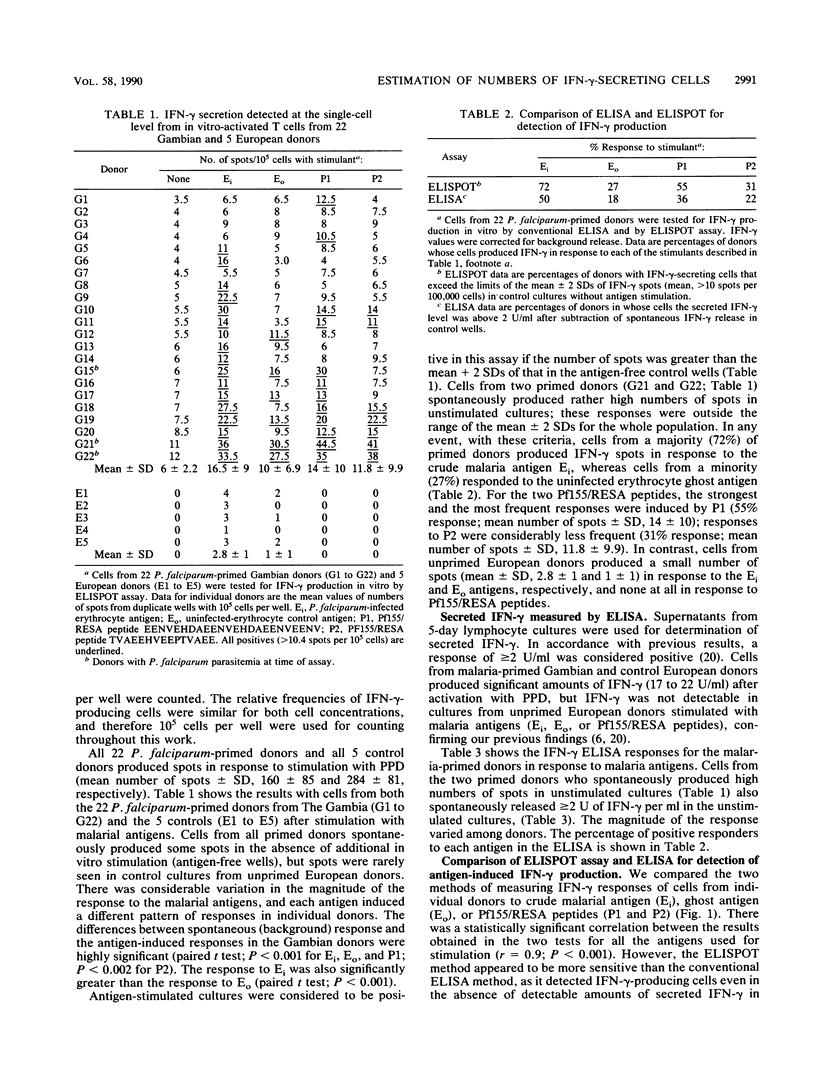
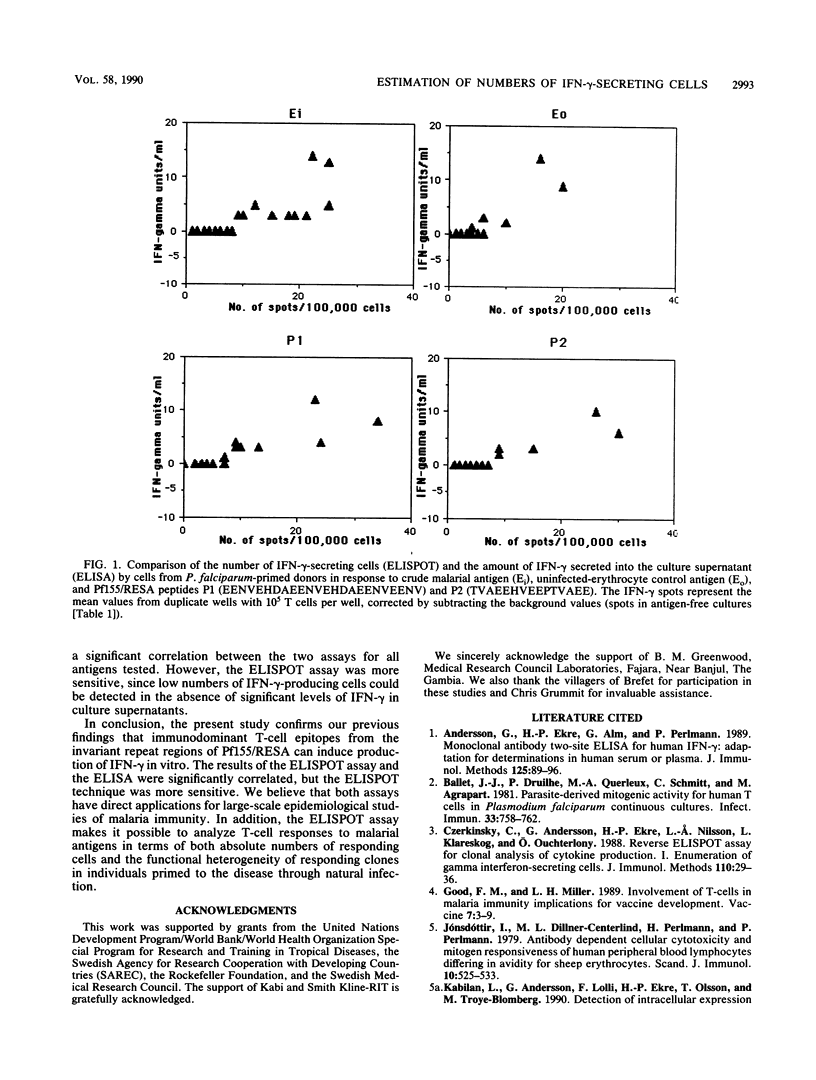
Abstract
Secretion of gamma interferon (IFN-gamma) in response to stimulation of Plasmodium falciparum-primed T cells by specific antigens may be a useful indicator of cellular immunity to malaria. An enzyme-linked immunospot (ELISPOT) assay designed to detect IFN-gamma at the single-cell level was used to study IFN-gamma-producing cells from P. falciparum-primed donors from The Gambia after in vitro stimulation with various malarial antigens. IFN-gamma secreted into the culture supernatant was measured by conventional enzyme-linked immunosorbent assay (ELISA). There was a good correlation in individual donors between the level of IFN-gamma secreted into the culture supernatant and the number of IFN-gamma-secreting cells. However, the ELISPOT assay was apparently more sensitive in demonstrating low levels of IFN-gamma production than the ELISA was. Thus after stimulation with crude P. falciparum antigen from infected erythrocytes, 72% of the primed donors responded positively in the ELISPOT assay but only 55% responded positively in the ELISA. When stimulated with synthetic peptides representing immunodominant epitopes of the malarial antigen Pf155/RESA, a vaccine candidate, 31 to 55% responded in the ELISPOT assay and 21 to 36% responded in the ELISA. Unprimed Europeans did not respond positively to these antigens in either of the assays, and background in antigen-free controls was generally low. These results indicate that measurement of IFN-gamma by the ELISPOT assay or ELISA should have wide applications in large-scale epidemiological studies of malaria immunity. In addition, the ELISPOT assay makes it possible to analyze the T cells responding to malarial antigens in terms of both numbers and functional heterogeneity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Ekre H. P., Alm G., Perlmann P. Monoclonal antibody two-site ELISA for human IFN-gamma. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989 Dec 20;125(1-2):89–96. doi: 10.1016/0022-1759(89)90081-1. [DOI] [PubMed] [Google Scholar]
- Ballet J. J., Druilhe P., Querleux M. A., Schmitt C., Agrapart M. Parasite-derived mitogenic activity for human T cells in Plasmodium falciparum continuous cultures. Infect Immun. 1981 Sep;33(3):758–762. doi: 10.1128/iai.33.3.758-762.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Good M. F., Miller L. H. Involvement of T cells in malaria immunity: implications for vaccine development. Vaccine. 1989 Feb;7(1):3–9. doi: 10.1016/0264-410x(89)90002-9. [DOI] [PubMed] [Google Scholar]
- Jónsdóttir I., Dillner-Centerlind M. L., Perlmann H., Perlmann P. Antibody dependent cellular cytotoxicity and mitogen responsiveness of human peripheral blood lymphocytes differing in avidity for sheep erythrocytes. Scand J Immunol. 1979;10(6):525–533. doi: 10.1111/j.1365-3083.1979.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Kabilan L., Troye-Blomberg M., Perlmann H., Andersson G., Högh B., Petersen E., Björkman A., Perlmann P. T-cell epitopes in Pf155/RESA, a major candidate for a Plasmodium falciparum malaria vaccine. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5659–5663. doi: 10.1073/pnas.85.15.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Gillard S., Simon B., Slade S., Eichmann K. Frequencies of CD4+ T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1(4):416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- Langhorne J., Simon B. Limiting dilution analysis of the T cell response to Plasmodium chabaudi chabaudi in mice. Parasite Immunol. 1989 Sep;11(5):545–559. doi: 10.1111/j.1365-3024.1989.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. K., Czarniecki C. W., Dutta G. P., Puri S. K., Dhawan B. N., Friedman R. M. Recombinant human gamma interferon inhibits simian malaria. Infect Immun. 1986 Sep;53(3):628–630. doi: 10.1128/iai.53.3.628-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Vilcek J., Cochrane A. H., Nussenzweig R. S. Plasmodium berghei sporozoites are mitogenic for murine T cells, induce interferon, and activate natural killer cells. J Immunol. 1984 Aug;133(2):1005–1009. [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Perlmann P., Berzins K., Wåhlin B., Troye-Blomberg M., Hagstedt M., Andersson I., Högh B., Petersen E., Björkman A. Dissection of the human antibody response to the malaria antigen Pf155/RESA into epitope specific components. Immunol Rev. 1989 Dec;112:115–132. doi: 10.1111/j.1600-065x.1989.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B. Recombinant gamma interferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987 Jan;67(1):5–10. [PMC free article] [PubMed] [Google Scholar]
- Rzepczyk C. M., Ramasamy R., Ho P. C., Mutch D. A., Anderson K. L., Duggleby R. G., Doran T. J., Murray B. J., Irving D. O., Woodrow G. C. Identification of T epitopes within a potential Plasmodium falciparum vaccine antigen. A study of human lymphocyte responses to repeat and nonrepeat regions of Pf155/RESA. J Immunol. 1988 Nov 1;141(9):3197–3202. [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Andersson G., Stoczkowska M., Shabo R., Romero P., Patarroyo M. E., Wigzell H., Perlmann P. Production of IL 2 and IFN-gamma by T cells from malaria patients in response to Plasmodium falciparum or erythrocyte antigens in vitro. J Immunol. 1985 Nov;135(5):3498–3504. [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann P. T cell functions in Plasmodium falciparum and other malarias. Prog Allergy. 1988;41:253–287. doi: 10.1159/000415226. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Riley E. M., Perlmann H., Andersson G., Larsson A., Snow R. W., Allen S. J., Houghten R. A., Olerup O., Greenwood B. M. T and B cell responses of Plasmodium falciparum malaria-immune individuals to synthetic peptides corresponding to sequences in different regions of the P. falciparum antigen Pf155/RESA. J Immunol. 1989 Nov 1;143(9):3043–3048. [PubMed] [Google Scholar]
- Versteegen J. M., Logtenberg T., Ballieux R. E. Enumeration of IFN-gamma-producing human lymphocytes by spot-ELISA. A method to detect lymphokine-producing lymphocytes at the single-cell level. J Immunol Methods. 1988 Jun 28;111(1):25–29. doi: 10.1016/0022-1759(88)90055-5. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


