SUMMARY
Neurexin and neuroligin, which undergo heterophilic interactions with each other at the synapse, are mutated in some patients with autism spectrum disorder, a set of disorders characterized by deficits in social and emotional learning. We have explored the role of neurexin and neuroligin at sensory-to-motor neuron synapses of the gill-withdrawal reflex in Aplysia that undergoes sensitization, a simple form of learned fear. We find that depleting neurexin in the presynaptic sensory neuron or neuroligin in the postsynaptic motor neuron abolishes both long-term facilitation and the associated presynaptic growth induced by repeated pulses of serotonin. Moreover, introduction into the motor neuron of the R451C mutation of neuroligin-3 linked to autism spectrum disorder blocks both intermediate-term and long-term facilitation. Our results suggest that activity-dependent regulation of the neurexin-neuroligin interaction may govern trans-synaptic signaling required for the storage of long-term memory, including emotional memory that may be impaired in autism spectrum disorder.
INTRODUCTION
The storage of long-term memory is associated with altered gene expression and synthesis of new proteins, centrally in the cell body as well as locally at the synapse where the new gene products lead to structural remodeling and the growth of new synaptic connections (Kandel 2001; Bailey et al., 2004; Bailey and Kandel, 2008). In addition to their roles in de novo synapse formation during development, an increasing body of evidence suggests that synaptic cell adhesion molecules also are critically involved in the functional expression and plasticity of the synapse in the adult brain (reviewed in Yamagata et al., 2003; Dalva et al., 2007).
Neuroligin (NLG) and neurexin (NRX) undergo a heterophilic interaction with each other and are among the most studied synaptic cell adhesion molecules in the nervous system. The neuroligins are postsynaptic transmembrane proteins produced from at least four genes in mammals (NLG-1 to 4; Ichtchenko et al., 1995; Ichtchenko et al., 1996). The neurexins are predominantly presynaptic transmembrane proteins generated from at least three genes in mammals (NRX-1 to 3), each with two promoters (thus α - and β-neurexins) and many alternative splice sites (Ushkaryov et al., 1992; Ushkaryov and Südhof, 1993). The cytoplasmic domain of both neurexin and neuroligin contains PDZ-binding motifs that can recruit signaling molecules thought to mediate differentiation of the presynaptic and the postsynaptic compartment, respectively. Indeed, in vitro, neurexin and neuroligin promote synapse formation by inducing post- and presynaptic differentiation, by interacting with each other (Scheiffele et al., 2000; Graf et al., 2004; Chih et al., 2005).
However, in vivo studies using gene ablation of neurexins or neuroligins in mice found no obvious changes in synapse number leading to the suggestion that in vivo neurexin and neuroligin affect synaptic remodeling and maturation rather than initial synapse formation (Missler et al., 2003; Varoqueaux et al., 2006; reviewed in Südhof, 2008). The finding that chronic inhibition of NMDA receptors suppresses the synaptogenic activity of neuroligin-1 in vitro further supports the idea that neuroligin contributes to the activity-dependent modification of developing neural circuits (Chubykin et al., 2007).
In light of these experimental results, it is particularly interesting that human neuroligin (NLG-3 and NLG-4) and neurexin (NRX-1α) have been linked to autism spectrum disorder (ASD: Jamain et al., 2003; Laumonnier et al., 2004; The autism genome project consortium, 2007; Kim et al., 2008). Since children with ASD often develop normally up to a point and only then regress in their social and emotional development, ASD is thought not to affect initial synapse formation but rather the synaptic remodeling that accompanies maturation of the nervous system and the subsequent stabilization of these synaptic connections (Zoghbi, 2003).
The postulated role of neurexin and neuroligin in synaptic remodeling and maturation and in the pathogenesis of ASD makes it interesting to explore their role in emotional learning and memory. As a first step in this direction, we examined the role of neuroligin-1 in mammals and found it to be important for memory of learned fear and for associated LTP at mature neural circuits in the amygdala (Kim et al., 2008). More recently, neuroligin-1 has also been found to contribute to hippocampus-dependent spatial memory (Dahlhaus et al., 2009; Blundell et al., 2010). However, there have been no detailed molecular studies thus far of how neuroligin contributes to the different stages of emotional memory formation or how it contributes to the learning-induced structural remodeling that leads to the growth of new synaptic connections associated with the storage of long-term emotional memory. Moreover, although neurexin-1α knockout mice have enhanced motor learning despite a defect in excitatory neurotransmission (Etherton et al., 2009), there are also no studies examining the role of neurexins in learning-related synaptic plasticity.
To explore the role of the neurexin-neuroligin trans-synaptic interaction in learning and memory, we have paralleled our study of emotional learning in mammals (Kim et al., 2008) with studies of an elementary neural circuit that underlies a simple form of learned fear in Aplysia – sensitization of the gill-withdrawal reflex. A critical component of this reflex that contributes importantly to the behavior is a direct monosynaptic connection from the siphon sensory neurons to the gill motor neurons. The sensory-to-motor neuron synapse can be reconstituted in dissociated cell culture where it is modulated, as in the intact animal, by serotonin (5-HT), a modulatory transmitter released during the learning of fear (Marinesco et al. 2006). Five applications of 5-HT over a period of 1.5 hr - designed to simulate 5 shocks to the tail that produce behavioral sensitization - produce both a long-term increase in the strength of the sensory-to-motor neuron synaptic connection lasting several days (long-term facilitation: LTF, Montarolo et al., 1986) and structural remodeling and growth of new sensory-to-motor neuron synapses (Glanzman et al., 1990; Bailey et al., 1992; Kim et al., 2003). Our recent finding that neurexin and neuroligin are cargo of kinesin transport and are upregulated by 5-HT at the Aplysia sensory-to-motor neuron synapse (Puthanveettil et al., 2008) further indicate the utility of this model system to study directly the role of the neurexin-neuroligin trans-synaptic interaction in learning and memory.
We here report that the Aplysia homologs of neurexin (ApNRX) and neuroligin (ApNLG) exhibit strong similarities with their vertebrate counterparts both in domain structure and subcellular localization. Next, we find that depleting ApNRX in the presynaptic sensory neuron or ApNLG in the postsynaptic motor neuron abolishes both LTF and the associated 5-HT-induced presynaptic structural changes. In addition to their role in the initiation of LTF, we find that ApNRX and ApNLG also play a critical role in the stabilization and persistence of LTF. Finally, we find that overexpression of the ApNLG autism-linked mutant in the postsynaptic motor neuron blocks both intermediate-term and long-term facilitation.
RESULTS
Cloning of the Aplysia Homolog of Neuroligin (ApNLG)
We used a primer design strategy for PCR amplification of distantly related gene sequences based on consensus-degenerate hybrid oligonucleotide primers (CODEHOPs – (http://blocks.fhcrc.org/codehop.html), Rose et al., 2003) to clone a single Aplysia homolog of neuroligin (ApNLG). Comparison of the deduced amino acid sequence of ApNLG with vertebrate and invertebrate homologs shows that ApNLG shares 39% identities with human NLG-3 (Figure S1). Despite repeated and rigorous efforts utilizing PCR-based cloning as well as screening cDNA libraries prepared from the Aplysia CNS extracts, we did not find any splice isoforms of ApNLG. The domain structure of ApNLG is similar to vertebrate neuroligins. As in vertebrates, the sequence of ApNLG is composed of a cleavable signal peptide, a single extracellular domain that is homologous with acetylcholinesterase followed by the single transmembrane region and then a short cytoplasmic region (Figure 1A). A highly conserved arginine (R) residue that is mutated in NLG-3 R451C linked to ASD (Jamain et al, 2003) is present in the ApNLG (Figure 1B).
Figure 1. Cloning and Subcellular Localization of ApNLG.
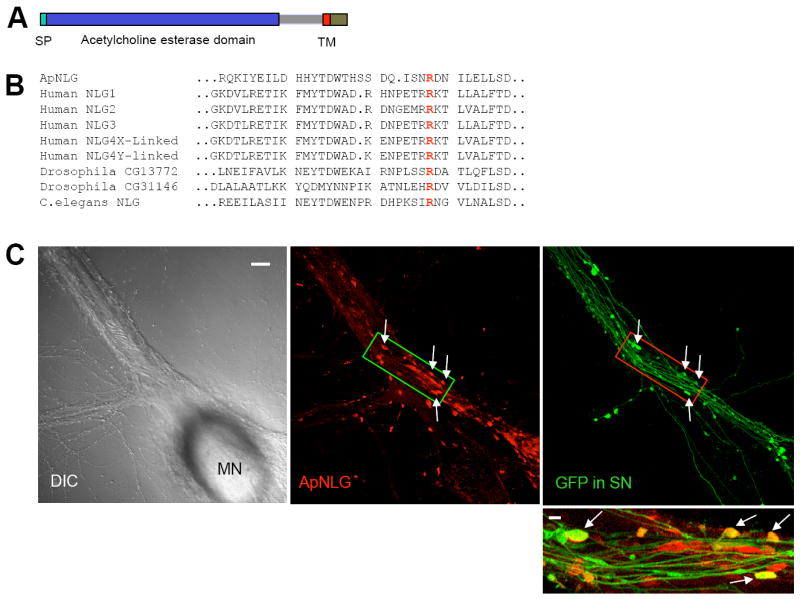
(A) The domain structure of ApNLG is similar to vertebrate neuroligin. SP= signal peptide. TM=transmembrane domain. (B) Comparison of the deduced amino acid sequence of ApNLG (partial) with vertebrate and invertebrate homologs. A highly conserved arginine (R) residue that is mutated in human NLG-3 R451C linked to ASD is present in the ApNLG (bold red). (C) Left: A DIC image shows a sensory-to-motor neuron co-culture. MN denotes the motor neuron cell body. Sensory neuron cell body is located outside of the field of view. Middle: ApNLG immunostaining (red) shows clustering of ApNLG at the initial segment and proximal regions of the major axons of the postsynaptic motor neuron. Right: GFP (green) as a whole cell marker outlines presynaptic sensory neuron processes and varicosities. Scale bar 20 μm. Bottom: A merged image in a magnified view shows some sensory neuron varicosities that partially or completely overlap with ApNLG clusters (yellow, arrows). Magnified view, scale bar 5 μm.
See also Figure S1 and S2.
Subcellular Localization of Endogenous ApNLG
We next determined the subcellular localization of endogenous ApNLG by immunocytochemical analysis using an affinity-purified polyclonal antibody generated against the extracellular region of ApNLG (Figure S2). In sensory-to-motor neuron co-cultures, immunostaining with ApNLG antibody showed clustering of ApNLG at the initial segment and the proximal regions of major axons of the postsynaptic motor neuron where the majority of functionally competent synaptic connections are found in sensory-to-motor neuron co-cultures (Figure 1C). Immunostaining in non-permeabilized condition showed a similar ApNLG staining pattern suggesting they are clusters at the cell surface (data not shown). The subcellular localization of endogenous ApNLG is consistent with the exclusive localization of mammalian neuroligins in the postsynaptic density (Song et al., 1999). When GFP was expressed in sensory neurons as a whole cell marker, it became readily evident that presynaptic sensory neuron varicosities, especially the ones in contact with the initial segment and major axons of postsynaptic motor neurons and thus containing functional presynaptic compartments (Kim et al., 2003), partially or completely overlap with ApNLG clusters (Figure 1C).
Cloning of the Aplysia Homolog of Neurexin (ApNRX)
Employing a PCR-based strategy and using the partial sequence homologous to known neurexin sequences found in the Aplysia EST database (Moroz et al., 2006), we cloned a single Aplysia homolog of neurexin (ApNRX). ApNRX also shares a high degree of sequence conservation with other invertebrate and with mammalian neurexins (35% identity and 52% similarity with human neurexin-1α) (Figure S1). The domain organization of ApNRX is very similar to mammalian α-neurexins. It has a cleavable signal peptide, a large extracellular domain that contains three repeats consisting of two LNS (Laminin-Neurexin-Sex hormone globulin) motifs flanking an EFG motif, followed by single transmembrane domain, and then a short cytoplasmic tail (Figure 2A). Furthermore, four out of the five alternative splice sites present in mammalian α-neurexin, including splice site 4 which is common to both α- and β-neurexins and determines binding affinity to neuroligin (Ichtchenko et al., 1995), are also present in equivalent locations in ApNRX suggesting a high degree of functional conservation (Figure S1 and Table S1). The high degree of conservation extends to the PDZ binding motif at the C-terminal end which is conserved and characteristic for “true” neurexins as opposed to the related but different neurexin IV which shares a number of the other domains of neurexin (Figure 2B). Despite repeated and rigorous efforts, we have not found a shorter β-like neurexin variant expressed in the Aplysia CNS. This is consistent with the absence of β-neurexins from the invertebrate genomes of Drosophila and C. elegans (Tabuchi and Südhof, 2002).
Figure 2. Cloning and Subcellular Localization of ApNRX.
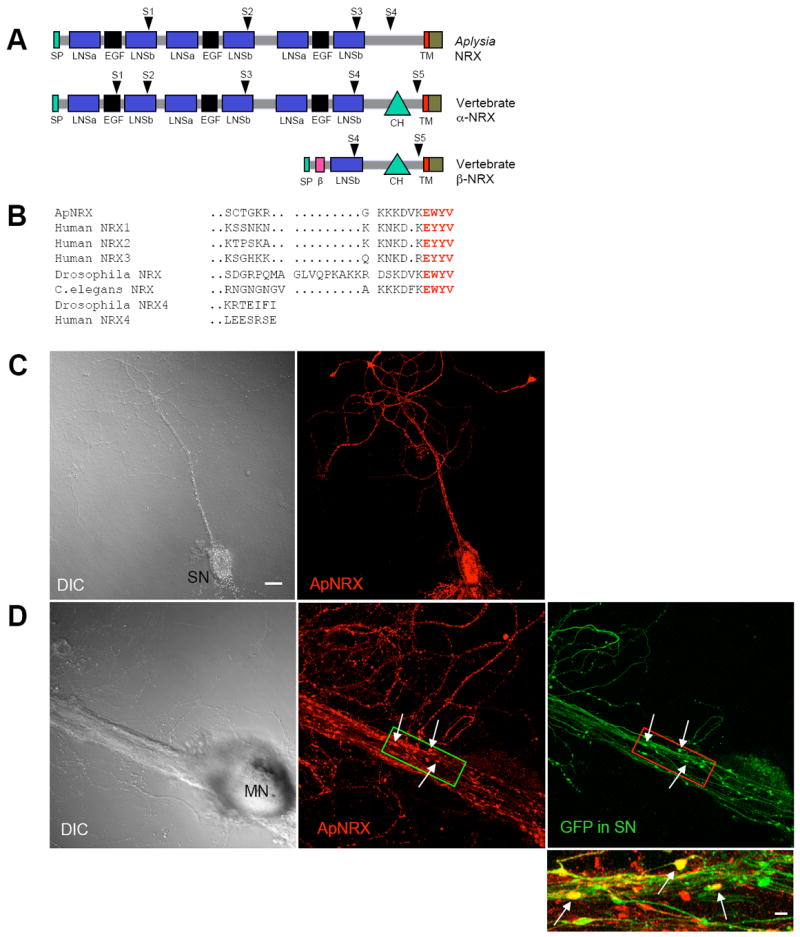
(A) The domain structure of ApNRX is similar to vertebrate α -neurexins. SP=signal peptide, β = β-neurexin-specific domain, LNSa and LNSb= LNS domain preceding and following EGF domain, respectively. CH=carbohydrate rich domain. TM=transmembrane domain. Arrowheads indicate splicing sites. (B) Comparison of the deduced amino acid sequence of the C-terminal end of ApNRX with vertebrate and invertebrate homologs. Bold red letters indicate the conserved residues in the C-terminal end PDZ domain. (C) Left: A DIC image of an isolated sensory neuron (SN). Right: ApNRX immunostaining (red) shows clustering of endogenous ApNRX along the neurites. (D) Left: A DIC image shows a sensory-to-motor neuron co-culture. MN denotes the motor neuron cell body. Sensory neuron cell body is located outside of the field of view. Middle: ApNRX immunostaining (red) shows clustering of ApNRX at the main neurites of postsynaptic motor neuron. Right: GFP (green) as a whole cell marker outlines presynaptic sensory neuron processes and varicosities. Scale bar 20 μm. Bottom: A merged image in a magnified view shows some sensory neuron varicosities that partially or completely overlap with ApNLG clusters (yellow, arrows). Magnified view, scale bar 5 μm.
See also Figure S1 and S2 and Table S1.
Subcellular Localization of Endogenous ApNRX
We next determined the subcellular localization of endogenous ApNRX by immunocytochemical analysis using an affinity-purified polyclonal antibody generated against the cytoplasmic tail region of ApNRX (Figure S1). In isolated sensory neurons, ApNRX immunostaining was distributed along neurites in a punctate pattern (Figure 2C). The punctate appearance of endogenous ApNRX cluster is similar to the distribution reported for endogenous neurexin in mammals (Dean et al., 2003). Interestingly, clusters of ApNRX also appear to be distributed along the neurites of the motor neuron in sensory-to-motor co-cultures (Figure 2D). When GFP was expressed in sensory neurons as a whole cell marker, it became clear that some ApNRX clusters do not co-localize with the neurites of the sensory neuron outlined by GFP fluorescence, thus they presumably are located on the motor neuron. These ApNRX clusters in the motor neuron may represent a pool of ApNRX in postsynaptic compartments since there is a significant amount of neurexin in postsynaptic compartments in mammals (Taniguchi et al., 2007). Moreover, it became clear that some ApNRX clusters overlap with some presynaptic sensory neuron varicosities in contact with the major receptive surface of the postsynaptic motor neuron where the majority of functionally competent synaptic connections are found in culture (Figure 2D, Kim et al., 2003). These ApNRX clusters overlapping with presynaptic varicosities may represent enrichment of ApNRX in presynaptic sensory neuron varicosities, clustering of ApNRX in postsynaptic motor neuron in apposition to presynaptic sensory neuron varicosities, or perhaps both.
Binding of Recombinant ApNRX and ApNLG to Each Other and Colocalization of Endogenous ApNRX and ApNLG
Since many known functions of neurexin and neuroligin require their binding to each other across the synaptic cleft, we set out to find whether ApNRX and ApNLG also bind to each other. To address this point, we generated Ig fusion constructs that contain the extracellular domain of either HA-tagged ApNLG or VSV-G-tagged ApNRX with the Ig Fc at their C-termini (ApNLG-Fc and ApNRX-Fc). We then incubated soluble ApNRX-Fc with cell lysates prepared from GFP-ApNLG transfected HEK293 cells and soluble ApNLG-Fc with cell lysates prepared from GFP-ApNRX transfected HEK293 cells, respectively. We found that ApNRX-Fc specifically binds to GFP-ApNLG, but not to GFP control (Figure 3A) and conversely, ApNLG-Fc binds to GFP-ApNRX, but not to GFP control (Figure 3B). These results provide evidence that recombinant ApNRX and ApNLG bind to each other, as is the case for their vertebrate counterparts.
Figure 3. Binding of Recombinant ApNRX with ApNLG and Enhancement of Basal EPSP by Simultaneous Overexpression of ApNRX in Presynaptic Sensory Neuron and ApNLG in Postsynaptic Motor Neuron.
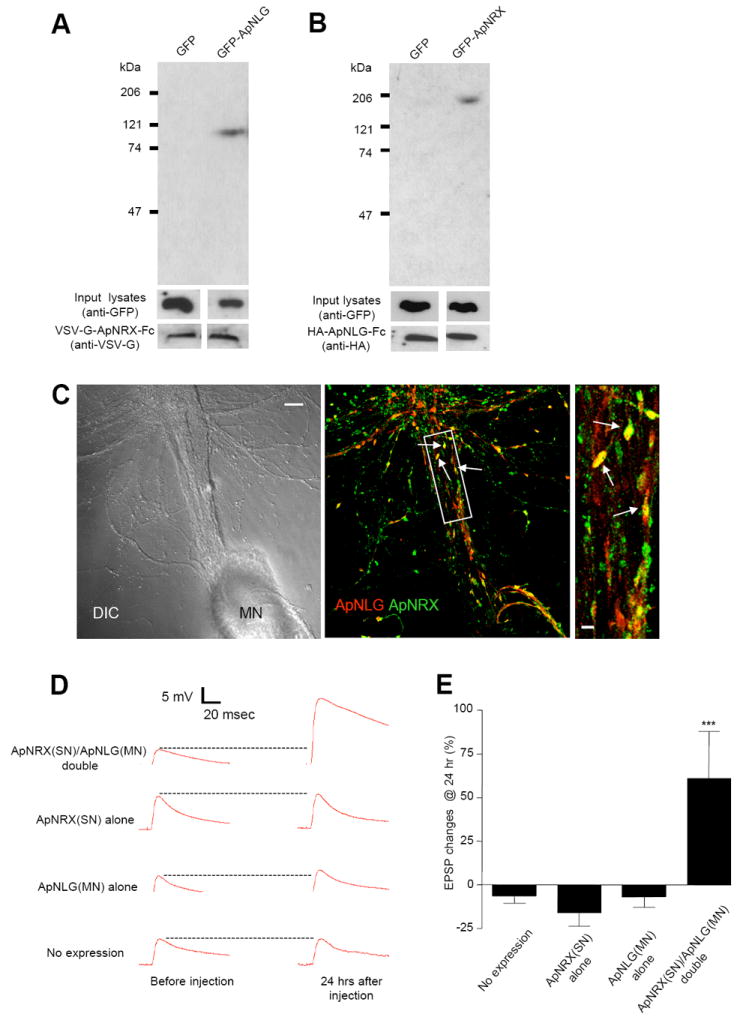
(A) Pull-down of GFP-ApNLG by VSV-G-ApNRX-Fc fusion protein. Immunoblotting with an anti-GFP antibody shows the presence of a 110 kDa band in the precipitate from HEK 293 cell lysates transfected with GFP-ApNLG. Similar amounts of input lysates and VSV-G-ApNLG-Fc fusion protein are shown when probed with an anti-GFP antibody and an anti-VSV-G antibody, respectively. (B) Pull-down of GFP-ApNRX by HA-ApNLG-Fc fusion protein. Immunoblotting with an anti-GFP antibody shows the presence of a 200 kDa band in the precipitate from HEK 293 cell lysates transfected with GFP-ApNRX. Similar amounts of input lysates and HA-ApNLG-Fc fusion protein are shown when probed with an anti-GFP antibody and an anti-HA antibody, respectively. (C) Left: A DIC image shows a sensory-to-motor neuron co-culture. MN denotes the motor neuron cell body. Sensory neuron cell body is located outside of the field of view. Middle: A merged co-immunostaining image shows clusters of ApNLG (red) and ApNRX (green) overlap or juxtapose at the initial segment and proximal regions of the major axons of the postsynaptic motor neuron (yellow, arrows). Scale bar 20 μm. Right: A magnified view, scale bar 5 μm. (D) EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before and 24 hr after injection of ApNRX to sensory neurons and ApNLG to motor neurons. Representative EPSP traces before and 24 hr after injections. (E) Changes in EPSP amplitudes are shown as a bar graph.
Next, we wondered whether endogenous ApNRX and ApNLG colocalize at the synapse. Thus, we immunostained sensory-to-motor neuron co-cultures with both ApNRX and ApNLG antibodies. In this fashion, we were able to demonstrate that clusters of ApNLG and ApNRX do indeed overlap or juxtapose each other at the initial segment and the proximal regions of major axons of the postsynaptic motor neuron where the majority of functionally competent synaptic connections are found (Figure 3C). However, there are the clusters of ApNRX and ApNLG that do not colocalize especially at the distal neurites which may represent, in part, mobile clusters that contribute to preformed scaffolding transport complexes and/or extra-synaptic clusters.
Simultaneous Overexpression of ApNRX in the Presynaptic Sensory Neuron and ApNLG in the Postsynaptic Motor Neuron Induces a Long-Term Increase in Synaptic Strength
Overexpression of neurologin-1 in cultured mammalian neurons increases excitatory postsynaptic currents induced by local extracellular stimulation (Chubykin et al., 2007). Thus, we examined the effect of overexpressing ApNLG in the postsynaptic motor neuron or ApNRX in the presynaptic sensory neuron on the strength of the sensory-to-motor neuron synaptic connection. Overexpression of ApNRX alone in the presynaptic sensory neuron or ApNLG alone in the postsynaptic motor neuron did not lead to an increase in the amplitude of the evoked excitatory post-synaptic potentials (EPSPs) measured at 24 hours after the injection. However, simultaneous overexpression of ApNRX in the presynaptic sensory neuron and ApNLG in the postsynaptic motor neuron led to a significant increase in the strength of the sensory-to-motor neuron synaptic connection measured at 24 hours after the injection (Figure 3D and E; % increase in EPSP amplitude: no expression −6.3 ± 4.2, n = 27; ApNRX expression alone −15.6 ± 8.0, n = 6; ApNLG expression alone -6.7 ± 6.1, n = 8; ApNRX and ApNLG expression 61.1 ± 27.5, n = 10, p < 0.001 versus no expression).
Thus, the concomitant overexpression of ApNRX in the presynaptic sensory neuron and ApNLG in the postsynaptic motor neuron can, by itself in the absence of 5-HT training, induce long-lasting synaptic facilitation. These results support the idea of a functional trans-synaptic interaction between ApNRX and ApNLG since ApNRX and ApNLG bind to each other and the overexpression of either ApNRX or ApNLG alone does not induce long-lasting synaptic facilitation.
Redistribution of ApNRX in the Presynaptic Varicosities After 5-HT Treatment Inducing LTF
When the whole cell marker Alexa-594 was injected into sensory neurons in combination with presynaptic overexpression of the ApNRX-GFP construct, it became evident that some presynaptic sensory neuron varicosities are completely filled with ApNRX whereas other varicosities are only partially filled and some varicosities appear to lack ApNRX entirely (Figure 4A). This heterogeneous distribution is similar to the pattern reported for other presynaptic markers in Aplysia such as synaptophysin (Kim et al., 2003) and allowed us to examine, by time-lapse imaging of living cells in culture, the time course and spatial distribution of ApNRX that may be recruited to the individual presynaptic sensory neuron varicosities during the development of LTF. In addition to the formation of new varicosities filled with ApNRX, there is also the enrichment of ApNRX in previously existing empty varicosities when cells were re-imaged at 24 hrs after 5-HT treatment (Figure 4B). We have defined an empty varicosity as any varicosity that was labeled by Alexa-594 but contains no ApNRX-GFP (see Experimental Procedures). Such empty varicosities represent 45.6 % ± 5.5% (11.0/25.0 varicosities, n = 15) of the total varicosities. When cells were re-imaged 24 hours after 5-HT treatment, 46.4 % ± 7.4 % (5.6/11.0 varicosities) of the empty varicosities were filled with ApNRX-GFP. There was little change in the distribution of ApNRX-GFP over a 24 hr in control cultures that were not treated with 5-HT.
Figure 4. Regulation of Subcellular Distribution of ApNRX by 5-HT.
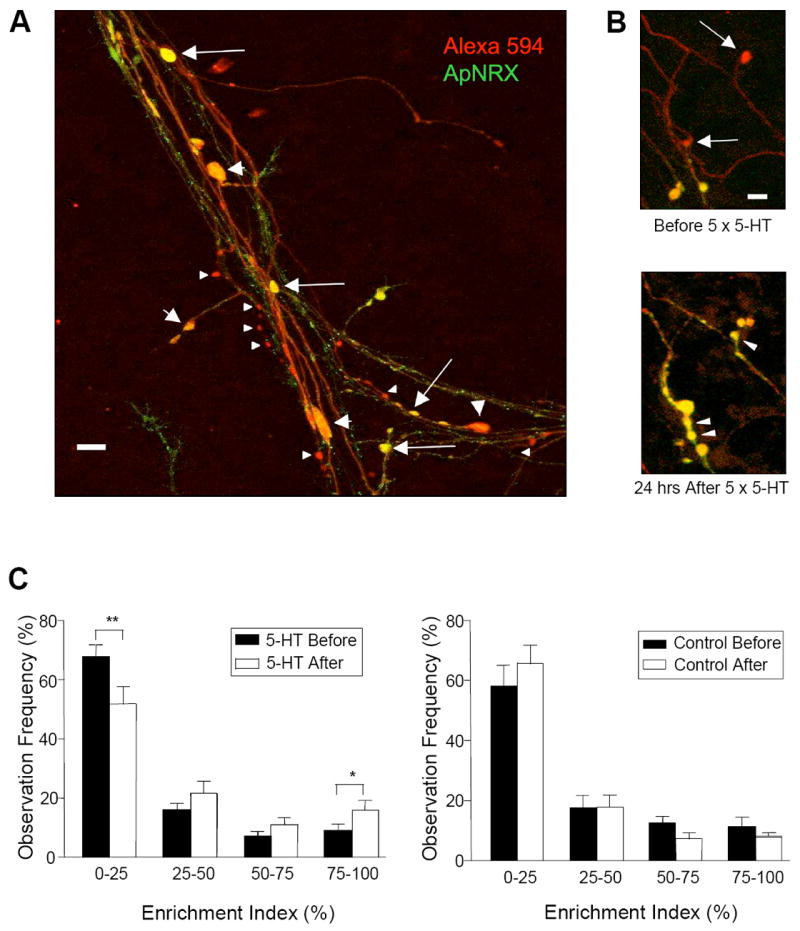
(A) ApNRX-GFP (green) is overexpressed in a sensory neuron and Alexa 594 (red) is injected into the same sensory neuron as a whole cell marker in sensory-to-motor co-cultures. Merged images illustrate that a few presynaptic sensory neuron varicosities are completely filled with ApNRX (yellow, large arrows), others are only partially filled (yellow and red, large arrowheads) and some have little or no apparent ApNRX (red, small arrowheads). Scale bar 10 μm. (B) After exposure to 5 × 5-HT (10 μM, 5 min), two empty (nascent) varicosities (upper panel: arrows) become filled with ApNRX-GFP. There is also the 5-HT-induced growth of new varicosities filled with ApNRX-GFP (lower panel: arrowheads). Scale bar 5 μm. (C) The total population of sensory neuron varicosities is grouped after binning according to the GFP mean pixel intensity. 5-HT treatment increases the percentage of varicosities highly enriched in ApNRX-GFP (75%-100% enrichment index) and decreases the percentage of varicosities containing little or no ApNRX-GFP (0%-25% enrichment index), but control cultures without 5-HT treatment do not show any significant changes in enrichment of ApNRX-GFP (n = 15 cells for 5-HT treatment and n = 10 cells for control, total of 633 varicosities in 7 independent experiments).
We quantified the distribution of ApNRX-GFP enrichment in the total population of sensory neuron varicosities. We found that 5-HT treatment that leads to LTF results in a net increase in the percentage of varicosities highly enriched in ApNRX-GFP (75%-100% enrichment group: before 5-HT, 9.1% ± 2.3 % versus after 5-HT, 16.1 % ± 3.1 %, n = 15, p < 0.05) and a net decrease in the percentage of varicosities containing little or no ApNRX-GFP (0%-25% enrichment group: before 5-HT, 67.3% ± 4.1 % versus after 5-HT, 51.4 % ± 5.8 %, n = 15, p < 0.01). In contrast, there were no significant changes in control groups that were not treated with 5-HT (75%-100% enrichment group: before 5-HT, 11.6 % ± 3.1 % versus after 5-HT, 8.3 % ± 1.2 %, n = 10, p = 0.17 and 0%-25% enrichment group: before 5-HT, 58.2% ± 6.6 % versus after 5-HT, 65.6% ± 6.1 %, n = 10, p = 0.22) (Figure 4C).
These results indicate that a subcellular redistribution of ApNRX accompanies the synaptic remodeling and growth that is induced by 5-HT in Aplysia sensory-to-motor neuron co-cultures.
Injection of ApNLG Anti-Sense Oligonucleotide into the Postsynaptic Motor Neuron Blocks LTF and Associated Presynaptic Structural Changes
To investigate the consequences of depleting ApNLG mRNA in sensory-to-motor neuron co-cultures, we used antisense oligonucleotides (Figure S3). Three hours after initial measurements of EPSPs and injection of the antisense oligonucleotide to ApNLG (50 ng/μl) in the postsynaptic motor neuron, we treated cultures with five pulses of 5-HT (10 μM) and measured EPSPs again 24 hr after 5-HT treatment. Injection of the antisense oligonucleotide to ApNLG leads to a significant reduction of LTF at 24 hr, but the injection of sense oligonucleotide did not have any significant effect on LTF (Figure 5A; % increase in EPSP amplitude: 5-HT 87.6 ± 13.4, n = 16; 5-HT + sense 95.9 ± 18.5, n = 8; 5-HT + antisense 32.0 ± 10.0, n = 28, p < 0.01 versus 5-HT). Basal synaptic transmission also was not affected by the oligonucleotide injections (% increase in EPSP amplitude: no injection −11.4 ± 7.4, n = 17; antisense alone −15.9 ± 10.5, n = 10; sense alone 6.7 ± 10.0, n = 6).
Figure 5. ApNLG Mediates LTF and Associated Presynaptic Structural Changes.
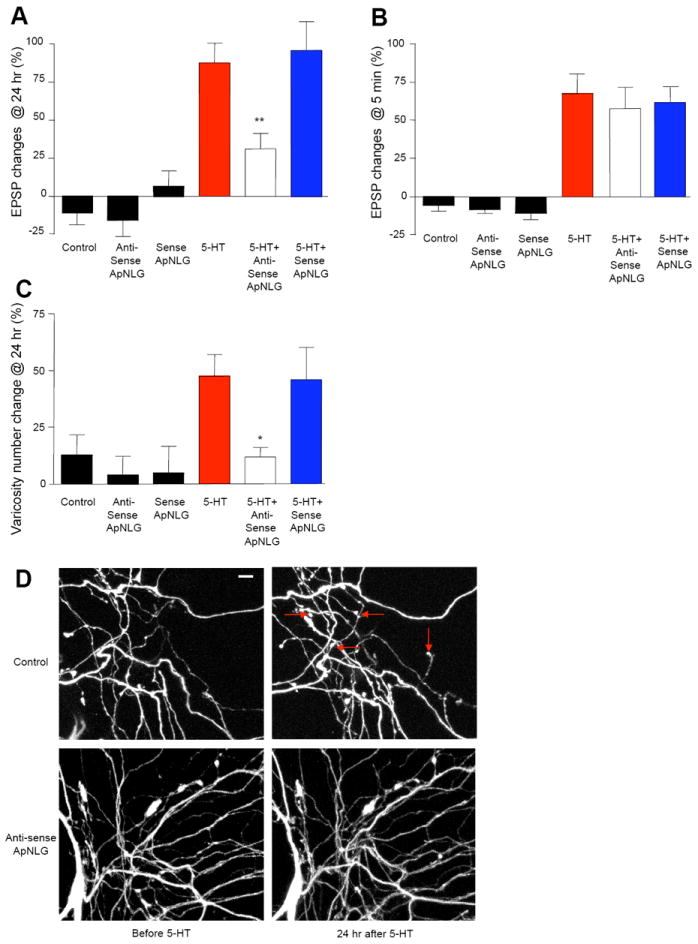
(A and B) Injection of ApNLG anti-sense oligonucleotide in the postsynaptic motor neuron to down-regulate the translation level of ApNLG blocks LTF (A) but has no effect on STF (B). EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before and 24 hr after exposure to 5 × 5-HT (10 μM, 5 min) for LTF or before and 5 min after exposure to 1 × 5-HT (10 μM, 5 min) for STF. Changes in amplitudes are shown as a bar graph. (C) Injection of ApNLG anti-sense oligonucleotide in the postsynaptic motor neuron also blocks growth of new presynaptic varicosities associated with LTF. The sensory neurons were injected a whole cell marker Alexa 594. The number of sensory neuron varicosities in apposition to the initial segment and major neurites of the motor neuron were counted before and 24 hours after exposure to 5 × 5-HT (10 μM, 5 min). Changes in numbers of varicosities are shown as a bar graph. Total of 123 cells and 1939 varicosities in 25 independent experiments were analyzed. (D) Representative images of presynaptic varicosities before and after 5-HT treatment. Red arrows indicate some newly formed varicosities. Scale bar 10 μm.
See also Figure S3.
Next, we treated cultures with one pulse of 5-HT (10 μM) for five minutes, 12 hours after oligonucleotide injections into the motor neurons, to induce short-term facilitation (STF) and measured EPSPs again 5 minutes after the 5-HT treatment (Figure 5B; % increase in EPSP amplitude: no injection −4.5 ± 7.5, n = 6; antisense alone −5.8 ± 5.3, n = 8; sense alone −8.8 ± 7.9, n = 5; 5-HT 61.9 ± 18.2, n = 12; 5-HT + antisense 63.6 ± 16.9, n = 11; 5-HT + sense 66.3 ± 14.2, n = 11). Thus, unlike LTF, STF was not affected by the antisense oligonucleotide to ApNLG.
Since 5-HT-induced LTF is accompanied by the growth of new sensory neuron presynaptic varicosities, we examined whether blocking ApNLG also blocks this learning-related synaptic growth. Indeed, we found a significant decrease in the number of new presynaptic varicosities when ApNLG was down-regulated in the postsynaptic motor neuron by injection of antisense oligonucleotides (Figure 5C-D; % increase in varicosity numbers: no injection 13.2 ± 8.6, n = 11; antisense alone 4.0 ± 8.4, n = 9; sense alone 5.2 ± 11.8, n = 6; 5-HT 47.7 ± 9.7, n = 24; 5-HT + antisense 12.0 ± 4.3, n = 45, p < 0.05 versus 5-HT; 5-HT + sense 46.4 ± 14.0, n = 25).
This result, showing that the depletion of ApNLG in the postsynaptic motor neuron blocks a structural change in the presynaptic sensory neuron, supports the idea that ApNRX and ApNLG have a trans-synaptic signaling role in long-term memory storage.
Injection of ApNRX Anti-sense Oligonucleotide into the Presynaptic Sensory Neuron Blocks LTF and Associated Presynaptic Structural Changes
To examine whether postsynaptic neuroligin acts through presynaptic neurexin to exert its effect on LTF and the associated presynaptic structural changes, we also used antisense oligonucleotides to ApNRX to investigate the consequences of depleting ApNRX mRNA in the sensory neurons of sensory-to-motor neuron co-cultures (Figure S3). Three hours after initial measurements of EPSPs and injection of the antisense oligonucleotide to ApNRX (100 ng/μl) in the presynaptic sensory neuron, we treated cultures with five pulses of 5 minutes of 5-HT (10 μM) and measured EPSPs again 24 hr after 5-HT treatment. The injection of the antisense oligonucleotide to ApNRX into presynaptic sensory neurons making functional synaptic connections with the postsynaptic motor neuron leads to a significant reduction of LTF at 24 hr, but the injection of sense oligonucleotide did not have any significant effect on LTF (Figure 6A; % initial EPSP amplitude: 5-HT 76.5 ± 12.7, n = 35; 5-HT + antisense 25.6 ± 7.4, n = 38, p < 0.01 versus 5-HT; 5-HT + sense 69.9 ± 11.3, n = 21). Basal synaptic transmission also was not affected by the oligonucleotide injections (% initial EPSP amplitude: no injection −7.8 ± 9.8, n = 19; antisense alone 5.1 ± 9.1, n = 13; sense alone 2.0 ± 11.3, n = 4).
Figure 6. ApNRX Mediates LTF and Associated Presynaptic Structural Changes.
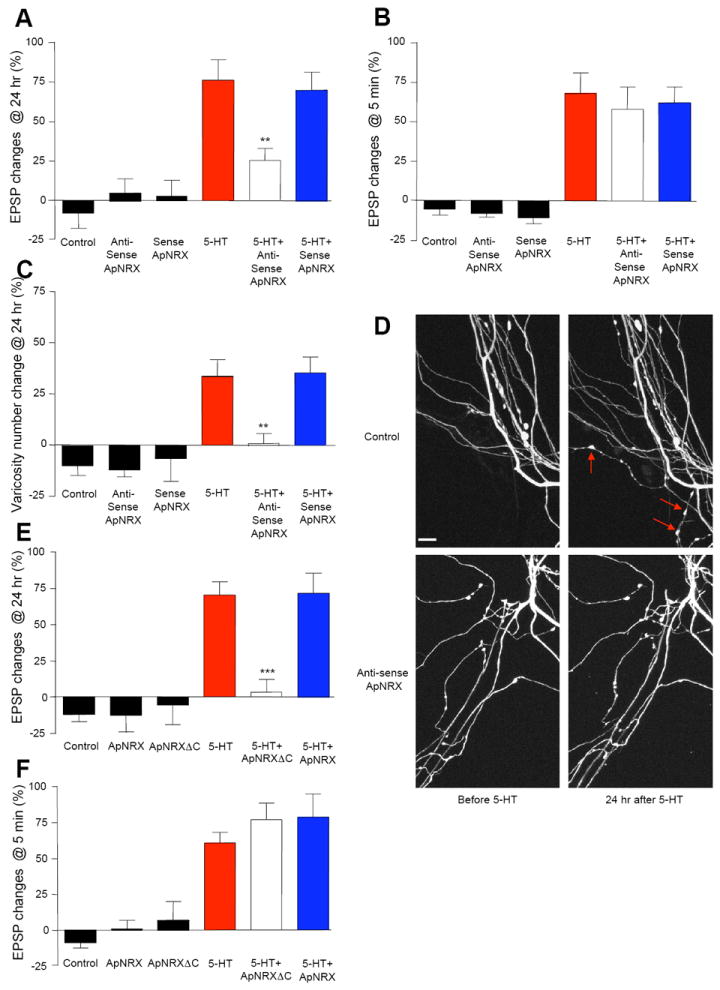
(A and B) Injection of ApNRX anti-sense oligonucleotide into the presynaptic sensory neuron to down-regulate the translation levels of ApNRX blocks LTF (A) but has no effect on STF (B). EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before and 24 hr after exposure to 5 × 5-HT (10 μM, 5 min) for LTF or before and 5 min after exposure to 1 × 5-HT (10 μM, 5 min) for STF. Changes in amplitudes are shown as a bar graph. (C) Injection of ApNRX anti-sense oligonucleotide in the presynaptic sensory neuron also blocks growth of new presynaptic varicosities associated with LTF. The sensory neurons were injected a whole cell marker Alexa 594. The number of sensory neuron varicosities in apposition to the initial segment and major neuritis of motor neuron were counted before and 24 hours after exposure to 5 × 5-HT (10 μM, 5 min). Changes in numbers of varicosities are shown as a bar graph. Total of 65 cells and 803 varicosities in 11 independent experiments were analyzed. (D) Representative images of presynaptic varicosities before and after 5-HT treatment. Red arrows indicate some newly formed varicosities. Scale bar 10 μm. (E and F) Expression of ApNRXΔC in the presynaptic sensory neuron blocks LTF (E) but has no effect on STF (F). EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before and 24 hr after exposure to 5 × 5-HT (10 μM, 5 min) for LTF or before and 5 min after exposure to 1 × 5-HT (10 μM, 5 min) for STF. Changes in amplitudes are shown as a bar graph.
See also Figure S3.
Next, we treated cultures with one pulse of 5-HT (10 μM) for five minutes to induce STF 12 hours after injection of the oligonucleotides into sensory neurons. We measured the EPSPs again 5 minutes after the 5-HT treatment (Figure 6B; % initial EPSP amplitude: no injection −5.6 ± 3.6, n = 12; antisense alone −8.4 ± 2.1, n = 8; sense alone -11.0 ± 3.7, n = 7; 5-HT 67.9 ± 13.0, n = 16; 5-HT + antisense 57.8 ± 14.4, n = 19; 5-HT + sense 62.0 ± 10.3, n = 11). Thus, injection of the antisense oligonucleotide to ApNRX in the presynaptic sensory neuron did not block STF and provides additional support for its selective role in the long-term process.
We next examined whether depleting ApNRX also blocks the 5-HT-induced synaptic growth that accompanies LTF. Again, we found a significant decrease in the number of new presynaptic varicosities when presynaptic ApNRX was downregulated by the injection of antisense oligonucleotides (Figure 6C-D; % increase in varicosity numbers: no injection −10.4 ± 4.8, n = 8; antisense alone −12.2 ± 3.8, n = 5; sense alone -6.8 ± 6.3, n = 4; 5-HT 35.2 ± 8.2, n = 13; 5-HT+antisense 0.6 ± 5.0, n = 17, p < 0.01 versus 5-HT; 5-HT+sense 36.8 ± 7.8, n = 9).
Expression of ApNRX C-Terminal Deletion Mutant in the Presynaptic Sensory Neuron Blocks LTF
We have hypothesized that the cytoplasmic tail of ApNRX is necessary to recruit new molecular components important for the learning-related assembly of the presynaptic active zone and its consequent 5-HT-induced remodeling and growth associated with long-term facilitation at the Aplysia sensory-to-motor neuron synapse. To test this idea, we generated a C-terminal deletion construct of ApNRX (ApNRXΔC) that lacks the cytoplasmic tail. We expressed this construct in the sensory neurons and found that there was no obvious difference in the expression of ApNRXΔC-GFP compared to ApNRX-GFP (Data not shown). To bring the expression level to a steady state, we waited two days after injecting ApNRX-GFP or ApNRXΔC-GFP into presynaptic sensory neurons and treated, sensory-to-motor neuron co-cultures with five pulses of 5 minutes of 5-HT (10 μM) and measured EPSPs before and 24 hr after 5-HT treatment. We found that the overexpression of ApNRXΔC in the presynaptic sensory neuron making functional connections with the postsynaptic motor neuron in culture leads to a significant reduction of LTF at 24 hr, but that overexpression of wild-type ApNRX did not enhance LTF (Figure 6E; % initial EPSP amplitude: 5-HT 70.4 ± 9.4, n = 51; 5-HT + ApNRXΔC overexpression 3.9 ± 5.6, n = 14, p < 0.001 versus 5-HT; 5-HT + ApNRX overexpression 71.8 ± 13.7, n = 10). Overexpression of wild-type ApNRX or ApNRXΔC had no effect on basal transmission (% initial EPSP amplitude: no expression -12.2 ± 4.2, n = 28; ApNRXΔC overexpression alone −12.4 ± 11.6, n = 5; ApNRX overexpression alone -5.5 ± 13.2, n = 6). Unlike its effect on LTF, ApNRXΔC overexpression had no effect on STF induced by one pulse of 5-HT (10 μM) (Figure 6F; % initial EPSP amplitude: no injection −8.5 ± 4.0, n = 19; ApNRXΔC overexpression alone 1.5 ± 5.7, n = 4; ApNRX overexpression alone 7.3 ± 12.8, n = 6; 5-HT 61.0 ± 7.3, n = 26; 5-HT + ApNRXΔC overexpression 77.0 ± 12.0, n = 9; 5-HT + ApNRX overexpression 78.9 ± 16.4, n = 11).
These results with ApNRXΔC provide additional support for the notion that ApNRX is an important regulatory component of long-term memory storage in Aplysia perhaps via intracellular signaling cascades mediated by the cytoplasmic domain.
ApNRX and ApNLG are Also Required for Persistence of 5-HT Induced Long-Term Facilitation
Using Aplysia sensory-to-motor neuron co-cultures, we have recently found that, in addition to the induction phase, there is a specific stabilization phase for the storage of long-term memory during which sustained CPEB-dependent local protein synthesis is required for the stabilization of 5-HT-induced synaptic growth and the persistence of LTF (Si et al., 2003; Miniaci et al., 2008). To test whether ApNRX and ApNLG are required for the stable maintenance of LTF, we injected antisense oligonucleotides directed against ApNRX into sensory neurons at 24 hr after repeated pulses of 5-HT and measured EPSPs at 48 hr and 72 hr. Basal synaptic transmission was not affected by the oligonucleotide injections (Figure 7A; % initial EPSP amplitude: no injection at 24 hr 8.6 ± 8.2, at 48 hr −4.1 ± 8.0, at 72 hr −4.2 ± 9.3, n = 14; antisense alone at 24 hr 3.5 ± 7.1, at 48 hr −17.5 ± 5.7, at 72 hr −13.7 ± 7.8, n = 11; sense alone at 24 hr −0.7 ± 5.5, at 48 hr −12.3 ± 5.6, at 72 hr −8.6 ± 10.0, n = 11). However, we find that injection of antisense oligonucleotides leads to a significant reduction of LTF measured at 48 and 72 hr (Figure 7A; % initial EPSP amplitude: 5-HT at 24 hr 99.2 ± 15.2, at 48 hr 75.1 ± 9.7, at 72 hr 52.2 ± 10.8, n = 16; 5-HT + antisense at 24 hr 89.2 ± 15.3, at 48 hr 24.6 ± 10.5, at 72 hr 10.6 ± 8.9, n = 19, p < 0.001 versus 5-HT at 48 and 72 hr; 5-HT + sense at 24 hr 103.7 ± 22.7, at 48 hr 69.0 ± 16.1, at 72 hr 56.8 ± 15.8, n = 14).
Figure 7. ApNRX and ApNLG Mediate Persistence of LTF.
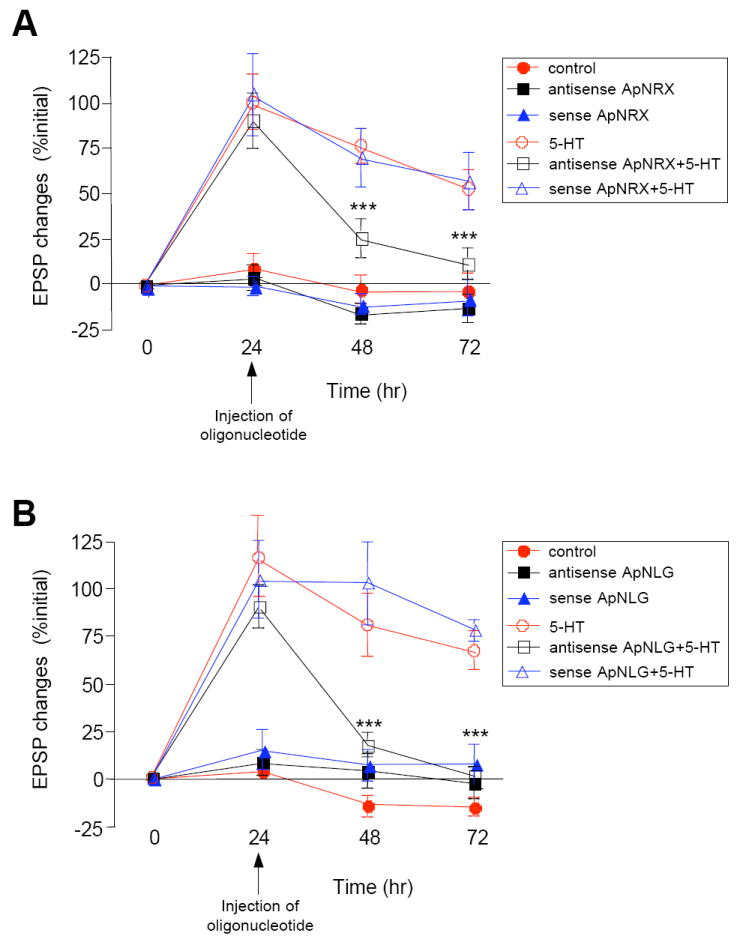
Injection of (A) ApNRX anti-sense oligonucleotide in the presynaptic sensory neuron or (B) ApNLG anti-sense oligonucleotide in the postsynaptic motor neuron at 24 hr after 5-HT blocks LTF measured at 48 hr and 72 hr. EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before, 24 hr, 48 hr, and 72 hr after exposure to 5 × 5-HT (10 μM, 5 min). Changes in amplitudes are shown as a line graph.
We also injected antisense oligonucleotides directed against ApNLG into the motor neuron at 24 hr after repeated pulses of 5-HT and measured EPSPs at 48 hr and 72 hr. We again found that injection of antisense oligonucleotides leads to a significant reduction of LTF measured at 48 and 72 hr (Figure 7B; % initial EPSP amplitude: 5-HT at 24 hr 115.2 ± 20.6, at 48 hr 81.5 ± 16.5, at 72 hr 67.2 ± 10.3, n = 11; 5-HT + antisense at 24 hr 89.8 ± 11.3, at 48 hr 18.6 ± 6.1, at 72 hr 1.8 ± 5.4, n = 18, p < 0.001 versus 5-HT at 48 and 72 hr; 5-HT + sense at 24 hr 103.7 ± 20.5, at 48 hr 103.0 ± 22.0, at 72 hr 78.0 ± 5.5, n = 11). Basal synaptic transmission was not affected by the oligonucleotide injections (% initial EPSP amplitude: no injection at 24 hr 4.1 ± 4.0, at 48 hr −13.6 ± 5.9, at 72 hr −14.4 ± 5.2, n = 7; antisense alone at 24 hr 8.4 ± 6.6, at 48 hr 5.0 ± 8.9, at 72 hr −1.9 ± 8.1, n = 7; sense alone at 24 hr 15.1 ± 10.7, at 48 hr 7.8 ± 8.3, at 72 hr 7.8 ± 10.6, n = 9).
These results indicate that ApNRX and ApNLG not only play a critical role in the initiation of LTF and the associated presynaptic structural changes but also in the stabilization and persistence of these learning-induced synaptic changes.
Expression of ApNLG Autism-Linked Mutant in the Postsynaptic Motor Neuron Blocks Intermediate and Long-Term Facilitation
The human neuroligin-3 R451C point mutation has been linked to ASD (Jamain et al., 2003). Since there has not been any study investigating the mutant’s effect in synaptic plasticity, we made an arginine (R) to cysteine (C) point mutation in ApNLG at the homologous position, overexpressed this mutant in the motor neurons, and investigated its effect on 5-HT-induced changes in the strength of the sensory-to-motor neuron synapse. We found that overexpression of the ApNLG autism-linked mutant in the postsynaptic motor neuron of sensory-to-motor neuron co-cultures inhibits LTF measured at 24 hr after repeated pulses of 5-HT (Figure 8A, % initial EPSP amplitude: 5-HT 55.0 ± 11.8, n=22, ApNLG overexpression + 5-HT 46.4 ± 14.6, n = 23, ApNLG mutant overexpression+ 5-HT 4.3 ± 12.9, n = 23, p < 0.001 versus ApNLG overexpression + 5-HT). Overexpression of wild-type ApNLG or ApNLG mutant had no effect on basal transmission (% initial EPSP amplitude: no expression 3.5 ± 7.0, n = 21; ApNLG mutant overexpression alone −6.8 ± 8.9, n = 9; ApNLG overexpression alone −9.3 ± 7.0, n = 17).
Figure 8. The Effect of ApNLG Autism-Linked Mutant Expressed in the Postsynaptic Motor Neuron on Intermediate-Term Facilitation and LTF.
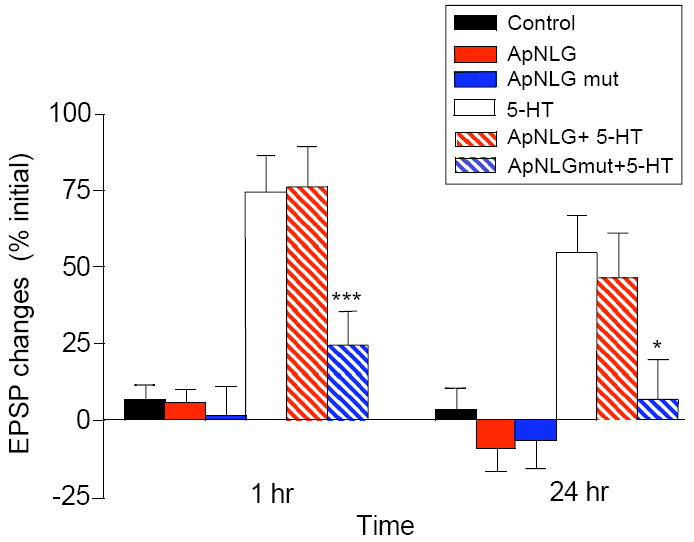
An ApNLG mutant harboring R to C mutation at the analogous position in human NLG-3 R451C mutant linked to ASD was overexpressed in the postsynaptic motor neuron in sensory-to-motor neuron co-cultures. EPSPs were recorded from motor neurons in response to extracellular stimulation of sensory neurons before, 1 hr, and 24 hr after exposure to 5 × 5-HT (10 μM, 5 min). Changes in amplitudes are shown as a bar graph.
Previous studies of the sensory-to-motor neuron synapse in Aplysia have revealed the existence of an intermediate-term phase of facilitation that requires protein synthesis, but does not require nuclear transcription (Ghirardi et al., 1995; Sutton and Carew, 2000). We therefore wondered whether the ApNLG autism-linked mutant might also have an effect on intermediate-term facilitation as it precedes LTF and may serve to initiate the trans-synaptic signaling required for the long-term process. Indeed, we found that similar to its affect on LTF, there was a significant decrease in facilitation during the intermediate-term time domain measured at 1 hr after repeated pulses of 5-HT when the ApNLG autism-linked mutant was overexpressed in the postsynaptic motor neuron (Figure 8A, % initial EPSP amplitude: 5-HT 74.4 ± 11.7, n=22, ApNLG overexpression + 5-HT 76.2 ± 13.0, n = 23, ApNLG mutant overexpression+ 5-HT 24.8 ± 10.9, n = 23, p < 0.05 versus ApNLG overexpression + 5-HT). Overexpression of wild-type ApNLG or ApNLG mutant had no effect on basal transmission measured at 1 hr (% initial EPSP amplitude: no expression 7.1 ± 4.3, n = 21; ApNLG mutant overexpression alone 1.8 ± 9.1, n = 9; ApNLG overexpression alone 5.8 ± 4.3, n = 17).
The findings that the ApNLG autism-linked mutant blocks both intermediate-term and long-term facilitation indicate that the trans-synaptic interaction mediated by neurexin and neuroligin is a critical component of both memory phases and is essential for the normal progression of long-term memory storage.
DISCUSSION
To investigate the role of the neurexin-neuroligin trans-synaptic interaction in activity-dependent synaptic plasticity, we have cloned Aplysia homologs of neurexin (ApNRX) and neuroligin (ApNLG). We found that they are indeed necessary components of 5-HT-induced long-term facilitation and the associated presynaptic structural remodeling and growth of sensory-to-motor neuron synapses of the Aplysia gill-withdrawal reflex reconstituted in culture.
ApNRX splice isoforms
The presence of neurexin and neuroligin in Aplysia as well as in the genomes of Drosophila and C. elegans further supports the view that the neurexin-neuroligin trans-synaptic interaction is highly conserved throughout evolution (Tabuchi and Südhof, 2002). Like other invertebrates, ApNRX has a domain structure similar to that of vertebrate α–neurexin with the likely absence of β-neurexin-like isoforms. In addition, four out of the five alternative splice sites present in vertebrate α-neurexin are conserved in ApNRX. Since the complete Aplysia genome is not yet available, we cannot directly compare the intron-exon structure of ApNRX with neurexins from other species. However, we find that the two splice sites–ApNRX sites 1 and 3–are located at precisely conserved positions corresponding to vertebrate neurexin sites 2 and 4 indicating that both the splicing mechanism and the underlying gene structure are likely to be similar between the Aplysia and vertebrate neurexins.
Alternative splicing determines binding affinities of neurexins to neuroligins (Ichtchenko et al., 1995; Boucard et al., 2005; Graf et al., 2006; Chih et al., 2006), but there has not as yet been detailed study of how the splice variants are functionally different. It will be interesting in future studies to investigate if the different ApNRX splice variants may serve differential roles in regulating activity-dependent synaptic plasticity.
Role of the Neurexin and Neuroligin Trans-Synaptic Interaction in Long-Term Memory and Associated Synaptic Remodeling and Growth
The current view regarding neurexin and neuroligin is that they are more likely to participate in activity-dependent modulation of the maturation, remodeling and specification of synapses rather than in de novo synaptogenesis (reviewed Südhof, 2008). This proposed role of neurexin and neuroligin suggested to us that they might be critical molecular components in regulating the synaptic plasticity that underlies learning and memory storage. Indeed, there is emerging evidence supporting the role of neurexin and neuroligin in learning and memory (Kim et al., 2008; Dahlhaus et al., 2009, Etherton et al., 2009, Blundell et al., 2010). By taking advantage of the monosynaptic sensory-to-motor neuron connection of the gill-withdrawal reflex of Aplysia, where a direct link between the activity-dependent changes in synaptic function and structure and the behavioral modification underlying a simple form of learned fear is firmly established, we provide direct evidence for an essential role of neurexin and neuroligin in the strengthening of synaptic connections that underlies the different stages of long-term memory storage. Furthermore, by time-lapse imaging of living cells in culture, we have found that the ApNRX-ApNLG trans-synaptic interaction also is important for the 5-HT-induced remodeling and growth of new synaptic structures associated with long-term memory. Our results in Aplysia support the idea that neurexin and neuroligin have an inherent, latent ability to remodel preexisting synapses and to generate new synapses under certain conditions and that this capacity can be induced and reutilized by learning and memory in mature neural circuits.
Our findings that either the knockdown of ApNRX in the presynaptic sensory neuron or knockdown of ApNLG in the postsynaptic motor neuron significantly blocks both the functional and structural expression of LTF at 24 hr suggest that the coordinated trans-synaptic actions of both molecules are required for the initial formation and subsequent stabilization of new synapses induced by 5-HT. One of the likely cellular mechanisms of how neurexin and neuroligin are mobilized by 5-HT stimulation is through a coordinated increase in both the pre- and postsynaptic neurons of kinesin-mediated axonal transport of neurexin and neuroIigin to the synapse. This assertion is based on the previous findings that neurexin and neuroligin are cargoes of kinesin transport from the cell body to the synapse and that 5-HT treatment, which induces LTF, leads to an increased kinesin-mediated anterograde transport of both neurexin and neuroligin (Puthanveettil et al., 2008). In support of this idea, we find that 5-HT treatment that induces LTF leads to the enrichment with ApNRX of some “empty” presynaptic sensory neuron varicosities.
The finding that overexpression of ApNRX alone or ApNLG alone does not inducing long-lasting synaptic facilitation further supports the importance of a coordinated increase and subsequent functional trans-synaptic interaction between ApNRX and ApNLG. The concomitant overexpression of ApNRX in the presynaptic sensory neuron and ApNLG in the postsynaptic motor neuron likely provides a similar “permissible condition”, perhaps mimicking a 5-HT-induced recruitment of both molecules to the sensory-to-motor neuron synapse, and thus leading to a more prolonged increase in synaptic strength.
The long-term maintenance of LTF and synaptic growth requires local protein synthesis (Martin et al, 1997) and is dependent on the translational regulator, cytoplasmic polyadenylation element-binding protein (CPEB, Si et al., 2003). Our finding that knockdown ApNRX or ApNLG protein 24 hrs after 5-HT treatment blocks the persistence of LTF measured at 72 hrs support the idea that newly synthesized neurexin and neuroligin are required continuously beyond 24 hrs for persistence of LTF. Our lab has previously shown that only the 5-HT-induced newly formed sensory neuron varicosities (and not preexisting varicosities) require sustained CPEB-dependent local protein synthesis for a period of approximately 2 days (24hr -72hrs) to acquire the more stable properties of “mature” varicosities. This selective stabilization of learning-induced synaptic growth leads to the persistence of LTF (Miniaci et al., 2008). It is therefore interesting that ApNRX mRNA has CPEB binding elements in the 3’ untranslated region (UTR, unpublished data) and mRNA of neuroligin is a target of CPEB in Drosophila (Mastushita-Sakai et al., 2010). Thus, we are in a position to test the idea that ApNRX and ApNLG are regulated by CPEB-mediated local protein synthesis and that this local synthesis of trans-synaptic signaling molecules is required for the stabilization of synaptic growth and the persistence of long-term memory storage.
In contrast, we show that the knockdown of ApNRX in the presynaptic sensory neurons or ApNLG in the postsynaptic motor neurons has no effect on basal synaptic transmission. This result implies that a 5-HT-induced increase in neurexin and neuroligin may not play a significant regulatory role in the maintenance of functional competency in the population of preexisting synapses.
The 5-HT-induced increase in kinesin-mediated transport of ApNRX and ApNLG and the postulated increase in CPEB-mediated local translation of ApNRX and ApNLG during LTF are not necessarily mutually exclusive. For example, it is possible that the enrichment of ApNRX after 5×5-HT treatment could be regulated by both processes in the same population of varicosities or that perhaps an increase in kinesin-mediated transport only occurs in some varicosities whereas an increase via CPEB-mediated local protein synthesis occurs in other varicosities.
Relevance of Neurexin and Neuroligin to Autism Spectrum Disorder
As an attempt to produce animal models of ASD, transgenic mice that contain the human NLG-3 R451C mutation linked to ASD have been generated. These mice have a modest impairment in social interactions and an enhancement in spatial learning ability (Tabuchi et al., 2007, but see Chadman et al., 2008). Moreover, electrophysiological recordings from the somatosensory cortex of these mice showed enhanced inhibitory synaptic transmission (Tabuchi et al., 2007). Since the patients with ASD having R451C substitution exhibit learning disabilities (Jamain et al., 2003), we made an ApNLG mutant containing the arginine to cysteine point mutation at the analogous position and investigated its effect on various stages of memory storage in Aplysia. We find that this mutation inhibits both intermediate-term and long-term facilitation. These findings are important for two reasons: First, our results further validate the utility of transgenic mice harboring the NLG-3 R451C mutation in ASD research and suggest a deeper understanding of how this defect relates to ASD can be accomplished by a more detailed examination of its role in the experience-dependent synaptic plasticity that underlies learning, including emotional learning that may be impaired in ASD. Second, these findings suggest the interesting point that the defect caused by this mutation in neurexin-neuroligin trans-synaptic signaling may first become apparent during the intermediate-term phase of memory storage and becomes further evident in the subsequent expression of facilitation at later time points. This interruption can account for a dysfunction in the normal progression of long-term memory storage.
It is becoming clear that aspects of ASD may be the result of a dysfunction of remodeling and stabilization at specific synapses, perhaps those involved in the acquisition of emotional and social cognition. Although the ASD-linked mutations in neuroligin and neurexin genes are thought to be rare, it is likely that here, as in other neuropsychiatric diseases such as early-onset Alzheimer’s disease and Parkinson’s disease, one may be able to obtain valuable insights into the mechanisms underlying the pathophysiology of ASD from the detailed cellular and molecular characterization of a set of clearly identified mutant genes such as neurexin and neuroligin involved in synapse formation, maturation, remodeling, and stabilization.
EXPERIMENTAL PROCEDURES
Cloning
Details about the cloning of ApNLG and ApNRX and DNA constructs used in the study are described in the supplementary information. The sequences of ApNLG (accession number HM448446) and ApNRX (accession number HM461999) were deposited into GenBank.
Antibody Production and Immunocytochemistry of Aplysia Neurons
Details about the ApNLG and ApNRX antibody production and immunocytochemistry are described in the supplementary information. Rabbit polyclonal antibodies were raised against a synthetic peptide derived from the intracellular cytoplasmic tail of ApNRX and against the extracellular domain of ApNLG recombinantly expressed and purified from E.Coli. Immunocytochemistry of Aplysia cultures were carried out as described previously (Martin et al., 1997).
Binding Assays
The ApNRX and ApNLG Fc fusion proteins were collected from the media of transfected HEK293 cells. Lysates from GFP-ApNLG or GFP-ApNRX transfected HEK 293 cells and Fc fusion proteins were incubated with Protein A agarose. Subsequent precipitates were analyzed by immunoblotting with an anti-GFP antibody. Details are described in the supplementary information.
Aplysia Cell Culture, Microinjection and Electrophysiology
We prepared Aplysia sensory-to-motor neuron co-cultures and measured excitatory postsynaptic potentials (EPSPs) as previously described (Montarolo et al., 1986). To induce LTF, we treated cultures with five 5 min pulses of 5-HT (10 μM) at 20 min intervals. For intermediate-term facilitation, EPSPs were measured again 1 hr after the conclusion of 5-HT treatment. To induce STF, we treated cultures with one 5 min pulse of 5-HT (10 μM) after the initial EPSP measurement. EPSP was measured again 5 min after 5-HT treatment. DNA constructs, oligonucleotides, or dyes were injected under visual guidance into the cytoplasm (for oligonucleotides or dyes) or into the nucleus (for DNAs) of Aplysia neurons.
Cell Imaging and Quantification of the Structural Changes that Accompany 5-HT-Induced LTF
We acquired images of Aplysia neurons using a Zeiss LSM 5 Pascal laser confocal scanning microscopes. We assessed the long-term structural changes by comparing the images of each sensory neuron before and 24 hr after 5-HT treatment as previously described (Kim et al., 2003). The maximum mean intensity among all the varicosities in one culture was designated as 100% enrichment index of GFP and the background intensity was designated as 0% enrichment index. The varicosities were binned according to their average fluorescence intensities. We considered varicosities in 0%-10% enrichment index to be “empty varicosities.”
Statistical Analysis
Results are denoted as means ± SEM. We used a paired or unpaired Student’s t test to determine statistical significance between two data sets, and one-way ANOVA followed by Tukey post hoc test for multiple comparisons. The statistical significance was indicated by * p < 0.05, ** p < 0.01,or *** p < 0.001.
Supplementary Material
Acknowledgments
We thank Professor Timothy Rose of University of Washington for help with CODEHOP PCR and Huixiang “Vivian” Zhu and Edward Konstantinov for Aplysia culture preparation. This work is supported by grants from the Howard Hughes Medical Institute (to E.R.K.), National Institute of Health grant NS053415 (to Y.-B.C.) and the Simons Foundation (to E.R.K, Y.-B.C. and C.H.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- The Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983;220:91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Montarolo PG, Chen M, Kandel ER, Schacher S. Inhibitors of protein and RNA synthesis block the structural changes that accompany long-term heterosynaptic plasticity in the sensory neurons of Aplysia. Neuron. 1992;9:749–758. doi: 10.1016/0896-6273(92)90037-e. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. In: Sossin W, Lacaille J-C, Castellucci VF, Belleville S, editors. The Essence of Memory Progress in Brain Research. Vol. 169. Elsevier Press; 2008. pp. 179–198. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, Bollinger MF, Südhof TC, Powell CM. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to α- and β-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Südhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhaus R, Hines RM, Eadie BD, Kannangara TS, Hines DJ, Brown CE, Christie BR, El-Husseini A. Overexpression of the cell adhesion protein neuroligin-1 induces learning deficits and impairs synaptic plasticity by altering the ratio of excitation to inhibition in the hippocampus. Hippocampus. 2010;20:305–322. doi: 10.1002/hipo.20630. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signaling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berg J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–716. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Kandel ER, Schacher S. Identified target motorneuron regulates neurite outgrowth and synapse formation of aplysia sensory neurons in vitro. Neuron. 1989;3:441–450. doi: 10.1016/0896-6273(89)90203-1. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–4265. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Nguyen T, Südhof TC. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J Biol Chem. 1996;271:2676–2682. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T Paris Autism Research International Sibpair Study. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jung SY, Lee YK, Park S, Choi JS, Lee CH, Kim HS, Choi Y-B, Scheiffele P, Bailey CH, Kandel ER, Kim J-H. Neuroligin-1 is required for normal expression of LTP and associative fear memory in the amygdala of adult animals. Proc Natl Acad Sci USA. 2008;105:9087–9095. doi: 10.1073/pnas.0803448105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Udo H, Li H-L, Youn TY, Chen M, Kandel ER, Bailey CH. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Molzard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthélémy C, Moraine C, Briault S. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Wickremasinghe N, Carew TJ. Regulation of behavioral and synaptic plasticity by serotonin release within local modulatory fields in the CNS of Aplysia. J Neurosci. 2006;26:12682–12693. doi: 10.1523/JNEUROSCI.3309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu H, Yaping Y, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific long-term facilitation of Aplysia sensory to motor synapses: A function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Mastushita-Sakai T, White-Grindley E, Samuelson J, Seidel C, Si K. Drosophila Orb2 targets genes involved in neuronal growth, synapse formation, and protein turnover. Proc Natl Acad Sci USA. 2010;107:11987–11992. doi: 10.1073/pnas.1004433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniaci MC, Kim J-H, Puthanveettil SV, Si K, Zhu H, Kandel ER, Bailey CH. Sustained CPEB-dependent local protein synthesis is required to stabilize synaptic growth for persistence of long-term facilitation in Aplysia. Neuron. 2008;59:1024–1036. doi: 10.1016/j.neuron.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthanveettil SV, Monje FJ, Miniaci MC, Choi Y-B, Karl KA, Khandros E, Gawinowicz MA, Sheetz MP, Kandel ER. A New Component in Long-Term Synaptic Plasticity: Upregulation of Kinesin in the neurons of gill-withdrawal reflex. Cell. 2008;135:960–1073. doi: 10.1016/j.cell.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose TM, Henikoff JG, Henikoff S. CODEHOP (COnsensus-DEgenerate Hybrid Oligonucleotide Primer) PCR primer design. Nucleic Acids Res. 2003;31:3763–3766. doi: 10.1093/nar/gkg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim J-H, Zhu H, Kandel ER. A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in Aplysia. Cell. 2003;115:893–904. doi: 10.1016/s0092-8674(03)01021-3. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Südhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Südhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Südhof TC. Neurexin III alpha: extensive alternative splicing generates membrane-bound and soluble forms. Proc Natl Acad Sci U S A. 1993;90:6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY. Postnatal neurodevelopmental disorders: meeting at the synapse? Science. 2003;302:826–830. doi: 10.1126/science.1089071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


