Abstract
Onion (Allium cepa) is regarded as a nonclimacteric vegetable. In onions, however, ethylene can suppress sprouting while the ethylene-binding inhibitor 1-methylcyclopropene (1-MCP) can also suppress sprout growth; yet, it is unknown how ethylene and 1-MCP elicit the same response. In this study, onions were treated with 10 μL L−1 ethylene or 1 μL L−1 1-MCP individually or in combination for 24 h at 20°C before or after curing (6 weeks) at 20°C or 28°C and then stored at 1°C. Following curing, a subset of these same onions was stored separately under continuous air or ethylene (10 μL L−1) at 1°C. Onions treated with ethylene and 1-MCP in combination after curing for 24 h had reduced sprout growth as compared with the control 25 weeks after harvest. Sprout growth following storage beyond 25 weeks was only reduced through continuous ethylene treatment. This observation was supported by a higher proportion of down-regulated genes characterized as being involved in photosynthesis, measured using a newly developed onion microarray. Physiological and biochemical data suggested that ethylene was being perceived in the presence of 1-MCP, since sprout growth was reduced in onions treated with 1-MCP and ethylene applied in combination but not when applied individually. A cluster of probes representing transcripts up-regulated by 1-MCP alone but down-regulated by ethylene alone or in the presence of 1-MCP support this suggestion. Ethylene and 1-MCP both down-regulated a probe tentatively annotated as an ethylene receptor as well as ethylene-insensitive 3, suggesting that both treatments down-regulate the perception and signaling events of ethylene.
Onion (Allium cepa) is traditionally classified as nonclimacteric (Downes et al., 2010). Both ethylene and 1-methylcyclopropene (1-MCP) have been shown to inhibit sprout growth (Chope et al., 2007a; Bufler, 2009), which decreases bulb quality. Onion quality is dependent on the rate of internal sprout growth during storage. To eliminate the use of artificial chemicals, such as maleic hydrazide, the use of the plant growth regulator (PGR) ethylene has been found to reduce sprout growth in onions when applied continuously throughout storage (10–15 μL L−1). Bufler (2009) found that Copra onions held in continuous ethylene (10.6 μL L−1) had reduced sprout growth compared with those held in air. Surprisingly, treatment with 1-MCP for 24 h after curing (6 weeks at 28°C prior to cold storage) reduced sprout growth in SuperSweet1 onions when stored at 4°C or 12°C (Chope et al., 2007a). Although ethylene and 1-MCP have both been shown to reduce sprout growth, biochemical and physiological responses to each stimulus differ (Downes et al., 2010). Ethephon is an ethylene-yielding chemical that, when applied directly to plants, can elicit a response characteristic of ethylene treatment (Warner and Leopold, 1969; Yang, 1969). Application of ethephon to onion plants 2 weeks prior to harvest was found to reduce sprout incidence by 5% after 32 weeks of storage at 0°C; however, no significant reduction in rooting was observed (Adamicki, 2005). Unlike ethephon treatment, continuous ethylene exposure has been found to increase shelf life after 14 d at 20°C (Adamicki, 2005; Johnson, 2006). The combination of ethylene and 1-MCP has not been investigated in onion, although it has in potato (Solanum tuberosum; Prange et al., 2005).
Onion is in the order Asparagales, which possess some of the largest genomes of the eukaryotes, especially in the genus Allium (Kuhl et al., 2004). Onion is diploid and comprises a large nuclear genome of 16,415 Mb (over five times that of the human genome) spread over eight chromosomes (Havey et al., 2008; NCBI, 2008). To date, the large size has hindered plans to sequence the onion genome; however, 20,180 ESTs are available, mainly from a cross of inbred cultivars, Bringham Yellow Globe 15-23 × Ailsa Craig 43 (NCBI, 2008). These ESTs have been used to develop the first onion microarray. Although literature exists on the effect of ethylene and 1-MCP on climacteric fruits and vegetables at the molecular level, the mechanisms by which exogenously applied ethylene and 1-MCP suppress sprout growth in onions are still unknown.
Here, we present novel transcriptional profiles and biochemical and physiological analyses of onion in response to short 24-h ethylene and/or 1-MCP treatment prior to storage, with or without the addition of long-term continuous ethylene during storage.
RESULTS
Ethylene and 1-MCP Treatments Reduce Sprout Development
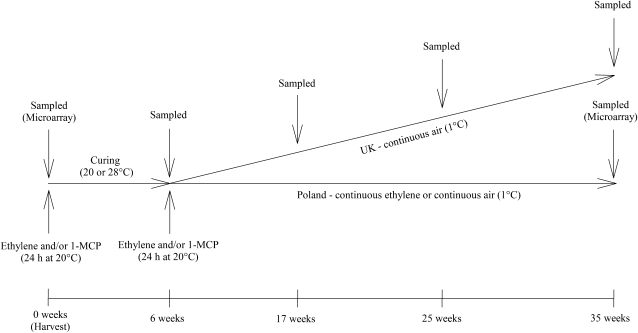
After harvest, onions were subjected to treatment with ethylene, 1-MCP, or ethylene and 1-MCP either before or after curing at either 20°C or 28°C. After curing, bulbs were placed in cold storage (1°C), with a subset of bulbs being stored under continuous ethylene supply (Fig. 1). Average sprout growth at 25 and 35 weeks was 29% and 58% of bulb height, respectively, with curing temperature affecting sprout length after 25 weeks only. Onions cured at 20°C had a mean sprout length of 38% of bulb height, whereas those cured at 28°C were 20% of bulb height at 25 weeks after harvest (Table I). Differences between treatments were only observed after 25 weeks, with the most significant reductions in sprout growth due to ethylene and 1-MCP in combination before (19% of bulb height) or after (12% of bulb height) curing compared with the control bulbs (45% of bulb height). In addition, onions treated with only 1-MCP before (23% of bulb height) or after (31% of bulb height) curing had shorter sprouts than the control.
Figure 1.
Schematic diagram of the experimental plan.
Table I. minus1 minus1Sprout length of Sherpa onions measured 25 and 35 weeks after harvest (6 weeks of curing and then transfer to cold storage) treated before or after curing with 10 μL L−1 ethylene and/or 1 μL L−1 1-MCP for 24 h at 20°C (n = 12).
Treatments are defined in “Materials and Methods.” lsd (P = 0.05) = 22.86.
| Sprout Length |
||||
| Treatment | 25 Weeks |
35 Weeks |
||
| 20°C | 28°C | 20°C | 28°C | |
|
% of bulb height |
||||
| Control | 51.3 | 39.2 | 55.4 | 58.9 |
| EB | 47.3 | 30.8 | 68.0 | 58.8 |
| MB | 39.8 | 6.1 | 58.0 | 42.9 |
| EMB | 24.8 | 13.9 | 60.7 | 53.5 |
| EA | 46.0 | 19.1 | 51.6 | 54.3 |
| MA | 40.1 | 22.0 | 62.5 | 56.1 |
| EMA | 15.3 | 7.9 | 61.6 | 64.0 |
Interactions between prestorage treatments and continuous storage treatments were observed in onions cured at 20°C only. Ethylene treatment throughout storage reduced sprout growth (43% of bulb height) compared with controls held in air (59% of bulb height) irrespective of prestorage treatments. Nevertheless, mean sprout length of onions pretreated with combined ethylene and 1-MCP treatment before curing was even shorter, at 29% of bulb height (Table II). In contrast, onions treated with ethylene and 1-MCP after curing and then treated continuously with ethylene had longer sprouts (64% of bulb height), yet those continuously stored in air had shorter sprouts, at 38% of bulb height (Table II).
Table II. minus1 minus1Sprout length of Sherpa onions measured 35 weeks after harvest treated before or after curing with 10 μL L−1 ethylene and/or 1 μL L−1 1-MCP for 24 h at 20°C and then transferred to air or continuous ethylene storage at 0°C to 1°C (n = 6).
Treatments are defined in “Materials and Methods.” lsd (P = 0.05) = 14.78.
| Sprout Length |
||||
| Prestorage Treatment | Air |
Ethylene |
||
| 20°C | 28°C | 20°C | 28°C | |
|
% of bulb height |
||||
| Control | 63.1 | 56.2 | 49.3 | 38.9 |
| EB | 68.3 | 61.0 | 33.2 | 51.3 |
| MB | 64.1 | 61.0 | 41.9 | 49.7 |
| EMB | 63.4 | 60.1 | 28.8 | 37.4 |
| EA | 63.0 | 51.6 | 36.0 | 40.6 |
| EMA | 37.6 | 62.8 | 64.2 | 43.9 |
Continuous Supply of Ethylene during Storage Reduces Root Development
There was no main effect of curing temperature or treatment on rooting; however, the interactions between treatment and curing temperature were significant (P = 0.028). The percentage of bulbs with roots was only significantly lower in onions treated with ethylene after curing at 28°C. However, several treatments resulted in a higher percentage of onions with roots, including bulbs treated with ethylene before curing at 20°C, bulbs treated with ethylene and 1-MCP after curing at 28°C, bulbs treated with ethylene and 1-MCP before curing at 20°C, and bulbs treated with 1-MCP alone before curing at 20°C (data not shown).
The continuous supply of ethylene during storage reduced the incidence of rooting (18%) compared with control bulbs stored in air (63%). Less rooting was also observed in bulbs cured at 20°C (29%) compared with 28°C (51%). Onions treated with ethylene and 1-MCP after curing at 20°C and then stored in air had no rooting (Table III); this treatment regime also had an inhibitory effect on sprout growth (Table II). Onions cured at 20°C and then stored in continuous ethylene had almost no rooting irrespective of the prestorage treatment. Onions cured at 28°C and then stored in continuous ethylene had more rooting, but this was absent in onions pretreated with ethylene and 1-MCP before curing (Table III).
Table III. minus1 minus1Root incidence of Sherpa onions measured 35 weeks after harvest (6 weeks of curing and then transfer to cold storage) treated before or after curing with 10 μL L−1 ethylene and/or 1 μL L−1 1-MCP for 24 h at 20°C and then transferred to air or continuous ethylene storage at 0°C to 1°C (n = 6).
Treatments are defined in “Materials and Methods.” lsd (P = 0.05) = 40.30.
| Root Incidence |
||||
| Prestorage Treatment | Air |
Ethylene |
||
| 20°C | 28°C | 20°C | 28°C | |
|
% of bulbs with roots |
||||
| Control | 83.3 | 50.0 | 0.0 | 16.7 |
| EB | 33.3 | 83.3 | 0.0 | 16.7 |
| MB | 83.3 | 66.7 | 0.0 | 83.3 |
| EMB | 66.7 | 83.3 | 0.0 | 0.0 |
| EA | 50.0 | 83.3 | 0.0 | 33.3 |
| EMA | 0.0 | 66.7 | 33.3 | 33.3 |
Curing Onions Reduces Respiration
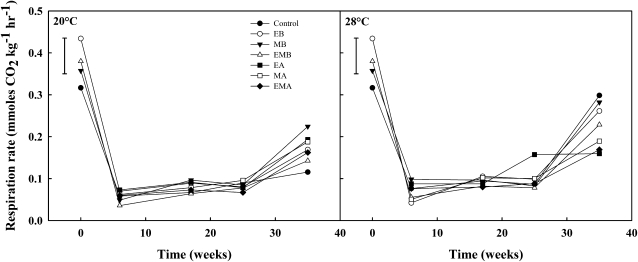
Respiration rate was measured throughout storage in onions stored in the United Kingdom only. Onion respiration rate was affected by curing, with a 6-fold decrease over 6 weeks. Before curing, control bulbs had the lowest respiration rate, bulbs treated with ethylene had the highest respiration rate, and onions treated with 1-MCP alone or in combination with ethylene fell between the two; however, this was not quite significant (Fig. 2). Control bulbs cured at 20°C had the lowest respiration rate at the end of storage, compared with control bulbs cured at 28°C, which had the highest respiration rate. Treatments applied before curing at 28°C gave higher respiration rates than in bulbs treated after curing at 28°C.
Figure 2.
Respiration rate (mmol CO2 kg−1 h−1) of Sherpa onions treated with ethylene before curing (EB), 1-MCP before curing (MB), ethylene and 1-MCP before curing (EMB), ethylene after curing (EA), 1-MCP after curing (MA), ethylene and 1-MCP after curing (EMA), or no treatment (control) for 24 h at 20°C (n = 12). lsd bars (P = 0.05) are shown.
Treatment with Ethylene and/or 1-MCP Does Not Affect Bulb Dry Matter
Onion bulb dry weight was not affected by prestorage treatments in the onions stored in air. However, onion dry weight was affected by curing temperature and time. There was no change in dry weight of onions cured at 20°C throughout storage, but those cured at 28°C had higher dry weight before curing (116 mg g−1 fresh weight) than the mean value of all postcured onions (110 mg g−1 fresh weight). No significant differences in dry weight were found between prestorage treatments (ethylene and/or 1-MCP), storage treatments (continuous ethylene/air), or curing temperatures (20°C or 28°C) in the onions stored in continuous ethylene treatment.
Curing Temperature and Postcuring Treatments Alter Carbohydrate Concentrations
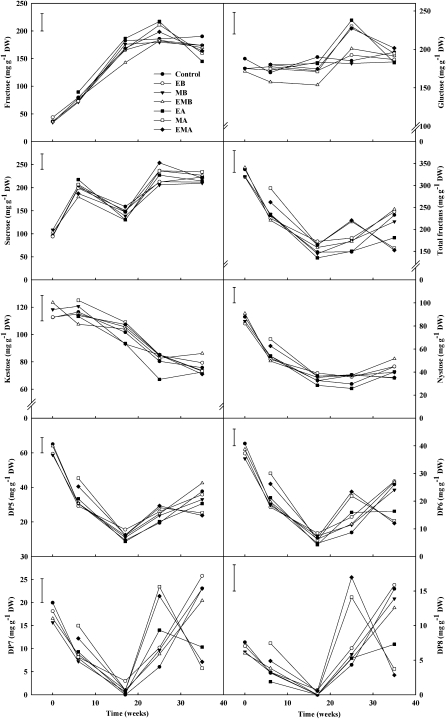
Nonstructural carbohydrates were measured in all samples to assess the impacts of treatments on carbohydrate metabolism during curing and storage. The Glc content of onions treated before curing with combined ethylene and 1-MCP was lower throughout storage, yet by 25 weeks, Glc had increased in line with the control. This lower Glc content in onions treated with ethylene and 1-MCP before curing was also observed in Fru, but only at 17 weeks after harvest. Onions treated after curing had higher Suc content at 25 weeks (254 mg g−1 dry weight) than those treated before curing (236 mg g−1 dry weight) and the control (212 mg g−1 dry weight). This trend was also observed in the onion Glc content coinciding with the initiation of sprout growth. Fru content tended not to vary much between treatments, but at the end of storage, onions treated with ethylene after curing had lower Fru content (145 mg g−1 dry weight) than the control (190 mg g−1 dry weight; Fig. 3). All other treated onions had lower Fru content compared with the control at the end of storage; however, those treated with ethylene after curing were the only onions with lower Fru content.
Figure 3.
Fru, Glc, Suc, and total fructans (DP3–DP8) of Sherpa onions treated with ethylene before curing (EB), 1-MCP before curing (MB), ethylene and 1-MCP before curing (EMB), ethylene after curing (EA), 1-MCP after curing (MA), ethylene and 1-MCP after curing (EMA), or no treatment (control) for 24 h at 20°C (n = 12). lsd bars (P = 0.05) are shown. DW, Dry weight.
Suc and total fructans were the only nonstructural carbohydrates affected by curing temperature (data not shown). Onions cured at 28°C had higher Suc content (206 mg g−1 dry weight) but lower total fructans (187 mg g−1 dry weight) than those cured at 20°C (188 and 203 mg g−1 dry weight, respectively). The lower content of total fructans in onions cured at 28°C was due to lower nystose, DP5 and DP6 (for degrees of polymerization) contents. Suc and total fructan contents were 1.2- and 1.5-fold higher, respectively, after 25 weeks, in onions treated in combination with ethylene and 1-MCP after curing. This peak was also observed in onions treated with 1-MCP after curing but only contained higher total fructans (1.5-fold increase) and not higher Suc content. Notably, the difference in total fructan content between treatments was due to the largest fructans, DP6 to DP8 (Fig. 3). This peak in total fructans at 25 weeks in onions treated with ethylene and 1-MCP in combination after curing or 1-MCP after curing did subsequently decrease by almost half during the final 10 weeks of storage. In contrast, an increase in total fructans was observed in the control onions or onions treated before curing or with ethylene after curing in the final 10 weeks of storage. It was difficult to compare the biochemical carbohydrate data with gene expression profiles, as very few genes classified as being involved in carbohydrate metabolism were differentially regulated in response to the treatments. Only one gene classified as being involved in carbohydrate metabolism was differently regulated in response to the short 24-h treatments. Cellulose synthase-like family C (CSLC9) was down-regulated in response to 1-MCP in the presence and absence of ethylene.
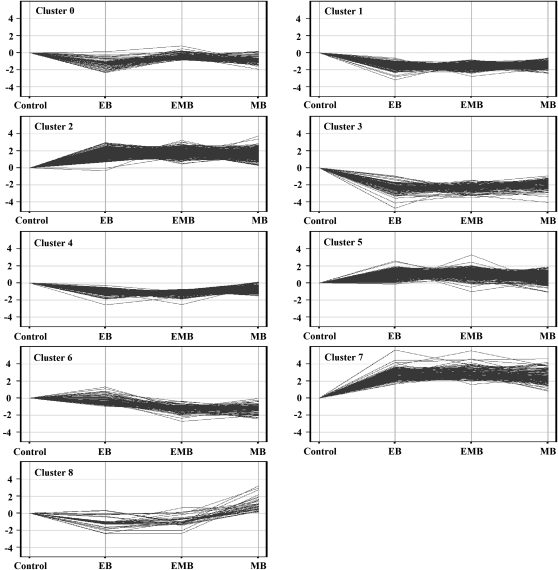
Ethylene and 1-MCP Elicit Unique Transcriptional Profiles
An onion microarray was utilized to characterize the transcriptional profiles of onions subjected to ethylene and/or 1-MCP treatments before and after curing and continuously treated with ethylene during storage (Fig. 1). In total, 1,228 probes representing transcripts with differential changes in expression were observed in response to ethylene and/or 1-MCP treatment as compared with the control. These probes were clustered into nine groups depending on their degree of response to each stimulus. Six of the clusters (clusters 1–5 and 7), representing 1,048 probes, had similar expression profiles across all precuring treatments (Fig. 4). The remaining 180 probes were divided into three clusters (clusters 0, 6, and 8), which showed differential expression when treated with ethylene and/or 1-MCP (Fig. 4). Cluster 0 represented 71 probes, including GA 20 oxidase 2, whose transcript abundance was lower in onions treated with ethylene or 1-MCP alone, but no change was observed in their abundance in onions treated with ethylene and 1-MCP together. Cluster 6 represented 87 probes whose transcript abundance was lower in onions treated with 1-MCP whether in the presence of ethylene or not, including the GA receptor GID1L2 and CSLC9. Finally, cluster 8 included 22 probes whose transcript abundance was lower in onions treated with ethylene irrespective of whether 1-MCP was present or not. These included precursors for expansin and a protease inhibitor/seed storage/LTP family protein.
Figure 4.
K-means cluster analysis of altered onion gene expression sampled following treatment before curing for 24 h at 20°C with ethylene (EB), 1-MCP (MB), ethylene and 1-MCP (EMB), or untreated (control).
Probes were classified into functional categories (Table IV) based on their similarity to a rice protein sequence database. Although probes representing transcripts characterized as being related to PGRs included those associated with auxins, cytokinins, and ethylene (Supplemental Table S1), the only PGR probes that were differentially expressed between ethylene and 1-MCP treatments were GA receptors and GA oxidase. Table V details the 30 most up- or down-regulated probes after short treatment precuring or postcuring with ethylene and/or 1-MCP treatments. A gene that appeared twice in the 30 most up- or down-regulated genes in response to treatment was 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR), which was up-regulated in response to ethylene alone or in combination with 1-MCP applied before curing.
Table IV. Functional classification of onion probes differentially expressed when treated before curing for 24 h at 20°C with ethylene, 1-MCP, ethylene and 1-MCP, or untreated (control).
| Functional Category | Cluster |
Row Totals | ||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Housekeeping | 11 | 20 | 40 | 17 | 49 | 37 | 21 | 14 | 6 | 215 |
| Stress and defense | 3 | 10 | 6 | 4 | 5 | 8 | 6 | 9 | 3 | 54 |
| Chaperones | 0 | 3 | 3 | 2 | 5 | 0 | 2 | 0 | 0 | 15 |
| Photosynthesis | 0 | 1 | 4 | 1 | 5 | 3 | 2 | 1 | 0 | 17 |
| Cell wall metabolism | 0 | 3 | 2 | 1 | 4 | 3 | 2 | 3 | 0 | 18 |
| Secondary metabolism | 3 | 8 | 7 | 1 | 4 | 3 | 3 | 4 | 2 | 35 |
| Cell death | 1 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 0 | 6 |
| Peptidase/kinase | 8 | 11 | 29 | 10 | 13 | 27 | 6 | 19 | 3 | 126 |
| Transport | 4 | 10 | 24 | 3 | 9 | 20 | 3 | 15 | 2 | 90 |
| Signaling | 3 | 0 | 4 | 1 | 8 | 6 | 2 | 4 | 0 | 28 |
| Metabolism | 8 | 22 | 29 | 4 | 20 | 23 | 8 | 13 | 2 | 129 |
| PGR | 1 | 6 | 7 | 0 | 5 | 2 | 2 | 5 | 0 | 29 |
| Cell cycle | 1 | 1 | 2 | 0 | 5 | 3 | 0 | 1 | 1 | 13 |
| Transcription factor | 9 | 11 | 34 | 2 | 15 | 15 | 6 | 14 | 1 | 107 |
| Unclassified | 19 | 44 | 75 | 19 | 49 | 64 | 21 | 34 | 2 | 327 |
| Phosphatase | 0 | 2 | 3 | 1 | 2 | 4 | 3 | 4 | 0 | 19 |
| Row totals | 71 | 152 | 271 | 66 | 200 | 219 | 87 | 140 | 22 | 1,228 |
Table V. The 30 most highly up- or down-regulated onion probes compared with controls after treatment with EB, MB, or EMB.
Treatments are defined in “Materials and Methods.”
| Probe | Tentative Annotation | Fold Changea | Regulation | Onion Sequence Identifier | Treatment |
| CUST_792_PI403527117 | Integral membrane protein | 51.1 | Up | TC4630 | EB |
| CUST_2054_PI403527117 | 3-Hydroxy-3-methylglutaryl-coenzyme A reductase | 48.1 | Up | TC5892 | EMB |
| CUST_390_PI403527117 | Transferase family protein | 42.0 | Up | TC4228 | MB |
| CUST_5592_PI403527117 | WD domain, G-β repeat domain-containing protein | 33.9 | Up | CF447771 | MB |
| CUST_3008_PI403527117 | Retrotransposon protein | 28.9 | Up | TC6846 | MB |
| CUST_2287_PI403527117 | LTPL121; protease inhibitor/seed storage/LTP family protein precursor | 26.4 | Down | TC6125 | EB |
| CUST_10068_PI403527117 | 1-Aminocyclopropane-1-carboxylate oxidase homolog 4 | 24.7 | Up | CF438875 | EMB |
| CUST_7201_PI403527117 | Starch synthase | 23.8 | Down | CF437167 | EMB |
| CUST_160_PI403527117 | Per1-like family protein | 22.6 | Up | TC3998 | EMB |
| CUST_3247_PI403527117 | CHIT5; chitinase family protein precursor | 21.8 | Up | TC7085 | EMB |
| CUST_4826_PI403527117 | Protein kinase family protein | 21.0 | Up | CF438357 | MB |
| CUST_7052_PI403527117 | S-Formylglutathione hydrolase | 18.4 | Up | CF448815 | MB |
| CUST_160_PI403527117 | Per1-like family protein | 18.2 | Up | TC3998 | EB |
| CUST_11478_PI403527117 | Stress-responsive protein | 18.1 | Up | BI095628 | EMB |
| CUST_10708_PI403527117 | Amino acid transporter | 17.9 | Up | CF440190 | EB |
| CUST_10973_PI403527117 | Monocopper oxidase | 17.7 | Down | BE205651 | EB |
| CUST_1451_PI403527117 | Dihydrodipicolinate synthase, chloroplast precursor | 17.1 | Up | TC5289 | MB |
| CUST_36_PI403527117 | Peroxidase precursor | 16.6 | Up | TC3874 | MB |
| CUST_10095_PI403527117 | Ser/Thr protein phosphatase family protein | 16.3 | Up | CF440115 | MB |
| CUST_600_PI403527117 | EF hand family protein | 15.9 | Up | TC4438 | EMB |
| CUST_6021_PI403527117 | OsWRKY48; superfamily of transcription factors with WRKY and zinc finger domains | 15.3 | Down | CF439568 | EMB |
| CUST_6801_PI403527117 | Zinc finger family protein | 15.3 | Down | CF435756 | EB |
| CUST_9681_PI403527117 | α-Soluble NSF attachment protein | 15.1 | Up | BQ580069 | EMB |
| CUST_792_PI403527117 | Integral membrane protein DUF6-containing protein | 15.0 | Up | TC4630 | EMB |
| CUST_11354_PI403527117 | Mitochondrial carrier protein | 14.8 | Up | CF441173 | MB |
| CUST_2054_PI403527117 | 3-Hydroxy-3-methylglutaryl-coenzyme A reductase | 14.5 | Up | TC5892 | EB |
| CUST_160_PI403527117 | Per1-like family protein | 14.4 | Up | TC3998 | MB |
| CUST_10095_PI403527117 | Ser/Thr protein phosphatase family protein | 12.7 | Up | CF440115 | EB |
| CUST_4897_PI403527117 | Aldehyde dehydrogenase | 12.6 | Up | CF442148 | EB |
| CUST_10269_PI403527117 | Myristoyl-acyl carrier protein thioesterase, chloroplast precursor | 12.6 | Up | CF445478 | EB |
Fold change compared with expression of control, calculated as 2x, where x = absolute value of (normalized treatment/normalized control).
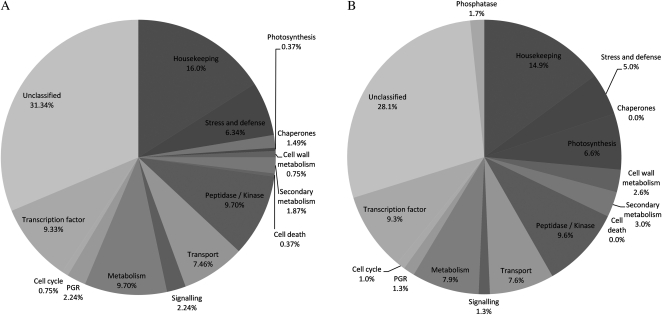
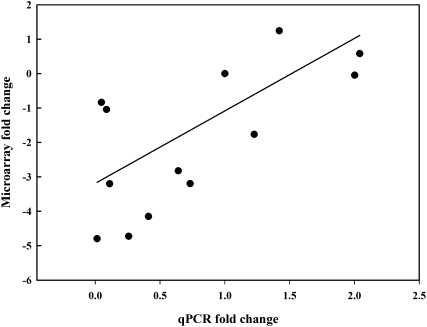
The abundance of transcripts represented by 574 probes was differentially regulated in response to continuous ethylene storage, with 272 having greater abundance and 302 having less abundance than the control treatment (Fig. 5). Functional characterization of these probes revealed that a relatively large proportion of the down-regulated genes were classified as involved in photosynthesis (6.6%), whereas only 0.4% of up-regulated genes were in this class. Interestingly, the transcript abundances of several probes related to PGRs were greater under continuous ethylene, including GA 2-β-dioxygenase, ethylene-insensitive 3 (EIN3; Supplemental Table S1), an auxin-responsive gene family member, and importantly, 1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE (ACO; Supplemental Table S1). Other probes annotated as being related to PGRs revealed the down-regulation of an auxin efflux carrier component and a cytokinin dehydrogenase by continuous ethylene. To test the sensitivity and consistency of the microarray analysis, the expression of five probes was determined using quantitative real-time PCR (qPCR). There was a qualitative relationship between the qPCR and microarray data, with a correlation coefficient of r = 0.68 (P < 0.001; Fig. 6), confirming that the data from the microarray presented an accurate indication of transcript abundances in the onion samples.
Figure 5.
Functional classification of 272 probes up-regulated by continuous ethylene treatment (A) and 302 probes down-regulated by continuous ethylene treatment (B) sampled after 29 weeks of storage.
Figure 6.
Correlation between the gene expression of five genes quantified using the onion microarray and qPCR. The expression of the five genes was quantified for each onion treatment (r = 0.68, P < 0.001).
DISCUSSION
Onions were treated before or after curing (6 weeks at 20°C or 28°C) with 24-h treatments of ethylene and/or 1-MCP before being transferred to cold storage at Cranfield University. A subset of these onions was removed following precuring treatment and 6 weeks of curing for cold storage in continuous air or ethylene (Fig. 1). Biochemical, physiological, and molecular techniques were used to identify the most successful methods of onion sprout suppression and the transcriptomic changes that occurred following each treatment to help identify possible mechanisms for sprout suppression.
Sprout Suppression through Exogenous Ethylene Down-Regulates Photosynthesis-Related Genes
Onions treated with ethylene and 1-MCP after curing had the shortest sprout length after 25 weeks in storage, and this was also found in the subset of onions stored in continuous air 35 weeks after harvest. The shorter sprout growth in onions treated with combined ethylene and 1-MCP after curing was also supported by the reduced utilization of Suc and the larger fructooligosaccharides, DP6, DP7, and DP8, which were higher at 35 weeks (Fig. 3). Treatment with ethylene and 1-MCP after curing resulted in no root growth in the onions held in continuous air. Although treatment with ethylene and 1-MCP before curing and 1-MCP before curing at 28°C also resulted in shorter sprouts, this was not consistent with the subset of onions stored separately. This said, onions treated with ethylene and 1-MCP in combination before curing and 1-MCP before curing had reduced expression of a probe annotated as coding for the protein CSLC9 following treatment. This protein is involved in cell wall polysaccharide synthesis; therefore, the down-regulation of this protein in response to the above-mentioned treatments may have played a role in the reduction of sprout growth by suppressing the production of new growth.
Comparison between onions stored in continuous air or ethylene revealed that those stored in ethylene had reduced sprout growth, which is consistent with similar previous work (Bufler, 2009). Reduced sprout growth in response to continuous ethylene storage was also supported by the microarray data. Onions treated with continuous ethylene had a higher proportion of down-regulated probes characterized as being involved in photosynthesis compared with onions stored in air. This suggests that the onions stored in ethylene had not yet reached the advanced stages of sprouting when the growing sprout becomes green. The question remains whether the down-regulation of probes characterized as being involved in photosynthesis in onions stored in continuous ethylene is a direct result of the ethylene or a result of the slowed sprout development. Immediately after treatment with ethylene for 24 h before curing, and therefore prior to sprout growth, only 17 probes characterized as being involved in photosynthesis were found to be differentially regulated as compared with the control. Nine of these genes were down-regulated and eight were up-regulated (0.7% and 0.6% of the total genes differentially regulated, respectively). This suggests that the greater proportion of down-regulated photosynthesis-related probes following continuous ethylene treatment are more likely to be due to the delay in sprout development rather than ethylene itself. That said, the molecular responses to a short 24-h treatment before curing and extended continuous ethylene treatment throughout storage are likely to differ; therefore, further investigation is required.
Ethylene Is Perceived in the Presence of 1-MCP in Onion
Short treatments with ethylene and 1-MCP individually have both been shown to reduce sprout growth in onion (Chope et al., 2007a; Downes et al., 2010), yet no work has investigated the effect of both ethylene and 1-MCP applied together. In Russet Burbank potato tubers, 1-MCP has been used to reduce the reported detrimental effect of ethylene on fry color darkening; 1-MCP did not interfere with ethylene-induced sprout suppression, and ethylene did not cause such a dark fry color when tubers were pretreated with 1-MCP (Prange et al., 2005). Prange et al. (2005) hypothesized that the 1-MCP may bind to the ethylene receptors and that the continuous ethylene then regulates sprout growth by binding to newly formed ethylene receptors in the sprout eyes, where mitotic activity is highest. It is possible that at these sites of high mitotic activity (e.g. potato eyes), in addition to the production of new ethylene receptors, greater 1-MCP metabolism may occur, since Huber et al. (2010) suggested that 1-MCP may be metabolized in planta.
In this study, treatment of onions with ethylene and 1-MCP together resulted in higher Suc and fructan concentrations than found in those treated with ethylene alone (Fig. 3). Also, sprout growth was reduced in onions treated with combined ethylene and 1-MCP but not in those treated with each compound separately. Therefore, this study suggests that ethylene and 1-MCP applied simultaneously for 24 h affects onion physiology and biochemistry differently than when applied individually, suggesting that ethylene and 1-MCP are both eliciting a response. This may be a consequence of different affinities of receptors for 1-MCP and ethylene. This is plausible, since there are five known receptors identified in Arabidopsis (Arabidopsis thaliana): ETR1, ETR2, ERS1, ERS2, and EIN4 (Chang et al., 1993; Hua et al., 1995; Hua and Meyerowitz, 1998; Sakai et al., 1998), and it is unknown whether 1-MCP binds similarly to each.
Ethylene has previously been shown to increase respiration rate in onion (Ecker and Davis, 1987; Downes et al., 2010); in this study, respiration rate of the treated onions was highest after treatment with ethylene and lowest after treatment with 1-MCP. The respiration rate of onions treated with both ethylene and 1-MCP together lay between those treated with either ethylene or 1-MCP alone, suggesting that the physiological response of onions to ethylene in the presence of 1-MCP was not as great as when 1-MCP was absent. This increase in respiration rate in response to ethylene in the presence or absence of 1-MCP may explain the increase in the expression of probes annotated as HMGR following these same treatments. In plants, HMGR may be related to sterol biosynthesis and membrane biosynthesis, and its activity has been positively correlated with rapidly dividing cells in maize (Zea mays; Ji et al., 1992). An increase in the expression of HMGR could suggest an increase in rapidly dividing cells and therefore sprout growth. However, ethylene alone or in combination with 1-MCP did not result in increased sprout growth as compared with the control onions. Following treatment with ethylene, respiration rate returned to levels in line with the control onions. Therefore, it would be interesting for future research to investigate whether the expression of HMGR also returns to baseline levels shortly after ethylene treatment. HMGR is also involved in the production of sesquiterpenes and, like ethylene, is involved in plant defense (Chappell et al., 1997). It is possible that the increase in expression of HMGR was a direct result of the ethylene treatment; however, HMGR was not up- or down-regulated by continuous ethylene treatment (Supplemental Table S2).
1-MCP May Not Bind All Ethylene Receptors
At the transcriptional level, three clusters representing 180 probes showed a differential response to ethylene and/or 1-MCP, suggesting that ethylene and 1-MCP probably do not elicit the same response by being perceived as the same molecule. In climacteric fruits, 1-MCP is believed to block the ethylene molecule from binding to the receptor, preventing the perception of ethylene, and it is unlikely that this mechanism differs in onion. Cluster 0 contained probes representing transcripts down-regulated by exogenous ethylene but not in the presence of 1-MCP, suggesting that these transcripts may only respond to a specific ethylene receptor or group of receptors that bind 1-MCP. In contrast, cluster 8 contained a set of probes representing transcripts only up-regulated by 1-MCP alone but down-regulated by ethylene alone or in the presence of 1-MCP. This suggests that these transcripts respond to ethylene perception by a receptor or group of receptors not bound by 1-MCP. Differences in ethylene and 1-MCP concentration, treatment duration, timing, and temperature may result in differential gene expression, since physiological and biochemical responses differ depending on these parameters (Blankenship and Dole, 2003; Watkins, 2006; Bufler, 2009). In addition, it is worth noting that given the differences in dormancy between various onion cultivars, it is difficult to make broad predictions of ethylene responses in onions (Yasin and Bufler, 2007).
Exogenously Applied Ethylene and/or 1-MCP Down-Regulate Ethylene Receptors
All microarray probes representing transcripts with differential expression characterized as being involved with ethylene showed a similar pattern in expression when treated with ethylene and/or 1-MCP. Ethylene and 1-MCP both appeared to have an effect on ethylene perception by down-regulating a transcript with similarity to an ethylene receptor. Other nonclimacteric species, such as citrus fruits, have low and continuous production of ethylene, which is autoinhibited following propylene treatment (Katz et al., 2004). Although citrus fruits exhibit some climacteric-like characteristics in the early stages of development, during the nonclimacteric later phase, Citrus Ethylene Response Sensor1 (CsERS1) expression remained constant following ethylene treatment (20 μL L−1). Treatment with 1-MCP was only applied after harvest, when the citrus fruits were in the climacteric-like phase; however, 1-MCP was found to down-regulate CsERS1, interfering with the autocatalytic production of ethylene. The results herein suggest that ethylene may actually reduce the expression of an ethylene receptor in onion. The other citrus ethylene receptor, Citrus Ethylene Receptor1 (CsETR1), was not affected by ethylene or 1-MCP, and Katz et al. (2004) concluded that this specific receptor may not be regulated by ethylene. Similarly, Rasori et al. (2002) found no change in the regulation of ETR1 but found down-regulation of ERS1 after treatment with 1 μL L−1 1-MCP (25°C for 24 h) in climacteric Maria Marta peach (Prunus persica).
The results in our study show that 1-MCP down-regulated an ethylene receptor in onion, yet this was also found after ethylene treatment. Taken together with previous findings, this suggests that exogenously applied ethylene and/or 1-MCP may mediate ethylene perception by down-regulating the production of some but not all ethylene receptors. That said, Ma et al. (2009) found that treatment of broccoli (Brassica oleracea) florets with 2.5 μL L−1 1-MCP for 12 h decreased gene expression of the broccoli ethylene receptors ETR1 and ETR2.
Ethylene and 1-MCP Down-Regulate EIN3 in the Early Stages of Storage
In Arabidopsis, the absence of ethylene usually results in the rapid degradation of EIN3 (Guo and Ecker, 2003), a transcription factor acting downstream of the ethylene receptors in the ethylene signaling pathway (Alonso et al., 1999). However, the results presented here have found that the presence of ethylene and 1-MCP appears to down-regulate EIN3. This down-regulation of both an ethylene receptor and ethylene transcriptional regulators by both ethylene and 1-MCP may help to explain why both compounds result in sprout suppression (Chope et al., 2007a; Downes et al., 2010), by down-regulating the perception and signaling events of ethylene. In direct contrast, gene expression analysis of onion treated with continuous ethylene for 29 weeks (plus 6 weeks of curing) revealed a greater transcript abundance for probes annotated as EIN3 and ACO, which is involved in ethylene biosynthesis. As well as an increase in the expression of these transcripts, an increase in the transcript abundance of a probe annotated as GA 2-β-dioxygenase was also observed, which is involved in GA biosynthesis. The probe representing a transcript annotated as cytokinin dehydrogenase was down-regulated; cytokinin dehydrogenase is an enzyme that deactivates cytokinins through the cleavage of their side chains (Galuszka et al., 2001). Although after 35 weeks of storage, sprout growth of onions stored in continuous ethylene was less than in those held in continuous air, it is possible that ethylene was no longer having an inhibitory effect on sprout growth at this advanced stage of storage. G.A. Chope. K. Cools, J.P. Hammond, A.J. Thompson, and L.A. Terry (unpublished data) found that onions may become less sensitive to ethylene and produce less endogenous ethylene the longer they are in storage. This was evidenced by a consistently low transcript abundance of probes with similarity to 1-aminocyclopropene-1-carboxylate synthase, involved in ethylene biosynthesis, and EIN3, a transcriptional regulator. It would be interesting to investigate at what stage of storage the inhibitory effects that ethylene has on the transcriptional regulation of PGRs take hold.
In conclusion, our experiments showed that treating onions with combined ethylene and 1-MCP after curing for just 24 h consistently reduced sprout and root growth for 25 weeks. Long-term storage over 25 weeks may require extended periods of ethylene treatment, although beyond this period, transcriptional changes suggest that continuous ethylene no longer controlled onion PGRs. Previous hypotheses have intimated that ethylene and 1-MCP may each be able to elicit a response in potato due to the production of new ethylene-binding sites (Prange et al., 2005). An alternative explanation, supported by our data, might be that ethylene and 1-MCP bind with different affinities to different ethylene receptors in onion. It appeared that ethylene and/or 1-MCP down-regulated probes representing transcripts annotated as ethylene receptors as well as ethylene transcriptional regulators (EIN3). Further research is required into the structures of different ethylene receptors to investigate whether 1-MCP can bind all receptors and with what affinity. Since microarray data were only gathered from onions immediately after treatment at the beginning of storage and at the end of storage in continuous ethylene, it would be interesting to further investigate the dynamic effect that ethylene/1-MCP has at the transcriptional level and indeed the metabolic level.
MATERIALS AND METHODS
Plant Material and Curing
Onion (Allium cepa ‘Sherpa’) seeds (medium pungency, medium dry matter) were drilled on sandy clay loam (Alistair Findlay’s; 1.2 × 0.3 ha) on March 5, 2008, at a rate of 57 seeds m−2 with pesticides applied as per commercial practice, although remaining maleic hydrazide free. Plants were machine harvested at 100% fall-down on September 17, 2009. Onions were stored in 72 large net bags (approximately 60 bulbs) and 24 half net bags (approximately 30 bulbs) buried among loose bulbs in 1-ton wooden crates for batch curing at Sutton Bridge Crop Storage Research (Lincolnshire UK). Bulbs were artificially cured at either 20°C or 28°C for 6 weeks as per normal commercial practice in the United Kingdom, with relative humidity controlled at 65% to 75%.
Experimental Design
The experiment was a completely randomized design with three replicates taken from three sections of the field. There were seven postharvest treatments per replicate: (1) 1 μL L−1 1-MCP before curing (MB); (2) 10 μL L−1 ethylene before curing (EB); (3) both 10 μL L−1 ethylene and 1 μL L−1 1-MCP before curing (EMB); (4) 1 μL L−1 1-MCP after curing (MA); (5) 10 μL L−1 ethylene after curing (EA); (6) both 10 μL L−1 ethylene and 1 μL L−1 1-MCP after curing (EMA); and (7) control (no treatment). Treatments were applied in water-sealed, air-tight polypropylene chambers (88 cm × 59 cm × 59 cm) that housed two 8- × 8-cm electric fans (Nidec beta SL) to circulate the gases during treatments. Onions were treated in the chambers for 24 h at 20°C, and the control bulbs were held at 20°C in air. In the treatment boxes, levels of CO2 did not rise above 0.30%. The 1-MCP was applied by adding 1.8 g of SmartFresh (0.14%; Rohm and Haas) to a 50-mL conical flask and sealed with Nescofilm (Bando Chemical Industries). To release 1 μL L−1 1-MCP gas, 20 mL of warm (50°C) water was injected into the conical flask through the Nescofilm using a needle and syringe prior to transfer to the chamber (Chope et al., 2007a). Ethylene treatment (10 μL L−1) was administered immediately after 1-MCP treatment by injecting 3.25 mL of ethylene (100% ethylene; SIP Analytical) directly into the chamber via a tapped tube (polyvinyl chloride) followed by repeated full withdrawal-injection displacements to flush the ethylene into the chamber.
Prestorage-Treated Onions
After curing, onions were transported to Cranfield University within 2.5 h. Diseased or damaged onions were removed, and the remaining onions were randomly placed in individual plastic stackable crates and stored in air for 29 weeks at 0°C to 1°C in the dark (Fig. 1). At each sampling time, four onions per treatment, curing temperature, and replicate (n = 168) were selected randomly, taken after harvest (day 0), immediately after curing (6 weeks), and then at intervals during cold storage (17, 25, and 35 weeks after harvest; n = 840).
Prestorage- and Storage-Treated Onions
After curing, a subset of the treated onions was transported (6 ± 1 h) at ambient temperature to the Research Institute of Vegetable Crops in Skierniewice, Poland, for continuous air or ethylene treatment (Fig. 1). Control onions and onions treated with EB, MB, EMB, EA, and EMA cured at either 20°C or 28°C for 6 weeks were placed in individual plastic trays and stored in air or 10 μL L−1 ethylene for a further 29 weeks at 0°C to 1°C in the dark. Six onions per prestorage treatment, postcuring treatment, and curing temperature (n = 144) were selected at random at the end of storage (35 weeks after harvest).
Sample Preparation
Onions stored in the United Kingdom were removed from storage 1 d prior to sample preparation for gas analysis. Each bulb was then halved, and visible sprout growth was recorded in mm and expressed as a percentage of the bulb height (Chope et al., 2007b). Two longitudinal wedges were cut and snap frozen in liquid nitrogen, and each was then stored at −40°C for biochemical analysis and −80°C for RNA extraction. Frozen tissue for biochemical analysis was lyophilized using an alpha 1-4 Christ LDC-1 freeze dryer and pump (Edwards Super Modulo) and powdered using a pestle (Chope et al., 2007b). Sprout and root growth and disease incidence were measured in the onions sent to Poland for continuous ethylene treatment. Onions were snap frozen in liquid nitrogen in Poland and returned to the United Kingdom on dry ice for microarray analysis.
Physiological Measurements
Respiration Rate
Respiration rate was measured immediately before and after curing and at each time point throughout cold storage. Four onions were placed in 3-L jars with air-tight lids and septum. The jars were sealed for 4 h at room temperature, and gas samples were removed with repeated full withdrawal-injection displacements using a 30-mL plastic syringe (Chope et al., 2007a). Gas samples were analyzed using gas chromatography (model 8340 gas chromatograph and DP800 integrator; Carlos Erba Instruments) coupled with hot-wire detection (Chope et al., 2007a; Terry et al., 2007a). The gas chromatograph was calibrated using 10.06% CO2 (10% CO2, 2% oxygen, 88% N2; certified standard from British Oxygen Company). The four onions were weighed, and respiration rate was expressed in mmol kg−1 h−1.
Biochemical Measurements
HPLC was used to quantify the concentration of sugars and fructans. All chemicals for these assays were purchased from Sigma unless otherwise stated.
Extraction and Quantification of Sugars
Fru, Glc, Suc, and fructans were extracted according to Downes and Terry (2010). Onion powder (150 mg) was extracted using 2.25 mL of HPLC-grade water for 10 min at 75°C to extract the fructans. To the slurry, 3.75 mL of methanol was added to give a final 62.5% methanol solution and extracted for 15 min at 55°C. The mixture was then passed through a 0.2-μm Millex-GV syringe-driven filter (Millipore). The extract was then stored at −40°C until further use. Glc, Fru, and Suc were quantified according to Chope et al. (2007a). Fructans were quantified according to Downes and Terry (2010). Extracts were thawed and loaded into a Dionex HPLC system with a P680 pump and an ASI-100 Automated Sampling Injector. The extract (10 μL) was injected into a Prevail Carbohydrate ES column of 250 mm × 4.6 mm diameter, 5-μm particle size (Alltech; part no. 35101) with a Prevail Carbohydrate ES guard cartridge of 7.5 mm × 4.6 mm diameter (Alltech; part no. 96435). The mobile phase consisted of HPLC-grade water (A) and ethanol (B). The gradient involved a linear increase/decrease of solvent B: 85% to 65%, 9 min; 65% to 85%, 3 min; 85%, 8 min, at a flow rate of 0.5 mL min−1 and column temperature of 40°C. An evaporative light-scattering detector (ELSD 2420; Waters) connected to the system via a UCI-50 universal chromatography interface detected the eluted carbohydrates. Carbohydrate concentrations were calculated against calibration standards: Fru, Glc, Suc, 1-kestose, and nystose ranging from 5 to 0.05 mg mL−1.
Microarray Analysis
RNA Extraction
Six samples were chosen for microarray analysis; four samples were taken before curing immediately after treatment with ethylene or 1-MCP or ethylene and 1-MCP in combination for 24 h at 20°C. The other two samples were taken after 29 weeks of cold storage (1°C) in continuous air or continuous ethylene. There were three biological replicates of each of the six treatments, making 18 samples in total. Total RNA was isolated according to Chang et al. (1993) with modifications.
Total RNA was extracted from frozen, ground onion tissue (100 mg) homogenized in 1 mL of extraction buffer (2% [w/v] cetyl trimethylammonium bromide, 0.8 m NaCl, 20 mm Na2EDTA, 0.2 m boric acid adjusted to pH 7.6 with TRIZMA base, and β-mercaptoethanol added to 1% [v/v] just prior to use) using a pestle and mortar. The mixture was transferred to a 2-mL microtube and incubated at 65°C for 10 min, then allowed to return to room temperature. Chloroform (1 mL) was added and mixed before being centrifuged at 13,000 rpm for 5 min at room temperature. The aqueous phase was removed to a clean tube, and an equal volume of precipitation buffer (0.5% [w/v] cetyl trimethylammonium bromide, 50 mm Na2EDTA, and 50 mm MES, adjusted to pH 5.8 with NaOH and filtered through a 0.2-μm sterile filter) was mixed and incubated on ice for 30 min. Samples were centrifuged at 13,000 rpm for 20 min at 4°C, and the supernatant was removed. The pellet was resuspended in SSTE (1.0 m NaCl, 0.5% SDS, 10 mm Tris-HCl [pH 8.0], and 1 mm Na2EDTA [pH 8.0]) and briefly incubated at 37°C before being allowed to return to room temperature. Chloroform (1 mL) was added and mixed before being centrifuged at 13,000 rpm for 5 min at room temperature. The aqueous phase was removed to a clean tube, and an equal volume of isopropanol was added and incubated on ice for 20 min. Samples were centrifuged at 13,000 rpm for 20 min at 4°C, and the supernatant was removed. The pellet containing total nucleic acid was washed with 1 mL of 70% (v/v) ethanol, left to air dry, and finally resuspended in 50 μL of diethyl pyrocarbonate-treated water. Then, 30 μL of 8 m lithium chloride solution was added, and the samples were incubated on ice overnight to selectively precipitate RNA. Samples were centrifuged at 13,000 rpm for 30 min at 4°C, the supernatant was removed, and the pellet was washed with 0.5 mL of 70% (v/v) ethanol and resuspended in 15 μL of RNase-free water. Sample purity and integrity were verified using the RNA 6000 Nano Assay on the Agilent 2100 BioAnalyzer (Agilent Technologies; Supplemental Data Set S1), and then samples were treated with Baseline Zero DNase (Epicentre) according to the supplier’s instructions.
Microarray
A total of 13,310 onion nucleotide sequences were available for the construction of a 60-mer oligonucleotide custom onion microarray. The majority were obtained from public databases: 13,154 from the Onion Gene Index (http://compbio.dfci.harvard.edu/cgi-bin/tgi/gimain.pl?gudb=onion) and 102 from GenBank (http://www.ncbi.nlm.nih.gov/genbank/), with the remaining 54 sequenced directly from onion bulb tissue. Microarrays were designed using Agilent Technologies e-array microarray design platform (https://earray.chem.agilent.com/earray/). The design process ensures that probes are designed to unique sequences within all sequences submitted for the design process, avoiding redundancy in the representation of sequences by probes. Initially, a prototype chip was designed in a 4 × 44K format where 60-mer oligonucleotide probes for ESTs and singletons were designed to both sense and antisense. Test hybridizations of RNA from a range of onion tissues (root, shoot, bulb, and leaf) were used to orientate these probes, thus reducing the number of probes, so the final format was 8 × 15K, consisting of eight independent arrays of 15K probes on a single glass slide. Each array consisted of 15,736 60-mer oligonucleotide probes in total, representing 536 internal control probes and 15,200 probes representing 13,310 unique onion sequences. In order to further our analyses of onion gene expression, the annotation for individual probes was populated with annotations from the closely related, fully sequenced genome of rice (Oryza sativa). Translated BLASTX alignments were made between onion sequences downloaded from the Onion Gene Index (release 2.0; http://compbio.dfci.harvard.edu/tgi/plant.html) and rice cDNA sequences from the Rice Genome Annotation project (version 6.1; http://rice.plantbiology.msu.edu/index.shtml). The TBLASTX alignments were performed with an E-value cutoff of 0.01 (Altschul et al., 1997). Annotations, including descriptions and Gene Onotology assignments, were then cross-referenced from rice sequences with significant homology to onion sequences, allowing Gene Onotology analysis and more informative descriptions on the putative role of onion genes.
The One Color Quick Amp Labeling Kit (Agilent Technologies) was used to amplify and label target RNA with cyanine 3-CTP to generate complementary RNA (cRNA) according to the manufacturer’s instructions. Purification of the labeled cRNA was performed using RNeasy mini spin columns (Qiagen) and quantified using a NanoDrop ND-1000 UV-VIS spectrometer. The One Color RNA Spike-In Kit (Agilent Technologies) was used as a positive control for monitoring sample amplification, labeling, and microarray processing. The cRNA was fragmented and hybridized to an onion oligonucleotide microarray, representing 13,310 unique onion sequences, using the Agilent Gene Expression Hybridization Kit and then washed with Gene Expression Wash Buffers 1 and 2, according to the manufacturer’s instructions (Agilent Technologies).
The microarray slides were scanned using an Agilent G2565BA Microarray Scanner with Agilent Scan Control version A8.4.1 at a resolution of 5 μm, using the extended dynamic range option. Signal values for individual probes were extracted using Feature Extraction version 10.5.1.1 software (Agilent Technologies).
qPCR Validation
To validate the microarray results, transcript levels of five differentially expressed transcripts identified in the microarray data were confirmed using qPCR (Fig. 6; Table VI). cDNA was synthesized using the ThermoScript RT-PCR System for First-Strand cDNA Synthesis Kit (Invitrogen; catalog no. 11146-024) from total RNA samples (1 μg) using a combination of random hexamers and oligo(dT) primers (20:80 mix, respectively). Gene-specific primers were designed using Primer 3 and PrimerSelect (Lasergene) software. Transcript abundance was detected by an ABI Prism 7900HT sequence detection system (Applied Biosystems) controlled by SBS 2.1 software (Applied Biosystems) using a SensiMix SYBR Green qPCR MasterMix (Bioline). qPCR was performed on 384-well plates using the “standard curve” method (Wong and Medrano, 2005) for mRNA quantification with normalization to the endogenous control gene, tumor protein TC4554 (CUST_716_P1403527117; forward, 5′-TCCGACTACAGGAACAACCAG-3′; reverse, 5′-AAACTCCTCTGCCTTCTCAGC-3′). The control gene was selected from six genes evaluated for stability within our samples using the geNorm software package (Vandesompele et al., 2002). Quantitative PCR conditions, efficiency calculations, and data normalizations were as described previously (Hammond et al., 2006).
Table VI. Primers used for qPCR analysis.
| Probe | Gene | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) |
| 3995_P1403527117 | ABTB1; armadillo repeat | TTGGCTCTTGCTCATCTTTG | ACCATCTTGCTGTTGCTTTG |
| 10973_P1403527117 | Monocopper oxidase | GATCGGAGAATTGGGAAAGAC | TTAGCTCGGCCACACAGAAG |
| 2287_P1403527117 | LTPL121; protease inhibitor/seed storage | CTGCACTCCTTGCCCTAAAC | CTCCCAGCTTCAGTGTATCG |
| 1126_P1403527117 | RNA polymerase | AAGTGGCGGTGGTCTGATAG | AGGCAGCAACAAAGATGGTAAG |
| 2252_PI403527117 | Starch synthase | ATGTTCGGGTTCTTTGTTCAG | GCCTCTTCTTCACTTACTTTCCAG |
Statistical Analysis
Statistical analyses were conducted using Genstat for Windows version 10.1.0.147 (VSN International). ANOVA was used to identify the main effects of cultivar, treatment, and time and the interactions between these factors to a value of P < 0.05 unless otherwise stated. The first sampling time (day 0; before curing) consisted of three treatments, and the outcomes thereafter consisted of five treatments. This imbalance was resolved by considering the first time point as a common baseline with which the remaining time points could be compared. lsd values (P = 0.05) were calculated from each analysis. Microarray data analysis was performed using GeneSpring GX11 (Agilent). There were three replicates for each treatment (control, ethylene before curing, 1-MCP before curing, ethylene and 1-MCP before curing, continuous storage in air, and continuous storage in ethylene), totaling 18 samples. The continuous treatment samples (n = 6) were analyzed separately from those treated before curing (n = 12). Raw expression data were subjected to quantile normalization, and then baseline normalization was applied to individual probes by dividing probe signal values by the median probe signal of control samples. Significantly differentially expressed transcripts were selected using one-way ANOVA (GeneSpring GX) with a Benjamini-Hochberg corrected value of P < 0.05 and a fold change cutoff greater than 2. Significantly differentially expressed transcripts were then grouped using the K-means clustering algorithm in GeneSpring GX.
All microarray data have been submitted to the online database Gene Expression Omnibus for public access and long-term storage (accession no. GSE27132).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Onion probes tentatively annotated as ethylene related differentially regulated in response to treatments.
Supplemental Table S2. Onion probes tentatively annotated as defense related differentially regulated in response to treatments.
Supplemental Data Set S1. Bioanalyzer data for RNA samples used.
Supplementary Material
Acknowledgments
We thank Sutton Bridge Crop Storage Research and Prof. F. Adamicki (Research Institute of Vegetable Crops) for use of their facilities.
References
- Adamicki F. (2005) Effects of pre-harvest treatments and storage conditions on quality and shelf-life of onions. Acta Hortic 688: 26–33 [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship SM, Dole JM. (2003) 1-Methylcyclopropene: a review. Postharvest Biol Technol 28: 1–25 [Google Scholar]
- Bufler G. (2009) Exogenous ethylene inhibits sprout growth in onion bulbs. Ann Bot (Lond) 103: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. (1993) Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science 262: 539–544 [DOI] [PubMed] [Google Scholar]
- Chappell J, VonLanken C, Vögeli U. (1997) Elicitor-induced 3-hydroxy-3-methylglutaryl coenzyme A reductase activity is required for sesquiterpene accumulation in tobacco cell suspension cultures. Plant Physiol 97: 693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chope GA, Terry LA, White PJ. (2007a) The effect of 1-methylcyclopropene (1-MCP) on the physical and biochemical characteristics of onion cv. SS1 bulbs during storage. Postharvest Biol Technol 44: 131–140 [Google Scholar]
- Chope GA, Terry LA, White PJ. (2007b) The effect of the transition between controlled atmosphere and regular atmosphere storage on bulbs of onion cultivars SS1, Carlos and Renate. Postharvest Biol Technol 44: 228–239 [Google Scholar]
- Downes K, Chope GA, Terry LA. (2010) Postharvest application of ethylene and 1-methylcyclopropene either before or after curing affects onion (Allium cepa L.) bulb quality during long term cold storage. Postharvest Biol Technol 55: 36–44 [Google Scholar]
- Downes K, Terry LA. (2010) A new acetonitrile-free mobile phase method for LC-ELSD quantification of fructooligosaccharides in onion (Allium cepa L.). Talanta 82: 118–124 [DOI] [PubMed] [Google Scholar]
- Ecker JR, Davis RW. (1987) Plant defense genes are regulated by ethylene. Proc Natl Acad Sci USA 84: 5202–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galuszka P, Frébort I, Šebela M, Sauer P, Jacobsen S, Peč P. (2001) Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals. Eur J Biochem 268: 450–461 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Hammond JP, Bowen HC, White PJ, Mills V, Pyke KA, Baker AJ, Whiting SN, May ST, Broadley MR. (2006) A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol 170: 239–260 [DOI] [PubMed] [Google Scholar]
- Havey MJ, McCallum J, Town CD, Jakse J, Shigyo M. (2008) The potential impact of genomes for Allium crop improvement. Acta Hortic 770: 139–146 [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. (1995) Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269: 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271 [DOI] [PubMed] [Google Scholar]
- Huber DJ, Hurr BM, Lee JS, Lee JH. (2010) 1-Methylcyclopropene sorption by tissues and cell-free extracts from fruits and vegetables: evidence for enzymatic 1-MCP metabolism. Postharvest Biol Technol 56: 123–130 [Google Scholar]
- Ji W, Hatzios KK, Cramer CL. (1992) Expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in maize tissues. Physiol Plant 84: 185–192 [Google Scholar]
- Johnson J. (2006) Onion storage revolution? The Vegetable Farmer 2: 25–26 [Google Scholar]
- Katz E, Lagunes PM, Riov J, Weiss D, Goldschmidt EE. (2004) Molecular and physiological evidence suggests the existence of a system II-like pathway of ethylene production in non-climacteric Citrus fruit. Planta 219: 243–252 [DOI] [PubMed] [Google Scholar]
- Kuhl JC, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, Catanach A, Rutherford P, Sink KC, Jenderek M, et al. (2004) A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. Plant Cell 16: 114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Wang R, Wang C-R, Kato M, Yamawaki K, Qin F, Xu H-L. (2009) Effect of 1-methylcyclopropene on expression of genes for ethylene biosynthesis enzymes and ethylene receptors in post-harvest broccoli. Plant Growth Regul 57: 223–232 [Google Scholar]
- NCBI (2008) National Centre for Biotechnology Information [online]. http://www.ncbi.nlm.nih.gov. November 23, 2009
- Prange RK, Daniels-Lake BJ, Jeong J-C, Binns M. (2005) Effects of ethylene and 1-methylcyclopropene on potato tuber sprout control and fry color. Am J Potato Res 82: 123–128 [Google Scholar]
- Rasori A, Ruperti B, Bonghi C, Tonutti P, Ramina A. (2002) Characterization of two putative ethylene receptor genes expressed during peach fruit development and abscission. J Exp Bot 53: 2333–2339 [DOI] [PubMed] [Google Scholar]
- Sakai H, Hua J, Chen QG, Chang C, Medrano LJ, Bleecker AB, Meyerowitz EM. (1998) ETR2 is an ETR1-like gene involved in ethylene signaling in Arabidopsis. Proc Natl Acad Sci USA 95: 5812–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. (2002) Accurate normalization of real-time quantification RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner HL, Leopold AC. (1969) Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol 44: 156–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CB. (2006) The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol Adv 24: 389–409 [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. (2005) Real-time PCR for mRNA quantification. Biotechniques 39: 75–85 [DOI] [PubMed] [Google Scholar]
- Yang SF. (1969) Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol 44: 1203–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin HJ, Bufler G. (2007) Dormancy and sprouting in onion (Allium cepa L.) bulbs. 1. Changes in carbohydrate metabolism. J Hortic Sci Biotechnol 82: 89–96 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.