Abstract
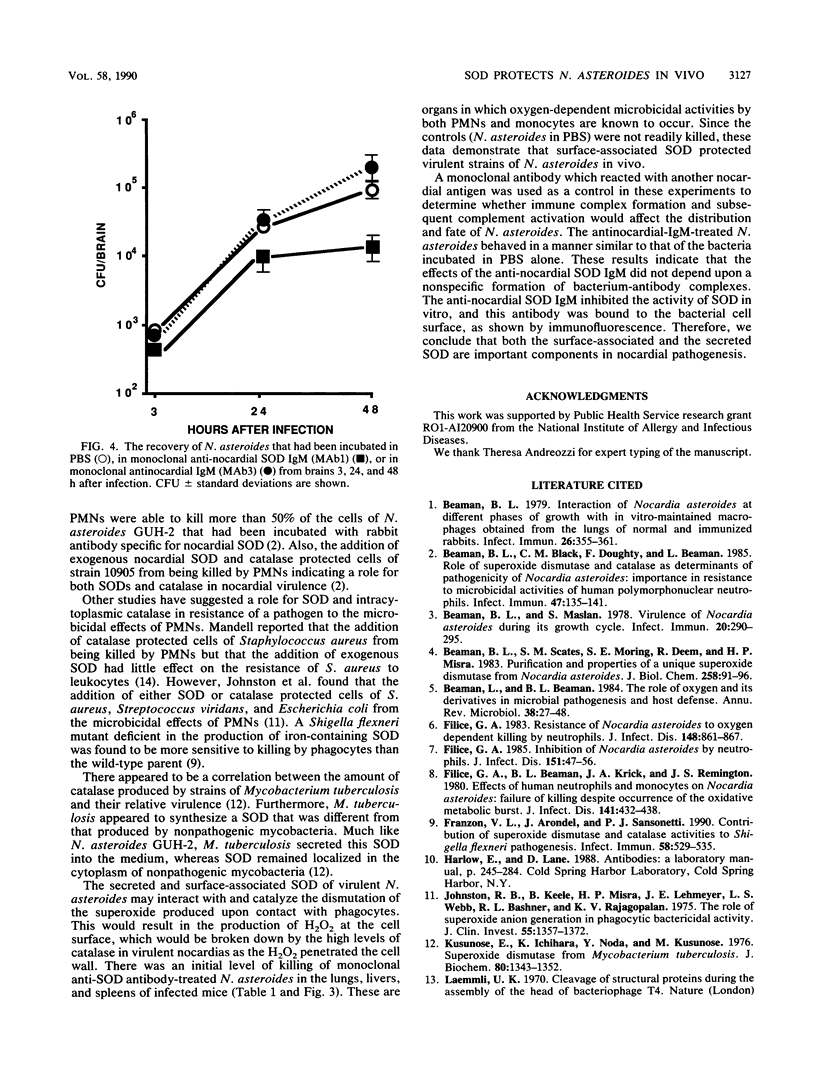
The importance of superoxide dismutase (SOD) in protecting cells of Nocardia asteroides from the oxidative killing mechanisms within the intact murine host was determined. Murine monoclonal antibodies specific for nocardial SOD and for another nocardial antigen were prepared. Both antibodies adhered to cell surface antigens, as shown by fluorescence-labeled-antibody staining. The anti-nocardial SOD antibody inhibited the effect of nocardial SOD on superoxide generated in vitro. Cells of N. asteroides GUH-2 in log phase of growth were incubated with monoclonal anti-nocardial SOD, another monoclonal antinocardial antibody (not reactive with SOD), or phosphate-buffered saline and then injected intravenously into mice. Total recovery of CFU and inhibition of growth were determined at 3, 24, and 48 h after infection. The brains, kidneys, spleens, lungs, and livers were weighed, homogenized, and plated in order to quantitate the number of organisms in each organ at each time period. There was an initial killing followed by enhanced clearance of N. asteroides from the lungs and livers of mice which had received anti-SOD antibody-treated nocardiae. There was also enhanced early killing in the spleen. At 48 h, there were fewer organisms recovered from the brains, kidneys, and livers of mice which had received anti-SOD antibody-treated nocardia. This was not true for mice which had received antinocardial antibody not specific for SOD. The data demonstrate that surface-associated SOD protects N. asteroides for oxidative killing in vivo during all stages of infection.
Full text
PDF

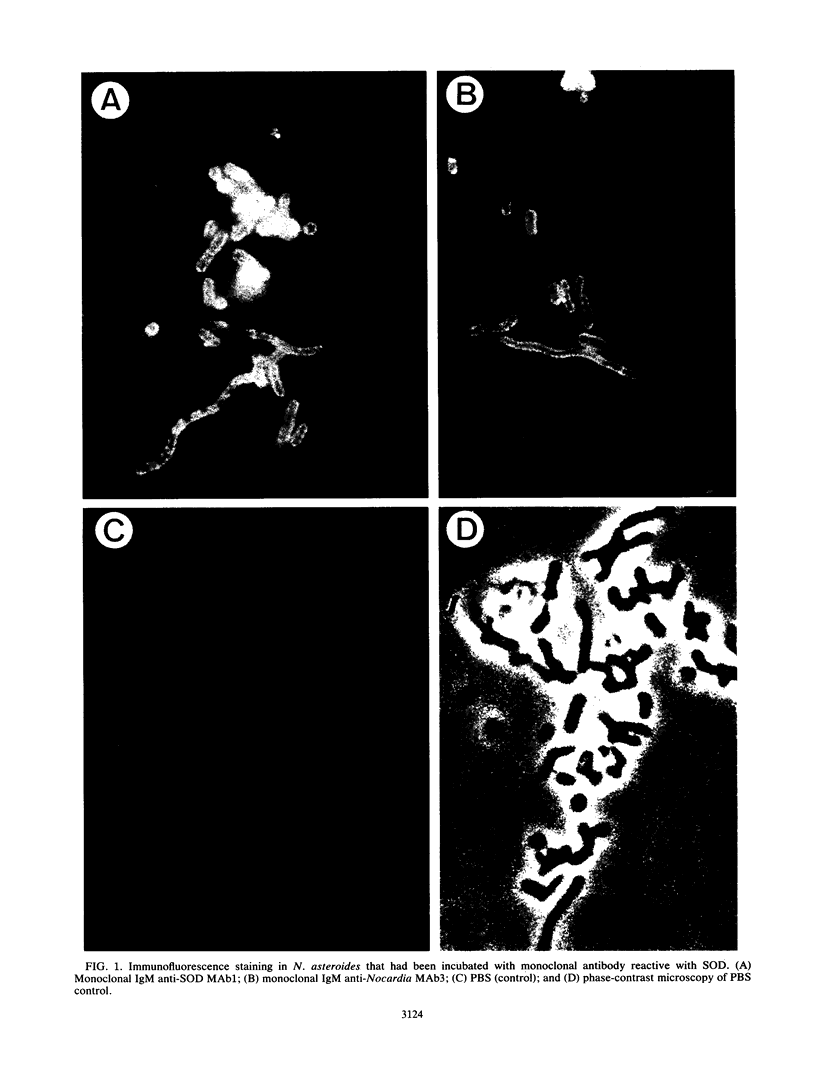
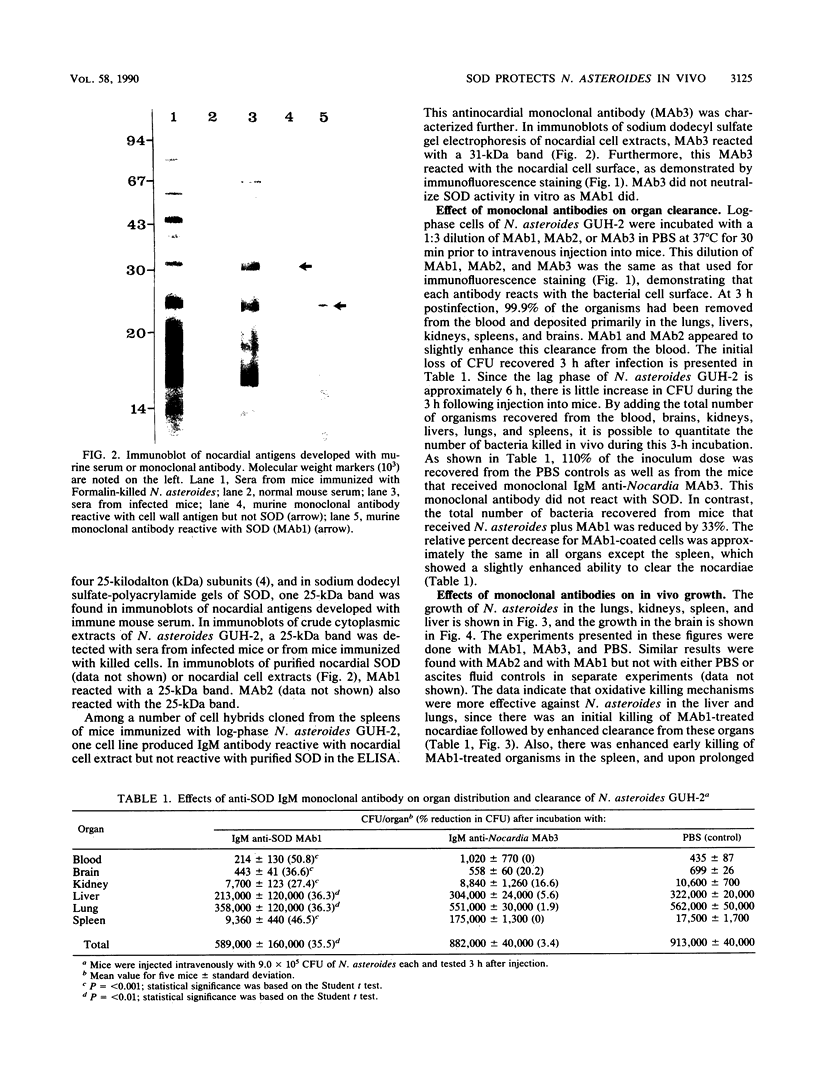
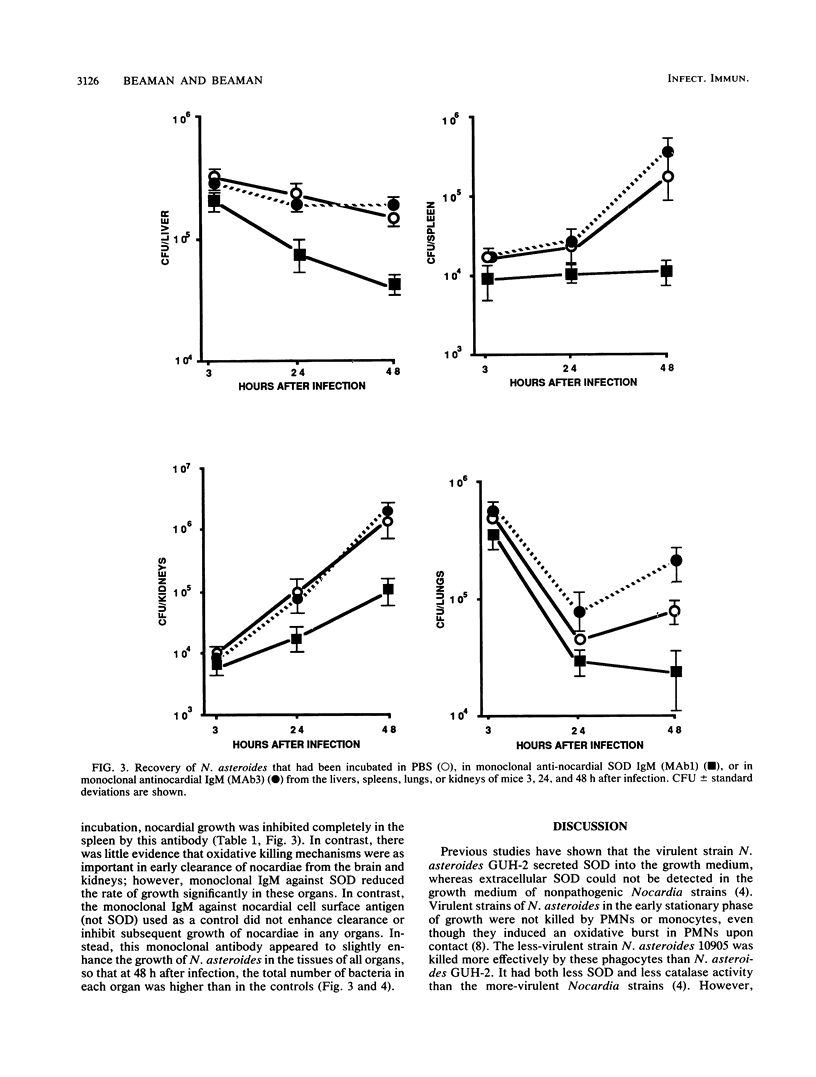

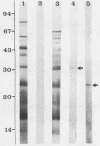
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Black C. M., Doughty F., Beaman L. Role of superoxide dismutase and catalase as determinants of pathogenicity of Nocardia asteroides: importance in resistance to microbicidal activities of human polymorphonuclear neutrophils. Infect Immun. 1985 Jan;47(1):135–141. doi: 10.1128/iai.47.1.135-141.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Interaction of Nocardia asteroides at different phases of growth with in vitro-maintained macrophages obtained from the lungs of normal and immunized rabbits. Infect Immun. 1979 Oct;26(1):355–361. doi: 10.1128/iai.26.1.355-361.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Virulence of Nocardia asteroides during its growth cycle. Infect Immun. 1978 Apr;20(1):290–295. doi: 10.1128/iai.20.1.290-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Scates S. M., Moring S. E., Deem R., Misra H. P. Purification and properties of a unique superoxide dismutase from Nocardia asteroides. J Biol Chem. 1983 Jan 10;258(1):91–96. [PubMed] [Google Scholar]
- Beaman L., Beaman B. L. The role of oxygen and its derivatives in microbial pathogenesis and host defense. Annu Rev Microbiol. 1984;38:27–48. doi: 10.1146/annurev.mi.38.100184.000331. [DOI] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Krick J. A., Remington J. S. Effects of human neutrophils and monocytes on Nocardia asteroides: failure of killing despite occurrence of the oxidative metabolic burst. J Infect Dis. 1980 Sep;142(3):432–438. doi: 10.1093/infdis/142.3.432. [DOI] [PubMed] [Google Scholar]
- Filice G. A. Inhibition of Nocardia asteroides by neutrophils. J Infect Dis. 1985 Jan;151(1):47–56. doi: 10.1093/infdis/151.1.47. [DOI] [PubMed] [Google Scholar]
- Filice G. A. Resistance of Nocardia asteroides to oxygen-dependent killing by neutrophils. J Infect Dis. 1983 Nov;148(5):861–867. doi: 10.1093/infdis/148.5.861. [DOI] [PubMed] [Google Scholar]
- Franzon V. L., Arondel J., Sansonetti P. J. Contribution of superoxide dismutase and catalase activities to Shigella flexneri pathogenesis. Infect Immun. 1990 Feb;58(2):529–535. doi: 10.1128/iai.58.2.529-535.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975 Mar;55(3):561–566. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]