and/or Arabidopsis (Feder et al., 1992; Rutherford and Lindquist, 1998; Queitsch et al., 2002). Thus, exploiting the diversity for the sensitivity to HSP90 inhibitors in stomata and during germination in presence of ABA in the large collection of Arabidopsis ecotypes could serve as a mean to identify genetically the first HSP90 clients in Arabidopsis and novel players in ABA signaling in seeds, stomatal closure, or plant innate immunity.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) genotypes used in this study are from the Col-0 ecotype but sgt1bedm1 (Tör et al., 2002) that is in the Col-5 ecotype: ost2-2D (Merlot et al., 2007), sgt1beta3 (Gray et al., 2003); sgt1a-2 (Cazalé et al., 2009); hsc70-1.1, hsc70-2.1, and hsc70-3.1 (Noël et al., 2007), rar1-21 (Tornero et al., 2002); hsp90.1-1 and hsp90.1-2 (Takahashi et al., 2003), and hsp90.2-2 and hsp90.2-3 (Hubert et al., 2003). Two independent homozygous Col-0 plants overexpressing HSC70-1 (lines 8.7 and 8.9) were studied (Sung and Guy, 2003). Plants were grown in soil in a walk-in chamber under short-day conditions (8 h light/16 h darkness) at 21°C/18°C (light/dark) and a light intensity of 200 μmol photons m−2 s−1. Seedlings were also grown in sterile conditions on vertically oriented Murashige and Skoog/10 medium containing 0.5% Suc in a white-light growth chamber under a 16 h photoperiod at 24°C/21°C (light/dark).
Quantitative RT-PCR
Ten-day-old in vitro-grown seedlings were sprayed with 10 μm ABA or water and harvested 3 h later. RNA extraction was performed using RNeasy mini kit according to manufacturer’s instructions (Qiagen). The RNA was then subjected to treatment with TURBO DNase (Ambion, Applied Biosystems) and confirmed by PCR to be free of detectable amounts of DNA. cDNA synthesis was done using the SuperScript VILO cDNA synthesis (Invitrogen). Quantitative RT-PCR was performed in 384-well plates using the Light Cycler 480 SYBR green I master and the LightCycler 480 real-time PCR system (Roche Diagnostics). The specificity of each primer pair was tested on a standard curve based on serial dilutions of the wild-type control cDNA and subsequently by melting curve analysis. The accumulation of each transcript was measured in three independent biological samples with three technical replicates. Actin8 and ROC3 expression was used to normalize the transcript levels for each sample. Primer sequences for each real-time reaction are listed in Supplemental Table S1. The bars represent mean values from three independent experiments. Statistically significant differences for values were determined by Student’s t test analyses.
Kinetics of Water Loss from Excised Rosettes
Hypocotyl of 4-week-old plants was cut and sealed with silicon grease. Water loss was evaluated by weighting rosette each 30 min during the first hour and each hour for the next 4 h. Four plants per lines were used per experiment. Rate of fresh weight loss was calculated over 3 h.
Infrared Thermal Imaging
Thermal imaging was performed using an infrared camera (FLIR, B20HS). The rosettes were imaged at room temperature under low relative humidity on 4- to 5-week-old plants kept in darkness for more than 14 h. The image analysis software provided with the camera (FLIR Researcher) was used to determine the leaf surface temperature from at least 10 positions per rosette for three different plants.
Leaf Conductance Measurement
Stomatal conductance was measured on attached leaves of 6-week-old plants using a LI 6400 portable photosynthesis system with the leaf chamber fluorometer (LI6400-40). The leaf temperature and the relative humidity were 22°C and 70%, respectively. Ambient CO2 was 400 μmol mol−1 unless stated otherwise. Illumination was set to 90% red, 10% blue, and 500 μmol photon m−2 s−1 irradiance. Three independent experiments were performed on at least three different plants.
Measurements of Stomatal Aperture and Density
Measurements were performed on epidermal peels from mature leaves of 3- to 4-week-old plants essentially as described (Leonhardt et al., 1997). Peels were placed in a solution (30 mm KCl, 10 mm MES, pH 5.6 at 22°C in light/darkness, 0–100 μm ABA, 0–5 μm flg22 peptide, and 0–100 nm radicicol). Stomatal apertures were measured with an optical microscope (Nikon) fitted with a camera lucida and a digitizing table (Houston instrument TG 1017) linked to a personal computer (Bull Micral 30). For each treatment, three peels were analyzed per condition and at least 50 stomatal apertures were measured at a magnification of 1,000. Experiments were performed at least twice. To measure stomatal density, three epidermal peels from three different plants of each genotype were briefly stained in ruthenium red, imaged using a bright-field microscope at a magnification of 400 fitted with a digital camera, and counted manually on the printed image. Experiments were performed at least three times.
Germination Assays
Fresh seeds harvested simultaneously were sown on Murashige and Skoog/10 with 0 to 5 μm ABA and 0 to 100 nm radicicol and stratified 2 d at 4°C. Radicule emergence was observed under the binocular after 5 d. Approximately 50 seeds per conditions were scored in triplicate samples. Experiments were performed at least twice.
Kinase Assays
Col-0 cell suspensions cultured as described (Droillard et al., 2002) were preincubated for 2 h with 0 or 10 nm radicicol and subsequently treated with/without 30 μm ABA for 10 min before harvest. Protein extracts were prepared and used for in-gel kinase assays as described (Boudsocq et al., 2007). Immunoprecipitation of endogenous SnRK2.6 before in-gel kinase assay was performed as described (Vlad et al., 2009).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Impact of SGT1b, HSC70-1, and HSP90 deregulation on average leaf surface temperature measured by thermal imaging.
Supplemental Figure S2. Effect of SGT1, HSC70, and HSP90 on leaf stomatal density and aperture under light.
Supplemental Figure S3. Transcriptional responses to ABA in sgt1 and hsp90.2 DN mutants and HSC70-1 OE lines.
Supplemental Table S1. Oligonucleotide primers used for real-time RT-PCR analysis.
Supplementary Material
Acknowledgments
We are grateful to Nathalie Pochon and Serge Chiarenza for technical assistance, Etienne Delannoy for expert advice in performing quantitative RT-PCR experiments, and Tina Romeis for contributing flg22 peptide and stimulating discussions. We thank Johannes Stuttmann, Jean-Philippe Galaud, and Didier Aldon for comments on the manuscript and Ken Shirasu and Jeff Dangl for contributing HSP90 mutant lines. We thank the Groupe de Recherches Appliquées en Phytotechnologie members for maintenance of the plant growth facility.
References
- Asselbergh B, De Vleesschauwer D, Höfte M. (2008) Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21: 709–719 [DOI] [PubMed] [Google Scholar]
- Austin MJ, Muskett P, Kahn K, Feys BJ, Jones JD, Parker JE. (2002) Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295: 2077–2080 [DOI] [PubMed] [Google Scholar]
- Azevedo C, Betsuyaku S, Peart J, Takahashi A, Noël L, Sadanandom A, Casais C, Parker J, Shirasu K. (2006) Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J 25: 2007–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M, Droillard MJ, Barbier-Brygoo H, Laurière C. (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63: 491–503 [DOI] [PubMed] [Google Scholar]
- Bukau B, Weissman J, Horwich A. (2006) Molecular chaperones and protein quality control. Cell 125: 443–451 [DOI] [PubMed] [Google Scholar]
- Catlett MG, Kaplan KB. (2006) Sgt1p is a unique co-chaperone that acts as a client adaptor to link Hsp90 to Skp1p. J Biol Chem 281: 33739–33748 [DOI] [PubMed] [Google Scholar]
- Cazalé AC, Clément M, Chiarenza S, Roncato MA, Pochon N, Creff A, Marin E, Leonhardt N, Noël LD. (2009) Altered expression of cytosolic/nuclear HSC70-1 molecular chaperone affects development and abiotic stress tolerance in Arabidopsis thaliana. J Exp Bot 60: 2653–2664 [DOI] [PubMed] [Google Scholar]
- de Torres Zabala M, Bennett MH, Truman WH, Grant MR. (2009) Antagonism between salicylic and abscisic acid reflects early host-pathogen conflict and moulds plant defence responses. Plant J 59: 375–386 [DOI] [PubMed] [Google Scholar]
- de Torres-Zabala M, Truman W, Bennett MH, Lafforgue G, Mansfield JW, Rodriguez Egea P, Bögre L, Grant M. (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO J 26: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. (2010) LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-kappaB. Cell Stress Chaperones 15: 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard M, Boudsocq M, Barbier-Brygoo H, Laurière C. (2002) Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions: involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett 527: 43–50 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K. (2001) Specific binding of vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125: 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbse A, Mayer MP, Bukau B. (2004) Mechanism of substrate recognition by Hsp70 chaperones. Biochem Soc Trans 32: 617–621 [DOI] [PubMed] [Google Scholar]
- Fan J, Hill L, Crooks C, Doerner P, Lamb C. (2009) Abscisic acid has a key role in modulating diverse plant-pathogen interactions. Plant Physiol 150: 1750–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. (1992) The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Genes Dev 6: 1402–1413 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Zhu JK. (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc Natl Acad Sci USA 106: 8380–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE. (2003) Arabidopsis SGT1b is required for SCF(TIR1)-mediated auxin response. Plant Cell 15: 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Torres PS, Vojnov AA. (2009) Xanthomonas campestris overcomes Arabidopsis stomatal innate immunity through a DSF cell-to-cell signal-regulated virulence factor. Plant Physiol 149: 1017–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Holt BF, III, Belkhadir Y, Dangl JL. (2005) Antagonistic control of disease resistance protein stability in the plant immune system. Science 309: 929–932 [DOI] [PubMed] [Google Scholar]
- Hubert DA, He Y, McNulty BC, Tornero P, Dangl JL. (2009) Specific Arabidopsis HSP90.2 alleles recapitulate RAR1 cochaperone function in plant NB-LRR disease resistance protein regulation. Proc Natl Acad Sci USA 106: 9556–9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert DA, Tornero P, Belkhadir Y, Krishna P, Takahashi A, Shirasu K, Dangl JL. (2003) Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J 22: 5679–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, Hashimoto M, Iba K, Schroeder JI. (2006) Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol 9: 654–663 [DOI] [PubMed] [Google Scholar]
- Jelenska J, van Hal JA, Greenberg JT. (2010) Pseudomonas syringae hijacks plant stress chaperone machinery for virulence. Proc Natl Acad Sci USA 107: 13177–13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenska J, Yao N, Vinatzer BA, Wright CM, Brodsky JL, Greenberg JT. (2007) A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr Biol 17: 499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Skowyra D, Elledge SJ, Harper JW, Hieter P. (1999) SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol Cell 4: 21–33 [DOI] [PubMed] [Google Scholar]
- Krishna P, Gloor G. (2001) The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones 6: 238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Marin E, Vavasseur A, Forestier C. (1997) Evidence for the existence of a sulfonylurea-receptor-like protein in plants: modulation of stomatal movements and guard cell potassium channels by sulfonylureas and potassium channel openers. Proc Natl Acad Sci USA 94: 14156–14161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BL, Wang JS, Liu HC, Chen RW, Meyer Y, Barakat A, Delseny M. (2001) Genomic analysis of the Hsp70 superfamily in Arabidopsis thaliana. Cell Stress Chaperones 6: 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, et al. (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310: 1180–1183 [DOI] [PubMed] [Google Scholar]
- Liu J, Elmore JM, Fuglsang AT, Palmgren MG, Staskawicz BJ, Coaker G. (2009) RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol 7: e1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Park SY, Weiner JJ, Fujii H, Chinnusamy V, et al. (2009) A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, He SY. (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46: 101–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Müller A, et al. (2007) Constitutive activation of a plasma membrane H(+)-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J. (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30: 601–609 [DOI] [PubMed] [Google Scholar]
- Miyazono K, Miyakawa T, Sawano Y, Kubota K, Kang HJ, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y, et al. (2009) Structural basis of abscisic acid signalling. Nature 462: 609–614 [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Noël LD, Cagna G, Stuttmann J, Wirthmüller L, Betsuyaku S, Witte CP, Bhat R, Pochon N, Colby T, Parker JE. (2007) Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell 19: 4061–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. (2002) Hsp90 as a capacitor of phenotypic variation. Nature 417: 618–624 [DOI] [PubMed] [Google Scholar]
- Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, Hong X, Zhu JK, Gong Z. (2010) ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J 63: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH. (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42: 260–266 [DOI] [PubMed] [Google Scholar]
- Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 16: 1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. (1998) Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342 [DOI] [PubMed] [Google Scholar]
- Sangster TA, Bahrami A, Wilczek A, Watanabe E, Schellenberg K, McLellan C, Kelley A, Kong SW, Queitsch C, Lindquist S. (2007) Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS ONE 2: e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Dupeux F, Round A, Antoni R, Park SY, Jamin M, Cutler SR, Rodriguez PL, Marquez JA. (2009) The abscisic acid receptor PYR1 in complex with abscisic acid. Nature 462: 665–668 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58: 221–227 [DOI] [PubMed] [Google Scholar]
- Shirasu K. (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol 60: 139–164 [DOI] [PubMed] [Google Scholar]
- Shirasu K, Schulze-Lefert P. (2003) Complex formation, promiscuity and multi-functionality: protein interactions in disease-resistance pathways. Trends Plant Sci 8: 252–258 [DOI] [PubMed] [Google Scholar]
- Song H, Zhao R, Fan P, Wang X, Chen X, Li Y. (2009) Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses. Planta 229: 955–964 [DOI] [PubMed] [Google Scholar]
- Sung DY, Guy CL. (2003) Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis: evidence for pleiotropic consequences. Plant Physiol 132: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Vierling E, Guy CL. (2001) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Casais C, Ichimura K, Shirasu K. (2003) HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc Natl Acad Sci USA 100: 11777–11782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JP, Willingham SB, Bergstralh DT. (2008) NLRs at the intersection of cell death and immunity. Nat Rev Immunol 8: 372–379 [DOI] [PubMed] [Google Scholar]
- Tör M, Gordon P, Cuzick A, Eulgem T, Sinapidou E, Mert-Türk F, Can C, Dangl JL, Holub EB. (2002) Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell 14: 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero P, Merritt P, Sadanandom A, Shirasu K, Innes RW, Dangl JL. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14: 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppalapati SR, Ishiga Y, Ryu CM, Ishiga T, Wang K, Noël LD, Parker JE, Mysore KS. (2011) SGT1 contributes to coronatine signaling and Pseudomonas syringae pv. tomato disease symptom development in tomato and Arabidopsis. New Phytol 189: 83–93 [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S. (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele H, Müller L, Buchner J. (2004) Hsp70 and Hsp90—a relay team for protein folding. Rev Physiol Biochem Pharmacol 151: 1–44 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. (2001) Hsp90: a specialized but essential protein-folding tool. J Cell Biol 154: 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al. (2005) Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120: 715–727 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.