Abstract
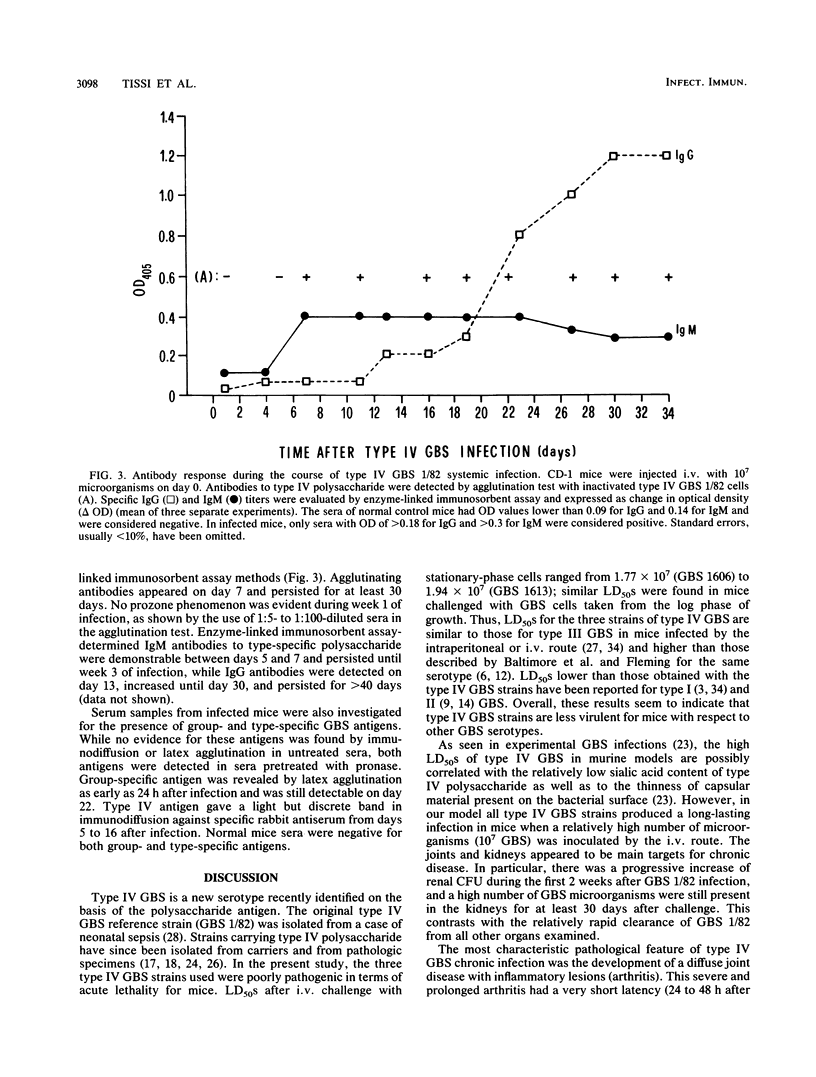
We have established an experimental murine model to gain insight into the pathogenicity and clinical features of type IV group B streptococcus (GBS) infections. Adult CD-1 mice were challenged intravenously with 10(7) type IV GBS cells, inducing systemic invasion. Most of the animals were able to clear the infection from the blood, brain, and lungs within 2 weeks and from the spleen and liver within 1 month. However, the animals were unable to clear the microorganism from the joints and kidneys during the 60-day observation period. About 80% of the mice challenged intravenously with type IV GBS manifested early septic arthritis, which evolved from an acute exudative synovitis to permanent lesions characterized by irreversible joint damage and ankylosis. Induction of persistent septic arthritis was dependent on the number and viability of microorganisms inoculated and was unrelated to the strain of type IV GBS and the growth phase of the inoculum. Type-specific antibodies of both immunoglobulin M and G classes could be detected by agglutination and enzyme-linked immunosorbent assay from days 7 and 14, respectively; immunoglobulin G antibodies persisted for more than 40 days. Complexes of antibodies and group- and type-specific antigens were detected in mouse sera 24 h after infection and persisted up to day 22. These results were obtained an experimental model of type IV GBS chronic infection with early development of septic arthritis, which could be useful in future studies of pathogenicity and immune mechanisms involved in the host resistance to this microorganism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Okada D. M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974 Nov 25;230(8):1158–1160. [PubMed] [Google Scholar]
- Baker C. J., Edwards M. S., Webb B. J., Kasper D. L. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J Clin Invest. 1982 Feb;69(2):394–404. doi: 10.1172/JCI110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L., Tager IRAB, Paredes A., Alpert S., McCormack W. M., Goroff D. Quantitative determination of antibody to capsular polysaccharide in infection with type III strains of group B Streptococcus. J Clin Invest. 1977 May;59(5):810–818. doi: 10.1172/JCI108703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Vecchitto J. Mouse protection test for group B Streptococcus type III. J Infect Dis. 1979 Jul;140(1):81–88. doi: 10.1093/infdis/140.1.81. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Fuselier P. A. Enhanced susceptibility of mice with streptozotocin-induced diabetes to type II group B streptococcal infection. Infect Immun. 1983 Feb;39(2):580–585. doi: 10.1128/iai.39.2.580-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Nicholson-Weller A., Baker C. J. The role of complement in opsonization of GBS. Antibiot Chemother (1971) 1985;35:170–189. doi: 10.1159/000410371. [DOI] [PubMed] [Google Scholar]
- Ferrieri P., Burke B., Nelson J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect Immun. 1980 Mar;27(3):1023–1032. doi: 10.1128/iai.27.3.1023-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. O. Mucin model for group B type III streptococcal infection in mice. Infect Immun. 1980 Feb;27(2):449–454. doi: 10.1128/iai.27.2.449-454.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciosi R. A., Knostman J. D., Zimmerman R. A. Group B streptococcal neonatal and infant infections. J Pediatr. 1973 Apr;82(4):707–718. doi: 10.1016/s0022-3476(73)80604-3. [DOI] [PubMed] [Google Scholar]
- Furtado D. Experimental group B streptococcal infections in mice: hematogenous virulence and mucosal colonization. Infect Immun. 1976 May;13(5):1315–1320. doi: 10.1128/iai.13.5.1315-1320.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr, Pritchard D. G. Interaction of group B streptococcal type-specific polysaccharides with wheat germ agglutinin and other lectins. J Immunol Methods. 1984 Aug 3;72(1):269–277. doi: 10.1016/0022-1759(84)90455-1. [DOI] [PubMed] [Google Scholar]
- HOOD M., JANNEY A., DAMERON G. Beta hemolytic streptococcus group B associated with problems of the perinatal period. Am J Obstet Gynecol. 1961 Oct;82:809–818. doi: 10.1016/s0002-9378(16)36146-4. [DOI] [PubMed] [Google Scholar]
- Jelínková J., Motlová J. The nomenclature of GBS. Antibiot Chemother (1971) 1985;35:49–52. [PubMed] [Google Scholar]
- Jelínková J., Motlová J. Worldwide distribution of two new serotypes of group B streptococci: type IV and provisional type V. J Clin Microbiol. 1985 Mar;21(3):361–362. doi: 10.1128/jcm.21.3.361-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald M. L. Animal models of infectious arthritis. Clin Rheum Dis. 1986 Aug;12(2):403–421. [PubMed] [Google Scholar]
- Motlová J., Wagner M., Jelínková J. A search for new group-B streptococcal serotypes. J Med Microbiol. 1986 Sep;22(2):101–105. doi: 10.1099/00222615-22-2-101. [DOI] [PubMed] [Google Scholar]
- Orefici G., Molinari A., Von Hunolstein C., Donelli G., Paradisi S., Recchia S., Arancia G. Pathogenic factors in group B streptococci types IV and V. Chemioterapia. 1987 Jun;6(2 Suppl):19–21. [PubMed] [Google Scholar]
- Perch B., Kjems E., Henrichsen New serotypes of group B streptococci isolated from human sources. J Clin Microbiol. 1979 Jul;10(1):109–110. doi: 10.1128/jcm.10.1.109-110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutrel B., Dore J. Virulence of human and bovine isolates of group B streptococci (types Ia and III) in experimental pregnant mouse models. Infect Immun. 1985 Jan;47(1):94–97. doi: 10.1128/iai.47.1.94-97.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit K. E., DeBiasio R. Kinetics of phagocyte response to group B streptococcal infections in newborn rats. Infect Immun. 1980 May;28(2):319–324. doi: 10.1128/iai.28.2.319-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Rote N. S., Santos J. I., Hill H. R. Assessment of the virulence factors of group B streptococci: correlation with sialic acid content. J Infect Dis. 1983 May;147(5):857–863. doi: 10.1093/infdis/147.5.857. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D. Am J Pathol. 1983 Jul;112(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Stanton B. F., Baltimore R. S., Shedd D. G. Effects of route and time of administration of antiserum on protection of mice from lethal infection due to group B Streptococcus type III. Infect Immun. 1981 Jan;31(1):391–395. doi: 10.1128/iai.31.1.391-395.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerstrom D. E., Schutt R. W. Adult mice as a model for early onset group B streptococcal disease. Infect Immun. 1978 Feb;19(2):741–744. doi: 10.1128/iai.19.2.741-744.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Benedí W. J., Jennings H. J., Michon F., DiFabio J. L., Kasper D. L. Isolation and characterization of type IV group B Streptococcus capsular polysaccharide. Infect Immun. 1989 Apr;57(4):1089–1094. doi: 10.1128/iai.57.4.1089-1094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Jones W. L. Radioimmunoassay for measuring antibodies specific for group B streptococcal types Ia, Ib, Ic, II, and III. J Clin Microbiol. 1976 May;3(5):480–485. doi: 10.1128/jcm.3.5.480-485.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]