Abstract
Exposure to endocrine disrupting compounds (EDCs), such as bisphenol A (BPA), may cause adverse health effects in wildlife and humans, but controversy remains as to what traits are most sensitive to EDCs and might serve as barometers of exposure. Expression of sexually selected traits that have evolved through intrasexual competition for mates and intersexual choice of mating partner are more dependent on developmental and physical condition of an animal than naturally selected traits and thus might be particularly vulnerable to disruption by developmental exposure to EDCs. We have used the deer mouse (Peromyscus maniculatus) as a model to test this hypothesis. Adult male–male competition for mates in this species is supported by enhanced spatial navigational and exploratory abilities, which enable males to search for prospective, widely dispersed females. Male deer mice exposed to BPA or ethinyl estradiol (EE) through maternal diet showed no changes in external phenotype, sensory development, or adult circulating concentrations of testosterone and corticosterone, but spatial learning abilities and exploratory behaviors were severely compromised compared with control males. Because these traits are not sexually selected in females, BPA exposure predictably had no effect, although EE-exposed females demonstrated enhanced spatial navigational abilities. Both BPA-exposed and control females preferred control males to BPA-exposed males. Our demonstration that developmental exposure to BPA compromises cognitive abilities and behaviors essential for males to reproduce successfully has broad implications for other species, including our own. Thus, sexually selected traits might provide useful biomarkers to assess risk of environmental contamination in animal and human populations.
Keywords: mate choice, sexual selection, spatial abilities, cognition, sex differences
Developmental exposure to endocrine-disrupting compounds (EDCs) has posed a major threat to wildlife since the large-scale production of these industrial chemicals (1). Numerous studies have documented disturbances of sex-typical development, reproductive tract pathologies, and abnormal adult behaviors through environmental contact with EDCs, including bisphenol A (BPA) (2–7). However, scant information is available regarding exposure to EDCs during development within the context of sexual selection (8, 9). Expression of sexually selected traits is critical to reproductive fitness and may be particularly vulnerable to EDCs because these traits show greater phenotypic variation than naturally selected traits, owing in part to dependence on more genetic loci and overall body condition (10, 11). Moreover, optimal expression of these traits in adulthood requires a complex orchestration of developmental exposure to estrogens and androgens, processes that can be compromised by EDC exposure (3, 4). We predicted that traits that evolved through intrasexual competition for mates and influence intersexual choice of mating partner would be particularly sensitive to EDCs (3, 12, 13) and might serve as useful barometers for detecting such chemicals in the environment.
To capture the variation in sexually selected traits found in wild populations, we used polygynous deer mice (Peromyscus maniculatus) to assess the effects of developmental exposure to BPA and ethinyl estradiol (EE), a synthetic estrogen, on male competitive behaviors and males’ attractiveness to females. Males of this species compete by expanding their territorial range during the breeding season, thereby increasing their prospects of locating prospective mates widely dispersed throughout the ecology. This enhanced adult male spatial ability and exploratory behavior requires not only seasonal increases in testosterone but prenatal exposure to this hormone (14), with the latter requirement rendering these traits potentially vulnerable to developmental EDC exposure. Once a prospective mate is found, females choose whether to mate with the male. Female choice is largely mediated by olfactory cues and behaviorally indicated by time engaged in nose-to-nose contact with and inspecting the male (15–17). On the basis of the sexually selected traits particular to this species, we assessed the effect of developmental exposure to BPA or EE on spatial navigational ability and exploratory behavior in male and female deer mice and female preference through a mate choice experiment.
Results
Litter Data.
Average litter size did not differ across maternal diet (2.95 ± 0.28, 2.76 ± 0.31, and 2.85 ± 0.20 for control, EE, and BPA groups, respectively) (Table S1). However, dams fed the EE diet had relatively fewer male offspring (41% male) than females receiving the control (62% male) and BPA-supplemented (65% male) diets (Table S1). Binomial tests, assessing whether the differences differed from the expected 50% sex ratio, were significant for the control (P = 0.009) and EE (P = 0.001) groups, and there was a trend for the dams on the BPA diet (P = 0.053). The more critical finding, however, was that the sex ratios for dams on the control and BPA-supplemented diets did not differ from each other (P = 0.83).
Assessments of Sensory and Neuromuscular Function.
Initial observations indicated offspring exposed to BPA and EE through maternal diet during prenatal development until weaning (day 25) demonstrated no visible abnormalities in phenotype, including coat color and weight. Males and females in all of the groups exhibited intact sensory systems, including olfaction, neuromuscular strength, response to sound, and vision (Table S2).
Spatial Learning and Memory.
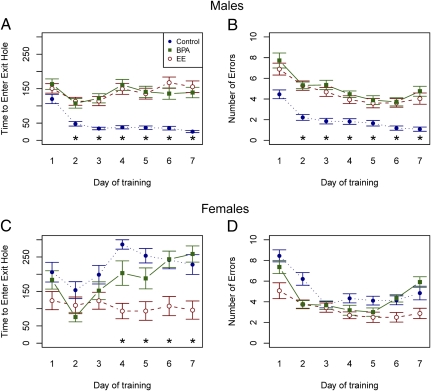
At sexual maturity (approximately day 60), spatial learning was assessed with the Barnes maze, a circular, dry-land maze that assesses the animal's ability to use visual cues to navigate out of the maze (Fig. S1). Behavior of each mouse was recorded and analyzed with automated tracking software (EthoVision XT; Noldus), allowing a composite tracking image to be assembled for every trial. After 2 d of habituation (5 min each), testing was performed on 7 successive days, with two trials per day. Fig. 1 shows the time required to reach the correct exit (latency) for males (Fig. 1A) and females (Fig. 1C) across training. After 2 d, control males exhibited shorter latencies to exit the maze than BPA- or EE-exposed males (P < 0.0001 for each) and females in all diet groups (P < 0.004). Males exposed to BPA or EE did not differ from each other on any day (P > 0.0834) (Fig. 1A). Except for day 2, females exposed to EE reached the exit hole sooner than control females (P < 0.0051). Frequency of entering the wrong exit hole for males (Fig. 1B) and females (Fig. 1D) was also affected by maternal diet. Control males and EE-exposed females did not differ in number of committed errors (P = 0.1767), but both groups committed fewer errors than males exposed to either BPA or EE (P < 0.0001 for each comparison) and control females (P < 0.0001).
Fig. 1.
Effects of early developmental exposure to BPA and EE on spatial learning and memory of adult male and female deer mice (Peromyscus maniculatus) in the Barnes maze. (A and C) Latency (i.e., time required to escape the maze) across days of training for males (A) and females (C) (mean ± SEM). (B and D) Number of escape errors across days of training for males (B) and females (D) (mean ± SEM, *P < 0.01) (see SI Results for detailed comparisons).
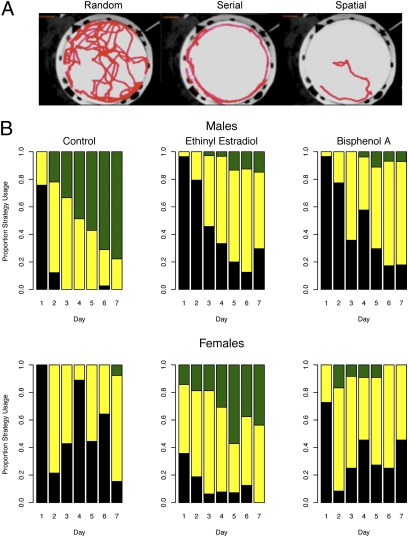
Automated tracking of movement in the Barnes maze allowed three types of search strategy to be identified: random (with no particular pattern), serial (in which the mice patrolled the perimeter), and spatial (in which the most direct approach to the exit hole was used, presumably based on learned use of visual cues) (Fig. 2A; further details supplied in SI Results). The dominant strategy on day 1 was random across the two trials, with the exception of EE-exposed females (Fig. 2B). Strategy differences between groups of males emerged within several days of training (Fig. 2B). On average, control males acquired the spatial-oriented strategy by day 3 of training, whereas the majority of males exposed to BPA or EE failed to shift to the spatial-oriented strategy by the end of training (P < 0.0002). Although behaviors for the BPA and EE male groups became less random, few demonstrated use of the more efficient spatial-oriented strategy used by control males (Movies S1–S3). EE-exposed females rapidly acquired the spatial-oriented strategy typical of control males (Movie S4) and, after day 2, outperformed control and BPA-exposed females (P < 0.0046 and P < 0.0084, respectively). Moreover, EE-exposed females did not differ from control males after day 1 (P > 0.20).
Fig. 2.
Effects of early developmental exposure to BPA and EE on spatial search strategy of adult male and female deer mice (Peromyscus maniculatus) in the Barnes maze. (A) Examples of composite images from single animals tracked from entry to escape, illustrating different spatial strategies used to exit the maze. (B) Distribution of different spatial strategies according to sex, diet exposure, and day of training. During the initial training period (day 1), most animals navigated by using a random strategy (black), followed by a serial search strategy (yellow). The most efficient spatial search strategy (green) emerged when the animals began to use directional and positional intramaze cues. By day 3 of training, control males used more efficient strategies than control females and EE- and BPA-exposed males, who in turn did not differ on any day (P < 0.0002). EE-exposed females used more efficient strategies than control and BPA-exposed females on all days except day 2 (see SI Results for further details).
These combined results reveal that spatial learning in males is compromised by developmental exposure to either BPA or EE in the maternal diet. Conversely, EE but not BPA exposure seemed to render female spatial behavior more like that of control males with respect to error frequency and spatial strategy; they also had shorter latencies than control females. When total distance traveled was used as a covariate, it did not alter the significance of the latency data (SI Results, Spatial Learning and Memory).
Exploratory and Anxiety-Like Behavior.
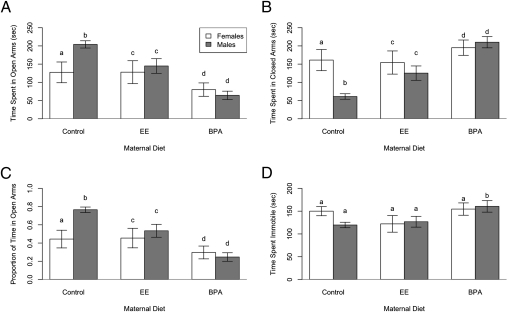
After completing the spatial learning and memory testing in the Barnes maze, exploratory behavior in the same group of mice was assessed by using the elevated-plus maze (EPM), which consisted of two open and two closed arms projecting from a central platform raised 1 m from the floor. Each mouse was placed on the center platform and allowed to explore the maze for 5 min. Each trial was scored for total time spent in the arms, and the proportion of total time spent in open arms was calculated. In addition, experiments measured time spent on the central platform and the number of closed and open arm entries and exits. Control males spent more time in the open arms, an indicator of exploratory behavior, than EE- or BPA-exposed males (P = 0.014 and P < 0.0001, respectively) and control females (P = 0.0039) (Fig. 3 A and C). However, male exploratory behavior was more strongly compromised by BPA than EE. Control males also entered open arms more frequently than either EE- or BPA-exposed males (P = 0.0044 and P = 0.0024, respectively) and control females (P = 0.0006), again emphasizing the potential demasculinizing effect of BPA and EE on male deer mice behaviors. In particular, BPA males spent a large proportion of their time in the closed arms of the maze (Fig. 3 B and C). Whether such sheltering in the closed arms reflects a lowered exploratory behavior, increased anxiety, or both, is unclear. Finally, males exposed to BPA spent more time immobile than control and EE-exposed males (means, 158 s, 135 s, and 124 s, respectively; P = 0.0591) (Fig. 3D).
Fig. 3.
Effects of early developmental exposure to BPA and EE on exploratory behavior in the EPM. Diet and sex differences in time spent in (A) open arms; (B) closed arms; (C) open arms in proportion to total time in open and closed arms; and (D) time spent immobile. Same letters indicate no difference, whereas different letters indicate significant differences (see SI Results for detailed comparisons).
Mate Choice Experiment.
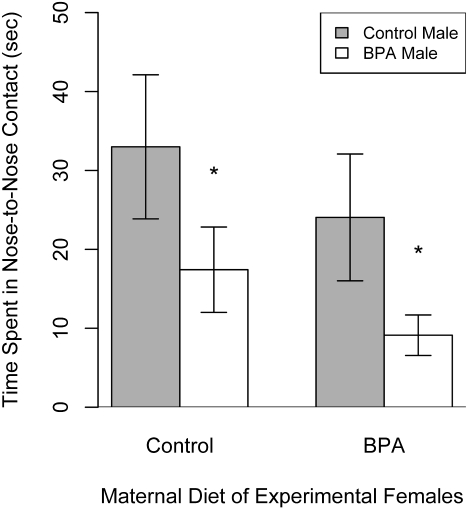
After a 2-wk resting period following EPM testing, female mate preference for control vs. BPA-exposed males was compared (Fig. S2). Control and BPA-exposed females spent more time engaged in nose-to-nose contact with control males than with males exposed to BPA (29.5 ± 5.86 s vs. 14.3 ± 5.76 s; P = 0.0114) (Fig. 4). Inspection time, however, did not vary with maternal diet of the females (P = 0.2727). We conclude that females have a reduced sexual preference for males exposed to BPA, although it remains to be determined whether this effect is due to behavioral cues, reduced chemical signaling through pheromones, or both (Movie S5). Male choice of female partners was not assessed because male choice is rare among species with polygynous mating systems, including Peromyscus (18).
Fig. 4.
Female choice of control vs. BPA-exposed males. Control and BPA-exposed females exhibited longer duration of nose-to-nose contact (mean ± SEM) with control males than with BPA-exposed males. *P < 0.05.
Adult Serum Testosterone and Corticosterone Concentrations in Male Deer Mice.
Finally, because spatial abilities and exploratory behavior in male deer mice are likely to be influenced by adult concentrations of circulating testosterone and corticosterone in the peripheral blood (19), we measured the concentration of these hormones in serum of adult males from the three diet groups. No differences in serum testosterone (P > 0.3180) or corticosterone (P > 0.2540) concentrations were observed between the control, EE, and BPA males (Fig. S3). Thus, the behavioral disturbances manifested in adult males caused by early exposure to BPA seem not to be linked to either changes in adult testosterone or corticosterone serum concentrations.
Discussion
Together, the results demonstrate that developmental exposure to BPA and the synthetic estrogen EE severely disrupts spatial learning of male P. maniculatus and simultaneously decreases their exploratory behavior, without altering adult concentrations of circulating testosterone and corticosterone (Fig. S3). In a mate choice experiment, females also seemed to detect the compromised quality of males exposed during early development to BPA and discriminated against them. Depressed male sexual vigor has also been observed in Japanese quail (Corturnix coturnix japonica) exposed in ovo to EE (20). In the latter study, there were also no accompanying changes in circulating testosterone concentrations to account for the abnormal behavior. The sex ratio bias in dams on the EE-supplemented diet might partially explain some of the effects of EE on male spatial cognition: males of this species born to female-biased litters have been previously demonstrated to exhibit some deficits in spatial abilities and a lower tendency to disperse during the mating season (14). However, these results do not explain the spatial deficits exhibited by BPA-exposed males, who, like the controls, were born into primarily male-biased litters. If anything, this sex ratio bias in the BPA group would have been anticipated to improve male spatial learning.
In our experiments, maternal diet exposure to BPA was 50 mg BPA/kg feed weight, which is consistent with prior studies that have demonstrated that such a dose can induce epigenetic (21) and behavioral changes (22) in laboratory rodents. It was designed to be well below the diet-administered maximum nontoxic dose for rodents (200 mg/kg body weight per day), within the presumptive no observed effect level for mice (23), and likely to provide circulating serum concentrations close to those observed for humans (24). We have recently demonstrated that dietary exposure of laboratory mice to 100 mg deuterated [dimethyl-d6]-BPA (BPA-d6)/kg feed weight leads to peak serum concentrations of 18.8 ± 4.4 ng/mL within 6 h after initiating the diets (24). On the basis of the linear response curve observed with BPA (25–28), the dietary exposure concentration tested here for the deer mouse was anticipated to yield serum concentrations in these animals within the range of human exposure (27, 29).
Although our initial assumption was that maternal consumption of BPA had a direct, endocrine-disrupting effect on pup brain development, it is also possible that maternal exposure to BPA led to decreased maternal investment in her pups, as has been observed in mice and rats (30, 31). Such reduced maternal care by dams receiving the BPA- and EE-supplemented diet could, in turn, account for the extended effects on offspring social and cognitive competencies, as has been proposed elsewhere (32). Further studies are therefore planned to examine maternal care by BPA- and EE-exposed female deer mice and to test whether such effects on maternal behaviors influence the subsequent spatial learning abilities of male offspring.
A feature of polygynous rodent species, in which males compete by expanding home range and searching for mates, is increased hippocampal cell density, volume, and spine density during the breeding season (14, 33–35). BPA exposure can also result in a variety of biochemical and structural changes in the hippocampus of laboratory rodents (36–38). Although these effects of BPA, if direct and not an indirect outcome of poor maternal care, might be due to its ability to act as an analog of estradiol and engage and inappropriately alter the expression of genes for estrogen receptors (Esr1 and Esr2) in the fetal brain (39, 40). It is also possible that the spatial navigational deficits in exposed males are an outcome of suppression of Leydig cell testosterone production at the time that androgens from the testes normally masculinize the developing brain through local conversion to estradiol by aromatase (41, 42). Another possibility is that BPA effects are more immediate and occur through membrane-initiated events that disrupt specific subset of neurons (43). Whatever the basis of the learning defects, the foundation of BPA pathologies in adult offspring seems likely to be epigenetic in origin (21, 44–48).
On the other hand, even though the proximate mechanisms mediating BPA effects on spatial learning and exploratory behaviors in P. maniculatus remain to be clarified, the reproductive consequences are quite apparent. The disruption of male spatial cognition and the supporting brain systems would severely compromise the ability of the male deer mice to find mates in natural settings, and even if they did locate females, such animals would seem to be less likely to be chosen as mates than males that had not been exposed to BPA. Moreover, these abnormalities in traits associated with the likelihood of male reproductive success cannot be explained by altered adult testosterone or corticosterone concentrations of affected males.
Spatial abilities among female deer mice were not affected by developmental exposure to BPA, most probably because such traits have not been elaborated by sexual selection and were, therefore, less sensitive to disruption than the same traits in males. These traits are not sexually selected in females because home range expansion and increased exploration is unlikely to confer reproductive benefit to females and could be costly in terms of predation risk. In other words, the relative insensitivity of some traits to BPA exposure, as demonstrated here for female spatial abilities in P. maniculatus, can be readily understood within the purview of sexual selection. Sex differences in expression of such traits provide a theoretical context within which to understand EDC exposure for a broad range of species. Subset examples include the decrease in sexually dimorphic physical size of alligators born in contaminated streams of Lake Apopka (49), the reduced plumage coloration around the facial region of male American kestrels (Falco sparverius) exposed to polychlorinated biphenyls (8), and the loss of attractiveness to females of male rats whose great grandmothers had been treated with the fungicide vinclozolin (9). The latter study also provides an example of the transgenerational effects of vinclozolin exposure on male quality and female choice.
In contrast to the effects of BPA, developmental exposure of female deer mice to the synthetic estrogen EE, itself a potent environmental contaminant with known adverse effects on a variety of species (39, 50–52), masculinized their spatial abilities and exploratory behaviors and led to a phenotype more similar to that of control males than control females. Laboratory mice (Mus musculus domesticus) and rats (Rattus norvegicus) exposed during prenatal through postnatal development to EE similarly demonstrate increased male-like patterns of behavior, and as in deer mice, a similar regimen of BPA exposure did not induce comparable responses (53, 54). These contrasting data obtained after EE and BPA exposure suggest that the endocrine-disrupting effects of the latter cannot be entirely attributed to its estrogenic actions.
In conclusion, we have shown that in a polygynous deer mouse species, sexually selected traits that drive intrasexual competition for mates and influence intersexual choice of mating partner are particularly sensitive to BPA. Such a paradigm might be extended to other species and provide a roadmap as to what sex-specific traits might be most vulnerable to such chemicals. In the case of P. maniculatus, male spatial navigational ability is disrupted by early BPA exposure, and the mate choice experiment revealed that females are sensitive to the compromised condition of such males and prefer males that were not exposed to BPA. In the wild, BPA exposure might thus reduce the chances of these males to reproduce successfully. To the extent that sexually selected traits are particularly vulnerable to disruption by BPA and other EDCs, they might constitute particularly useful biomarkers to assess for environmental contamination (13, 55), provide a means to resolve the accumulating, apparently contradictory accounts of BPA effects appearing in the EDC literature, and allow future studies of human risk assessments to be targeted in ways that are not currently considered.
Materials and Methods
Animals.
Thirty outbred female and 30 male deer mice (Peromyscus maniculatus bairdii) were purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC). All experiments were approved by University of Missouri Animal Care and Use Committee and performed in accordance with National Institutes of Health Animal Care and Use Guidelines. Virgin females, 8–12 wk of age, were randomly assigned to receive one of three diets: (i) a low phytoestrogen AIN 93G diet supplemented with 7% corn oil (control); (ii) AIN93G supplemented with 50 mg of BPA/kg feed weight (BPA); or (iii) AIN93G diet supplemented with 0.1 parts per billion feed weight of EE, as a positive control (56). Diets were administered 2 wk before mating, and dams remained on the diet through pregnancy and lactation, because early brain development extends into the postnatal period (57). The total number of F1 offspring analyzed was 57 males (n = 20, 18, 19, for control, EE, and BPA diets, respectively) to capture male variability and 32 females (n = 13, 9, 10, for control, EE, and BPA diets, respectively) to determine whether sexually selected traits demonstrate differential susceptibility to EDCs.
After weaning, all offspring were placed on control diet and housed with same-sex siblings until sexual maturity (age ≈60 d). Mice remained singly housed throughout behavioral testing and were culled at ≈90 d of age. In contrast to common laboratory rodent species, P. maniculatus does not respond well to repeated handling (58), and thus animals were only handled during weekly cage changes and behavioral testing. To minimize background exposure to BPA beyond treatment regimen (59), deer mice were housed in white polypropylene cages (32 × 18 × 24 cm), maintained at standardized environmental conditions, at 25 ± 2 °C and 50% ± 10% humidity, with ad libitum access to water from glass bottles and food specific to each treatment group. All animals were maintained on a long daylight cycle (16 h light/8 h dark) to induce sexual maturity in males and females.
Assessments of Sensory and Neuromuscular Function.
On postnatal day 25, offspring were assessed to ensure that neuromuscular, sensory, and other functional systems were intact, as described previously (60, 61). Olfaction was tested by placing a small piece of food (cookie crumbs, 20 mg) under the bedding in a clean mouse cage that was visually, but not physically, divided into nine quadrants. The time required to find the food (latency) was recorded (maximum of 10 min). Neuromuscular strength was tested by placing mice individually on a wire lid 15 cm above their home cage. The lid was gently turned upside down, and the latency to falling into their cage was recorded (maximum of 60 s). To test acoustic startle, the experimenter clapped his or her hands ≈0.65 m from the individually caged mouse and recorded whether the animal became startled by the noise. Vision was tested by holding the deer mouse ≈20 cm above a wire cage and slowly lowering it. Normal vision was indicated by an arched back posture and reaching for the lid.
Spatial Learning and Memory.
At 60 d of age, spatial navigational ability of F1 male and female deer mice was assessed with a modified black polypropylene Barnes maze for use with Peromyscus (62–64) (Fig. S1). Offspring were randomly assigned an escape hole number. Exit holes were alternated 90° to eliminate odor cues for consecutively tested mice, whereas the escape hole location and visual cues remained constant for any individual deer mouse across all test trials. All testing occurred late in the light phase, and animals were returned to the vivarium immediately after testing. Two days before testing, mice were provided two habituation trials to acclimate the mice to the maze design, followed by 7 d of two-trial evaluations per day for 300 s each, with a 30-min intertrial interval. At the beginning of each testing day, animals were transferred from the vivarium to testing room 30 min before behavioral assessments to reduce any confounding stress. A trial consisted of carefully placing the mouse in the center of the maze in an opaque starting box to allow the tracking software to detect the center body-point of the mouse. The starting box was lifted and a trial initiated once the mouse began to move in the maze. If the mouse failed to enter the escape box within 300 s, the observer gently guided the animal to the escape hole. Stimulatory light measured ≈1,200 lx (vivarium room lighting measured 420 lx). If a deer mouse did not enter the exit hole within 30 s, a recording of a barn owl (Tyto alba) screech was played to motivate predator avoidance (65). The maze platform was cleaned after every trial with 70% ethanol solution. Additional information is provided in SI Materials and Methods.
Exploratory and Anxiety-Like Behavior.
Two weeks after the Barnes Maze testing, exploratory and anxiety-related behaviors were assessed by using the EPM (66). Additional information is provided in SI Materials and Methods.
Mate Choice Experiment.
Control and BPA-exposed females were tested for their preference of control vs. BPA-exposed males, similar to a previous study (Fig. S2) (9). Additional information is provided in SI Materials and Methods.
Adult Male Serum Testosterone and Corticosterone Concentrations.
One week after completing mate-choice tests, males were killed, and cardiac serum from 12 males per group was used to quantify circulating testosterone and corticosterone concentrations by RIA (67). Males were 90 ± 7 d of age. Blood was always collected between 700 and 900 hours, Central Standard Time. Additional information is provided in SI Materials and Methods.
Statistical Analyses.
Sensory and EPM scores were submitted to a 2 (sex) × 3 (diet) ANOVA or analysis of covariance (ANCOVA); Barnes maze latencies were analyzed with a 2 (sex) × 3 (diet) × 7 (day) repeated-measures ANCOVA, with trial (nested within day) as covariate (see SI Materials and Methods for additional analyses). Hierarchical linear models (PROC MIXED, SAS, 2004), whereby males and females were treated as separate random variables, were used to assess female preference for control or BPA-exposed males. Additional information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Dennis Miller for his advice on the sensory battery tests; Dr. Rex Hess for his suggestions on the manuscript; Mr. Wayne Shoemaker, Bond Life Sciences Center, for constructing the behavior testing apparatuses; Lisa Caldwell and Denise Warzak for technical assistance; and Joseph Reddy and Scott Williams for their assistance with husbandry and care of the deer mice. This work was supported by a Mizzou Advantage grant (to D.C.G. and C.S.R.) and National Institutes of Health Challenge Grant RC1 ES018195 (to C.S.R.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 11305.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107958108/-/DCSupplemental.
References
- 1.U.S. Geological Survey Biology - contaminant biology program: Toxicology. 2011. Available at: http://biology.usgs.gov/contaminant/toxicology.html. Accessed June 8, 2011.
- 2.Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–316. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Palanza P, Morellini F, Parmigiani S, vom Saal FS. Prenatal exposure to endocrine disrupting chemicals: Effects on behavioral development. Neurosci Biobehav Rev. 1999;23:1011–1027. doi: 10.1016/s0149-7634(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 4.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res Brain Res Rev. 2008;57:352–362. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Schantz SL, Widholm JJ. Cognitive effects of endocrine-disrupting chemicals in animals. Environ Health Perspect. 2001;109:1197–1206. doi: 10.1289/ehp.011091197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–885. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortolotti G, Fernie K, Smits J. Carotenoid concentration and coloration of American kestrels (Falco sparverius) disrupted by experimental exposure to PCBs. Funct Ecol. 2003;17:651–657. [Google Scholar]
- 9.Crews D, et al. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson M. Sexual Selection. Princeton: Princenton Univ Press; 1994. [Google Scholar]
- 11.Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc Natl Acad Sci USA. 1996;263:1415–1421. [Google Scholar]
- 12.Crews D, Willingham E, Skipper JK. Endocrine disruptors: Present issues, future directions. Q Rev Biol. 2000;75:243–260. doi: 10.1086/393498. [DOI] [PubMed] [Google Scholar]
- 13.Hill G. Condition-dependent display traits hold promise as potent biomonitors: Condition-dependent display traits hold promise as potent biomonitors. Bioscience. 1995;45:25–31. [Google Scholar]
- 14.Galea LA, Kavaliers M, Ossenkopp KP. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exp Biol. 1996;199:195–200. doi: 10.1242/jeb.199.1.195. [DOI] [PubMed] [Google Scholar]
- 15.Doty RL. Odor preferences of female Peromyscus maniculatus bairdi for male mouse odors of P. m. bairdi and P. leucopus noveboracensis as a function of estrous state. J Comp Physiol Psychol. 1972;81:191–197. doi: 10.1037/h0033515. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg J. Studies on the behavior of Peromyscus maniculatus gambelii and Peromyscus californicus parasiticus. Behaviour. 1962;19:177–207. [Google Scholar]
- 17.Penn DJ. The scent of genetic compatibility: Sexual selection and the major histocompatibility complex. Ethology. 2002;108:1–21. [Google Scholar]
- 18.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- 20.Halldin K, Berg C, Brandt I, Brunström B. Sexual behavior in Japanese quail as a test end point for endocrine disruption: Effects of in ovo exposure to ethinylestradiol and diethylstilbestrol. Environ Health Perspect. 1999;107:861–866. doi: 10.1289/ehp.99107861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm Behav. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi O, Oishi S. Testicular toxicity of dietarily or parenterally administered bisphenol A in rats and mice. Food Chem Toxicol. 2003;41:1035–1044. doi: 10.1016/s0278-6915(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 24.Sieli PT, Jašarević E, Warzak DA, Mao J, Ellersieck MR, et al. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003385. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, et al. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: Relevance for human exposure. Environ Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teeguarden J, Gunawan R, Calafat A. Proceedings of the Fiftieth Society for Toxicology. Washington, DC: 2011. Blood and urinary elimination kinetics of bisphenol A in humans following dietary exposure. Abstract 1943. [Google Scholar]
- 28.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Vandenberg LN, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect. 2002;110(Suppl 3):415–422. doi: 10.1289/ehp.02110s3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Della Seta D, Minder I, Dessì-Fulgheri F, Farabollini F. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull. 2005;65:255–260. doi: 10.1016/j.brainresbull.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 33.Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci USA. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol. 2010;31:519–530. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav. 2010;58:326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 38.MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. doi: 10.1289/ehp.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceccarelli I, Della Seta D, Fiorenzani P, Farabollini F, Aloisi AM. Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol Teratol. 2007;29:108–115. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: Delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 41.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura D, et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett. 2010;194:16–25. doi: 10.1016/j.toxlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Tanabe N, Kimoto T, Kawato S. Rapid Ca(2+) signaling induced by Bisphenol A in cultured rat hippocampal neurons. Neuroendocrinol Lett. 2006;27:97–104. [PubMed] [Google Scholar]
- 44.Avissar-Whiting M, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaoi T, et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Commun. 2008;376:563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 48.Ho SM, Tang WY, Belmonte de Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66:5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillette LJ, Jr, Crain DA, Rooney AA, Pickford DB. Organization versus activation: The role of endocrine-disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995;103(Suppl 7):157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delclos KB, et al. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–132. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muller M, et al. Chemical and biological analysis of endocrine-disrupting hormones and estrogenic activity in an advanced sewage treatment plant. Environ Toxicol Chem. 2008;27:1649–1658. doi: 10.1897/07-519. [DOI] [PubMed] [Google Scholar]
- 52.Partridge C, Boettcher A, Jones AG. Short-term exposure to a synthetic estrogen disrupts mating dynamics in a pipefish. Horm Behav. 2010;58:800–807. doi: 10.1016/j.yhbeh.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol Sci. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- 54.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Carere C, Costantini D, Sorace A, Santucci D, Alleva E. Bird populations as sentinels of endocrine disrupting chemicals. Ann Ist Super Sanita. 2010;46:81–88. doi: 10.4415/ANN_10_01_10. [DOI] [PubMed] [Google Scholar]
- 56.Vom Saal FS, et al. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res A Clin Mol Teratol. 2005;73:140–145. doi: 10.1002/bdra.20120. [DOI] [PubMed] [Google Scholar]
- 57.Layne J. Ontogeny. In: King J, editor. Biology of Peromyscus (Rodentia) Stillwater, OK: American Society for Mammologist; 1968. pp. 148–253. [Google Scholar]
- 58.Martin LB, Trainor BC, Finy MS, Nelson RJ. HPA activity and neotic and anxiety-like behavior vary among Peromyscus species. Gen Comp Endocrinol. 2007;151:342–350. doi: 10.1016/j.ygcen.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howdeshell KL, et al. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 61.Jones KL, et al. Combined effect of maternal serotonin transporter genotype and prenatal stress in modulating offspring social interaction in mice. Int J Dev Neurosci. 2010;28:529–536. doi: 10.1016/j.ijdevneu.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 63.Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003;79:683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 64.Walker JM, et al. Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer's disease. Behav Brain Res. 2011;222:169–175. doi: 10.1016/j.bbr.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 65.Clarke J. Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus) Behav Ecol Sociobiol. 1983;13:205–209. [Google Scholar]
- 66.Fountain ED, et al. Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biol Reprod. 2008;78:211–217. doi: 10.1095/biolreprod.107.065003. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeld CS, et al. The differential fate of mesonephric tubular-derived efferent ductules in estrogen receptor-α knockout versus wild-type female mice. Endocrinology. 2000;141:3792–3798. doi: 10.1210/endo.141.10.7694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.