Abstract
Previous studies suggest that exogenous factors crucial for spermatogonial stem cell (SSC) self-renewal are conserved among several mammalian species. Since glial cell line-derived neurotrophic factor (GDNF) and fibroblast growth factor 2 (FGF2) are critical for rodent SSC self-renewal, we hypothesized that they might promote self-renewal of nonrodent SSCs. Therefore, we cultured testicular germ cells from prepubertal rabbits in the presence of GDNF and FGF2 and found they proliferated indefinitely as cellular clumps that displayed characteristics previously identified for rodent SSCs. The rabbit germ cells could not be maintained on mouse embryonic fibroblast (STO) feeders that support rodent SSC self-renewal in vitro but were rather supported on mouse yolk sac-derived endothelial cell (C166) feeder layers. Proliferation of rabbit germ cells was dependent on GDNF. Of critical importance was that clump-forming rabbit germ cells colonized seminiferous tubules of immunodeficient mice, proliferated for at least 6 mo, while retaining an SSC phenotype in the testes of recipient mice, indicating that they were rabbit SSCs. This study demonstrates that GDNF is a mitogenic factor promoting self-renewal that is conserved between rodent and rabbit SSCs; with an evolutionary separation of ∼60 million years. These findings provide a foundation to study the mechanisms governing SSC self-renewal in nonrodent species.—Kubota, H., Wu, X., Goodyear, S. M., Avarbock, M. R., Brinster, R. L. Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties.
Keywords: spermatogenesis, testis, growth factors
Long-term self-renewal of spermatogonial stem cells (SSCs) ensures continuous and highly productive spermatogenesis, which is the process of male gametogenesis from spermatogonia to mature spermatozoa. In rodents, SSCs are a small fraction of the most immature type A spermatogonia that consist of Asingle, Apaired, and Aaligned spermatogonia, which commonly are called undifferentiated spermatogonia (1, 2). Apaired and Aaligned spermatogonia are generated by incomplete cytokinesis from Asingle spermatogonia and are thought to be more differentiated than Asingle spermatogonia. However, recent studies demonstrated that Asingle spermatogonia could be produced by breaking up Aaligned spermatogonia, suggesting that commitment of differentiation in Aaligned spermatogonia may not be completely determined (3). Classically, Asingle spermatogonia are considered SSCs; however, stem cells can only be defined by their biological ability to both self-renew and differentiate into fully functional mature cells. Therefore, identification of SSCs requires proof of biological function. At present, unequivocal identification of SSCs can only be achieved by spermatogonial transplantation, which was first developed using mice (4, 5). When testicular germ cells are transplanted into seminiferous tubules of infertile male mice, only SSCs colonize the basement membrane and generated spermatogenesis for the remaining life of the recipient animal. Long-term spermatogenesis in the recipient is dependent on self-renewal of SSCs. Although the complete molecular mechanisms controlling self-renewal of SSCs is still largely unknown, in vivo and in vitro studies have clearly demonstrated that a critical extrinsic mitogenic factor for SSC self-renewal in mice is glial cell line-derived neurotrophic factor (GDNF) (6, 7). In the testes of GDNF-overexpressing transgenic mice, undifferentiated spermatogonia proliferated abnormally, whereas in the testes of GDNF-knockout mice, spermatogenesis is impaired (6, 8). In the testis, GDNF is produced by Sertoli cells and acts on undifferentiated spermatogonia, including SSCs, which express the receptor complex consisting of GDNF family receptor α-1 (GFRA1) and rearranged during transfection (RET) protooncogene (6, 9). GFRA1 is a glycosylphosphatidylinisotol (GPI)-anchored cell-surface molecule with GDNF binding ability, and RET is a transmembrane tyrosine kinase that transduces stimulatory signals into RET-expressing cells following binding of the GDNF-GFRA1 complex (10). In vitro studies using a serum-free culture medium have shown that supplementation of GDNF or GDNF plus soluble GFRA1 can support the unlimited proliferation of mouse SSCs that maintain their ability to differentiate, indicating that the GDNF/GFRA1/RET system promotes mouse SSC self-renewal (7). In addition to the critical role of GDNF, the serum-free culture system also demonstrated that fibroblast growth factor 2 (FGF2) enhanced proliferation of mouse SSCs in vitro (7). FGF2 appears to play a supportive role in self-renewal, because FGF2 alone cannot support proliferation and self-renewal of murine SSCs (11). Further investigation has revealed that GDNF and FGF2 are also the primary extrinsic factors for self-renewal of rat and hamster SSCs (12–14). Taken together, these studies establish that GDNF and FGF2 promote self-renewal and are evolutionally conserved in rodent SSCs.
Although rodent SSCs have been investigated intensively using the functional transplantation assay and in vitro culture techniques (15, 16), our knowledge about nonrodent SSCs is limited. Progress in the field has been hampered by a lack of long-term nonrodent culture systems, as well as an absence of a useful model animal to investigate SSC biology. Nevertheless, transplantation experiments of testis cells from various mammalian species have been informative regarding the biology of nonrodent SSCs. When testis cells from nonrodent mammals are transplanted into seminiferous tubules of infertile immunodeficient mice, a small portion of germ cells are shown to colonize the basement membrane and proliferate for several months to 1 yr, depending on the species (17–20). The source of the colonizing cells is considered to be SSCs of donor animals, because they reside on the basement membrane of the seminiferous tubule, likely in the SSC niche, and replicate for months. Testis germ cells from all species examined, including rabbit, dog, pig, bovine, baboon, rhesus monkey, and human, colonized the mouse testes, suggesting that mitogenic factors for SSCs or spermatogonial progenitors are conserved among many mammalian species (15, 16, 21). Although transplantation of nonrodent testis cells into infertile immunodeficient mouse recipients is commonly used to evaluate SSC activity, there have been no studies to investigate the nature of colonized donor germ cells in the mouse testes. If mitogenic factors for SSCs are conserved among mammalian species, colonized nonrodent germ cells will retain SSC characteristics for long periods in mouse seminiferous tubules.
The development of human SSC cultures remains challenging, undermining its importance in therapeutic applications in reproductive medicine. Recent studies reported that putative human SSCs could be cultured using conditions similar to those developed for rodent SSCs (22, 23). However, it is not clear whether the cultured cells were actually SSCs, because only partial characterization of the colonizing cells is possible in the absence of a functional transplantation assay, resulting in spermatogenesis. Furthermore, the culture medium used contained GDNF and FGF2, but it was not determined whether these factors were indispensable for the putative SSCs. In addition, the culture media in these studies were supplemented with a growth factor cocktail, including GDNF, FGF2, epidermal growth factor (EGF), and leukemia inhibitory factor (LIF), in addition to FBS and proprietary compounds (22, 23). Although there have been several reports demonstrating that nonrodent spermatogonia, including human, express GFRA1, it has not been shown whether GDNF is required for self-renewal (23, 24). At present, growth factor requirements of nonrodent SSCs, including human, remains unknown.
In this study, we focused on rabbit SSCs, because this species diverged phylogenetically from rodents ∼6 × 107 yr ago (25) and may, therefore, more closely resemble larger species, including humans. In addition, the rabbit has been used as an important animal model for basic and medical research, representing a valuable species to investigate the biology of nonrodent SSCs. The in vitro self-renewal of SSCs represents an important resource to examine the biological characteristics of SSCs. Therefore, we first established a testis-derived germ cell culture, enriched for putative rabbit SSCs, that was capable of continuous proliferation in a serum-free medium based on information from rodent SSCs culture (11, 12, 26). Subsequently, we investigated the characteristic phenotype of the putative SSCs and determined their growth factor requirements for self-renewal and proliferation in vitro. Finally, after introducing reporter genes, the putative rabbit SSCs were transplanted into the testes of immunodeficient recipient mice to investigate their colonization capability and their fate in the xenogeneic environment, including the retention of an antigenic profile characteristic of rodent SSCs. This study provides a foundation to develop similar strategies to investigate human SSCs, as well as various mammalian SSCs.
MATERIALS AND METHODS
Rabbit testicular germ cell culture
New Zealand White rabbits (3 to 3.5 mo old) were purchased from Covance (Princeton, NJ, USA). Rabbit testis cell suspensions were prepared by enzymatic digestion using collagenase and trypsin. Prior to digestion, the tunica albuginea was removed, and the testes were minced with scissors and then incubated with 1 mg/ml collagenase (Sigma-Aldrich, St. Louis, MO, USA) at 36°C. After 15 min of incubation, the tissue was rinsed with Hank's balanced salt solution (HBSS). Subsequently, the collagenase-treated pieces of tissue were incubated with 0.25% trypsin-EDTA (Invitrogen, Carlsbad, CA) and 7 mg/ml DNase (Sigma-Aldrich) solution at 36°C for 15 min. Digestion was stopped by adding 0.1 vol of FBS, and the testis cell suspension was filtered through a 40-μm nylon mesh (BD Biosciences, San Jose, CA, USA). The cell suspension was then incubated with red blood cell lysis buffer (0.15 M NH4Cl and 0.01 M KHCO3). To eliminate debris from lysed red blood cells and dead testis cells, the cell suspension was overlaid on 30% Percoll solution (Sigma-Aldrich) and centrifuged at 600 g for 8 min at 4°C (26). The pellet was resuspended in rat serum-free medium (RSFM) for SSCs (12) and placed on feeder cells at a concentration of 1.3 × 106 cells/well of a 12-well plate with 40 ng/ml human GDNF (R&D Systems, Minneapolis, MN), 300 ng/ml rat GFRA1 (GFRA1-Fc chimera; R&D Systems), and 1 ng/ml human FGF2 (BD Biosciences). Two types of feeder cells, STO (SIM mouse embryo-derived thioguanine and ouabain resistant, SNL76/7; a gift from Dr. Allan Bradley, Wellcome Trust Sanger Institute, Cambridge, UK) and C166 (CRL-2581; American Type Culture Collection, Manassas, VA, USA), were prepared with mitomycin C (Sigma-Aldrich) treatment to block cell division (26). For feeder preparation, STO and C166 cells were cultured in DMEM containing 10% FBS, and mitomycin C-treated SNL76/7 or C166 cells were seeded in gelatin-coated 12-well plates at a concentration of 2 × 105 or 1 × 105 cells/well, respectively. Although rabbit germ cells were initially cultured with 40 ng/ml GDNF, 300 ng/ml GFRA1, and 1 ng/ml FGF2, the concentrations of GDNF and GFRA1 were reduced to 20 and 150 ng/ml, respectively, following 7 mo in culture, when constant proliferation of rabbit germ cells was confirmed.
To examine the effect of GDNF, GFRA1, and FGF2 on proliferation of clump-forming rabbit germ cells, 2 × 105 cells that had been cultured on C166 feeders with the 3 factors for 8 to 10 mo were placed into wells of a 12-well plate seeded with C166 feeders and RSFM containing 4 different combinations of factors: GDNF/GFRA1/FGF2, GDNF/GFRA1, FGF2, or no factors. After 10 d, the cultured germ cells were counted and subcultured in the same media. Cell number was counted again 5 d after subculture.
All germ cell cultures were maintained in 10% O2/5% CO2/85% N2, because the 10% O2 condition was superior to the 21% O2 used for mouse SSC self-renewal (27). SNL76/7 and C166 cells were routinely maintained in 5% CO2 in air.
Lentivirus transduction
HIV1-based lentiviral vectors pseudotyped with the vesicular stomatitis virus G glycoprotein (VSV-G) were used for expression of a Escherichia coli lacZ (LacZ) or green fluorescent protein (GFP) reporter gene, which consisted of β-galactosidase-neomycin (βgeo) gene or GFP-neomycin (GFP-neo) fusion gene (Qbiogene, Montreal, QC, Canada) driven by the phosphoglycerate kinase (PGK) promoter (28). The lentiviral vectors were produced by triple transfection of the HIV helper packaging plasmid pCMVR8.2, encoding HIV helper functions (29), the transfer constructs pHIV-CPPT-PGK-GFP-neo-WP or pHIV-CPPT-PGK-βgeo-WP, and the VSV-G expression plasmid into HEK293T cells using the calcium phosphate precipitation method (Clontech, Mountain View, CA, USA). The detailed procedure was described previously (30). The lentiviral vectors were produced by the Penn Vector Core in the School of Medicine at the University of Pennsylvania. Germ cells continuously proliferating on C166 feeder cells were exposed to the lentiviruses for 18–22 h at a multiplicity of infection (MOI) of 400. Germ cells transduced with the lentivirus were selected with G418 (Invitrogen) at a concentration of 20 μg/ml for 40–42 d. C166-neoresistant cells were generated by introducing pMC1neo poly(A) vector (Stratagene, La Jolla, CA, USA) into C166 cells, and these cells were used as feeder cells for G418 selection of germ cells.
Germ cell transplantation
Single-cell suspensions of lacZ or GFP-expressing cultured germ cells were prepared by trypsin-EDTA treatment, as described previously (26). Digested germ cells were suspended in RSFM and transplanted into recipient mouse testes via efferent duct injection. Recipient mice were NCr nude (nu/nu; Taconic Farms, Germantown, NY, USA) treated with 50 mg/kg busulfan at least 6 wk prior to transplantation (4, 31). Approximately 10 μl of 30–39 × 106 cells/ml was transplanted into each testis. The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all experimental procedures.
Flow cytometry
Cell staining for flow cytometry of freshly isolated testis cells or cultured cells was performed as described previously (32). Antibodies used for surface antigens were biotin-conjugated anti-human THY1 (clone 5E10; BD Biosciences), biotin-conjugated anti-human CD9 (clone MM2/57, Serotec, Oxford, UK), anti-rat GFRA1 (clone 81401.11; R&D Systems), anti-human integrin αv/integrin β3 (ITGAV/ITGB3, clone LM609; Chemicon, Temecula, CA), R-phycoerythrin (PE)-conjugated anti-integrin α6 (ITGA6, clone GoH3; BD Biosciences), and PE-conjugated anti-mouse ITGAV (clone H9.2B8; BD Biosciences) antibodies. Alexa Fluor 647-conjugated streptavidin, Alexa Fluor 647-conjugated goat anti-mouse IgG2b antibody, and Alexa Fluor 647-conjugated goat anti-mouse IgG1 antibody (all from Invitrogen) were used as secondary reagents. Stained cells were analyzed by FACS Calibur (BD Biosciences).
Immunocytochemistry
Cultured germ cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.1% Triton X-100 in Dulbecco's PBS for 60 min at room temperature. After incubating with 20% normal donkey or goat serum for 60 min to avoid nonspecific interaction with antibodies, the cells were stained with primary antibodies overnight at 4°C. Primary antibodies used were goat anti-human POU class 5 homeobox 1 (POU5F1, AF1759, 1:50; R&D Systems), mouse anti-human RET (MAB718, 1:100; R&D Systems), rabbit anti-human GFRA1 (sc-10716, 1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human zinc finger, and BTB domain-containing protein 16 (ZBTB16, sc-22839, 1:50; Santa Cruz Biotechnology), and rabbit anti-DEAD box polypeptide 4 (DDX4, 1:500; a gift from Dr. Toshiaki Noce, Mitsubishi-Kasei Institute of Life Science, Tokyo, Japan). The following day, the cells were stained with secondary antibodies for 5 h at 4°C. Alexa Fluor 488-conjugated donkey anti-goat IgG, goat anti-mouse IgG1, and donkey anti-rabbit IgG antibodies (all from Invitrogen; 1:500) were used as secondary antibodies. The stained cells were analyzed using a Leitz Dialux 20 microscope (Leica Microsystems, Buffalo Grove, IL, USA), and images were obtained with a Spot Insight 2MP Firewire Color Mosaic Digital Camera (Diagnostic Instruments, Sterling Heights, MI, USA) or a Leica TCSSP2 confocal microscope (Leica Microsystems).
Histological analysis
Rabbit testis used for cell preparation and transplanted mouse testes stained with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal) were evaluated histologically (27). Briefly, rabbit testes were fixed in Bouin's solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Transplanted mouse testes were fixed with 4% paraformaldehyde, stained with X-gal, sectioned, and counterstained with nuclear fast red.
Alkaline phosphatase (AP) activity staining
For detection of endogenous AP activity, cultures were fixed with 66% acetone and stained with naphthol AS-BI phosphate/fast red violet solution (Chemicon, Temecula, CA, USA; ref. 7).
Western blot
Rabbit and mouse germ cell cultures, along with respective C166 and STO feeders, were harvested in modified RIPA buffer (1% Triton X-100, 50 mM Tris–HCL, 135 mM NaCl, 0.1% sodium deoxycholate, 2 mM EDTA, 50 mM NaF, 2 mM sodium orthovanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin and 1 mM PMSF). Protein lysates were resolved by SDS-PAGE gels and transferred to nitrocellulose membranes. Blots were blocked in 5% nonfat dry milk in 1× PBST (1× PBS and 0.1%Tween-20) for 2 h and incubated overnight with rabbit anti-DDX4 antibody (sc67185, 1:200; Santa Cruz Biotechnology), or mouse anti-β-actin antibody (sc10731, 1:1000; Santa Cruz Biotechnology). Blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000; Thermo Fisher, Pittsburgh, PA, USA), and proteins were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
All data are presented as means ± se. Data were analyzed by 1-way ANOVA followed by Tukey-Kramer multiple-comparison test.
RESULTS
Long-term culture of rabbit clump-forming germ (RCFG) cells
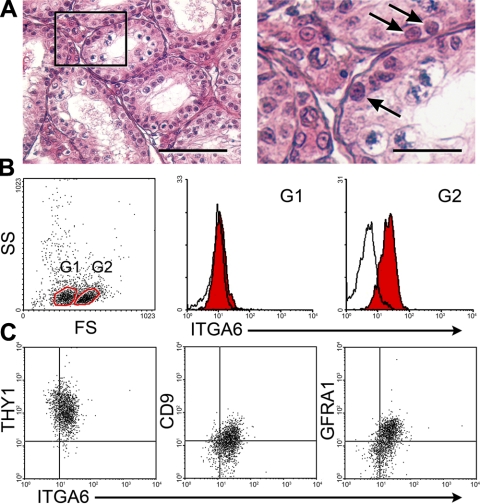
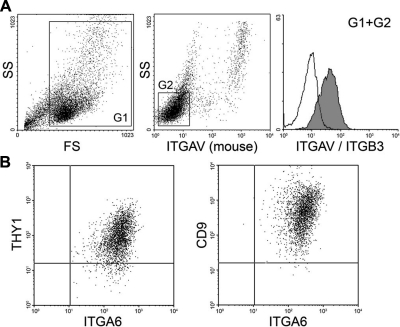
Testicular cells were isolated from prepubertal rabbit testes, in which spermatids had not developed (Fig. 1A). Flow cytometric analysis showed 2 subpopulations in the cell suspension based on forward scatter (FS), which represents cell size. The FShi cell population expressed ITGA6, a spermatogonial cell surface marker (32, 33), whereas the FSlo population was ITGA6− (Fig. 1B). The ITGA6+ cells expressed THY1 (Fig. 1C), a unique surface marker for the most primitive type A spermatogonia, including SSCs, in the mouse (11, 32). The ITGA6+ cells also expressed GFRA1 and CD9 (Fig. 1C), which is found on mouse and rat SSCs (7, 12, 34). These results indicate that prepubertal rabbit testes contain a subpopulation of cells, which has a similar antigenic profile to rodent SSCs.
Figure 1.
Analysis of rabbit spermatogonia. A) Histological examination of a 15-wk-old prepubertal rabbit testis. Left panel: no spermatids were found in the seminiferous tubules. Right panel: enlarged image of the boxed area in left panel. Arrows indicate spermatogonia. Scale bars = 50 μm (left panel); 15 μm (right panel). B) Flow cytometric analysis of testicular cells isolated from 15-wk-old rabbit testes. Gate 1 (G1) and gate 2 (G2) were created by analysis of forward scatter (FS) and side scatter (SS). Cells in G1 were FSlo ITGA6−; cells in G2 were FShi ITGA6+. C) Flow cytometric analysis of G2 cells of panel B. FShi ITGA6+ cells expressed THY1, CD9, and GFRA1. Proportions of ITGA6+ THY1+, ITGA6+ CD9+, and ITGA6+ GFRA1+ cell fractions in the total cells analyzed were 25, 11, and 17%, respectively. The x and y axes depict the relative fluorescence intensity of individual cells.
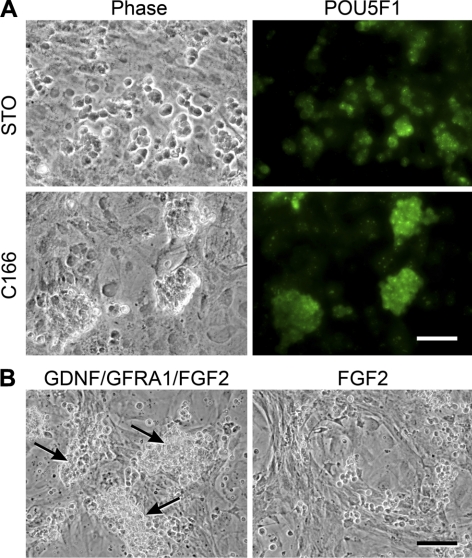
Subsequently, single-cell suspensions of rabbit testis were cultured in RSFM supplemented with GDNF, GFRA1, and FGF2 on mitotically inactivated feeders that were prepared from STO or C166 cells. STO cells are derived from mouse embryonic fibroblasts and are able to support replication of mouse and rat SSCs in vitro (7, 12, 26). C166 cells are mouse yolk sac-derived endothelial cells and have been used to maintain hematopoietic progenitors (35). At 4 d after the initiation of culture, testis cells formed clumps on C166, but not on STO feeder cells (Fig. 2A). Almost all rabbit cells within the clumps stained positive for POU5F1 (OCT3/4), strongly suggesting that the clumps consisted of germ cells (Fig. 2A). The germ cell clumps were successfully established on C166 feeders, and they could be maintained using GDNF/GFRA1, but FGF2 alone was not sufficient (Fig. 2B). The rabbit germ cells proliferated for ∼3 wk after initiation of culture at a slow speed in RSFM supplemented with GDNF/GFRA1/FGF2, making it challenging to readily scale up the culture. However, during 5 mo in culture, the RCFG cells gradually increased their replication rate and achieved an average doubling time of ∼11 d. They were then subcultured every 10 to 14 d at a ratio of 1:2. These long-term cultures were successfully established in 4 of 7 attempts. We hypothesized that the continuously proliferating clump-forming cells were rabbit germ cells, enriched for rabbit SSCs; therefore, cells were further characterized in detail.
Figure 2.
Culture of prepubertal rabbit testis cells. A) Testis cells were cultured on STO or C166 feeder cells in serum-free medium supplemented with GDNF, GFRA1, and FGF2. After 4 d in culture, cells were fixed and stained with an anti-POU5F1 antibody, followed by staining with an Alexa Fluor 488-conjugated secondary antibody. While POU5F1+ cells spread on STO feeders, POU5F1+ clumps formed on C166 feeders. B) Germ cell clumps were subcultured at d 11 after initiation of culture and placed on C166 feeder cells in the presence or absence of GDNF/GFRA1. At 6 d after subculture, germ cell clumps grew in the presence of GDNF and GFRA1 (arrows), but not in their absence. Scale bars = 50 μm (A); 100 μm (B).
Characterization of RCFG cells
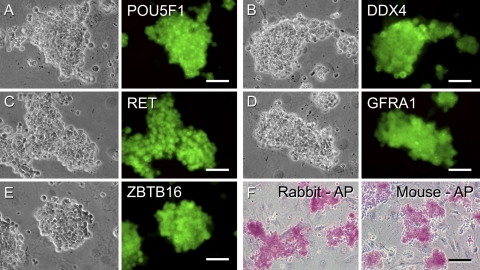
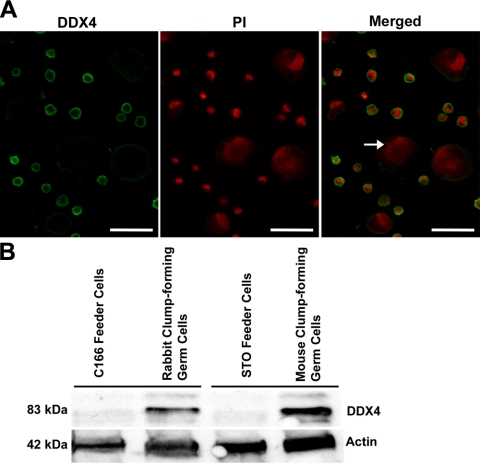
To confirm that the proliferating clump-forming cells were germ cells, immunocytochemistry was used to examine the expression of protein markers for both germ cells and SSCs. Immunostaining of the clumps (Fig. 3A–E) or individually dissociated RCFG cells (Supplemental Fig. S1) revealed that they were positive for the expression of POU5F1, DDX4, RET, GFRA1, and ZBTB16 (PLZF). In addition, the RCFG cells showed AP activity, which is expressed in undifferentiated ES cells and primordial germ cells, as well as in mouse and rat SSCs (Fig. 3F; refs. 7, 39). DDX4 is an evolutionally conserved germ cell-specific protein and is localized in the cytoplasm of spermatogenic cells, including spermatogonia (36). The dissociated RCFG cells, individually labeled, strongly expressed DDX4 in the cytoplasm (Fig. 4A), whereas no positive signal was detected in the C166 feeder cells (Fig. 4A, arrow). Moreover, Western blot analysis was carried out to further characterize its expression in RCFG cells, and a clear band of ∼83 kDa was observed in RCFG cells, which was the same molecular mass of mouse DDX4 in the SSC cultures (Fig. 4B). These results demonstrate that RCFG cells are indeed germ cells that developed from the primary testis cell-derived culture. Notably, there was expression of RET, GFRA1, and ZBTB16 in the rabbit clump-forming cells, which are SSC marker molecules and essential for their self-renewal in the mouse (6–8, 37, 38), suggesting that RCFG cells are enriched for SSCs.
Figure 3.
Characterization of rabbit clump-forming cells. Continuously proliferating cell clumps were cultured on C166 feeder cells in serum-free medium supplemented with GDNF, GFRA1, and FGF2. A–E) Phase contrast (left panels) and immunofluorescent images (right) of clump-forming cells stained with antibodies against POU5F1 (A), DDX4 (B), RET (C), GFRA1 (D), and ZBTB16 (E), which are expressed in mouse SSCs, followed by staining with Alexa Fluor 488-conjugated appropriate secondary antibodies. Rabbit clump-forming cells expressed the SSC markers. F) AP activity of rabbit clump-forming cells and cultured mouse SSCs. Scale bars = 50 μm (A–E); 100 μm (F).
Figure 4.
Examination of the expression of DDX4 protein in RCFG cells. A) Proliferating RCFG cells were dissociated into single cells and immunolabeled with antibodies against DDX4. Appropriate Alexa Fluor 488-conjugated secondary antibodies were used for visualization following primary antibody labeling; cells were counterstained with propidium iodide (PI) and visualized using confocal microscopy. Arrow indicates negative staining of C166 feeder cells. Scale bars = 37.5 μm. B) Western blot analysis of rabbit and mouse clump-forming germ cells cultured on C166 and STO feeders, respectively, for DDX4 expression. Protein lysates of germ cells from each culture and their respective feeders (i.e., C166 or STO) were used to determine expression of DDX4 in clump cells. Distinct band of ∼83 kDa was identified for germ cell cultures, but not feeder cells. Relatively equal loading of proteins in each lane was verified by β-actin expression (∼42 kDa).
Mouse SSCs that are self-renewing in culture proliferate to form cell clumps that maintain the expression of surface markers characteristic of SSCs in the testis (7, 26). The antigenic profile of mouse SSCs in vitro and in vivo is THY1+ ITGA6+ ITGAVlo/− (7, 11, 32). Therefore, flow cytometry was used to investigate whether the continuously proliferating RCFG cells possess a similar surface phenotype. While C166 feeder cells were ITGAV+, RCFG cells showed no reaction with anti-mouse ITGAV antibody (Fig. 5A), allowing RCFG cells to be readily distinguished from C166 feeder cells. Although RCFG cells did not react with an anti-mouse ITGAV antibody, they were weakly stained by an anti-human ITGAV/ITGB3 antibody, suggesting they were ITGAVlo. Flow cytometric analysis of mouse ITGAV− cells in the culture showed that RCFG cells were THY1+ ITGA6+ CD9+ (Fig. 5B). These results indicated that RCFG cells and mouse SSCs share a similar antigenic profile, characterized as THY1+ ITGA6+ CD9+ ITGAVlo.
Figure 5.
Flow cytometric analysis of RCFG cells cultured on C166 feeder cells. A) RCFG cells were harvested and stained with anti-mouse ITGAV and anti-human ITGAV/ITGB3 antibodies. Anti-mouse ITGAV antibody is specific for the mouse antigen; anti-human ITGAV/ITGB3 antibody reacts with the rabbit antigen. Gate 1 (G1) was created by analysis of forward scatter (FS) and side scatter (SS) to discriminate culture debris (left panel). Cells in G1 were analyzed for mouse ITGAV expression and SS (middle panel). Two populations were identified based on mouse ITGAV expression and SS. C166 feeder cells, represented as the SShi minor population, were mouse ITGAV+, while RCFG cells were identified as mouse ITGAV− SSlo major population in the culture. Gate 2 (G2) was created for RCFG cells and analyzed for ITGAV/ITGB3 expression. RCFG cells expressed ITGAV/ITGB3 weakly (right panel). Open histogram indicates RCFG cells unstained with the ITGAV/ITGB3 antibody. Filled histogram represents RCFG cells stained with the antibody. B) RCFG cells were stained with anti-mouse ITGAV, anti-ITGA6, and anti-THY1 or anti-CD9 antibodies. G2 gate for RCFG cells was created as shown in panel A and analyzed for THY1, ITGA6, and CD9 expression. RCFG cells were THY1+ ITGA6+ CD9+. The x and y axes depict the relative fluorescence intensity of individual cells.
GDNF-dependent proliferation of RCFG cells
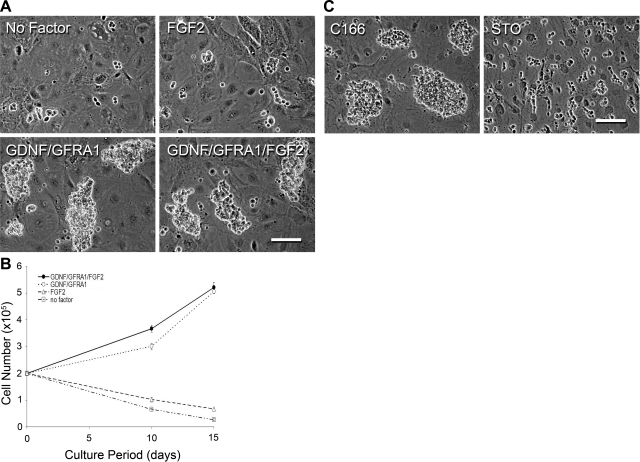
During the initial phase of rabbit testis cell culture, clump formation depended on GDNF/GFRA1 and C166 feeder cells (Fig. 2). Although GDNF, GFRA1, and FGF2 were added to the culture medium for long-term cultivation of RCFG cells that expressed GFRA1 and RET, the relative importance of GDNF and FGF2 was not clear. Therefore, RCFG cells that had been maintained for 10 mo in vitro were cultured without GDNF/GFRA1 or FGF2 for 15 d. After 15 d without GDNF/GFRA1, RCFG cells disappeared (Fig. 6A), whereas in the absence of only FGF2, RCFG cells continuously expanded (Fig. 6A). When 2 × 105 RCFG cells were seeded on C166 feeders, no significant difference (mean±se, n=3 cultures of 8, 8.5, and 10 mo of age) was observed in the number of RCFG cells cultured for 15 d in the presence of GDNF/GFRA1/FGF2 (5.21±0.21×105) or GDNF/GFRA1 (5.05±0.08×105) (Fig. 6B). In contrast, in the absence of GDNF/GFRA1, the number of RCFG cells supported only by FGF2 significantly decreased to 0.67 ± 0.04 × 105 cells. The complete lack of all 3 factors (GDNF/GFRA1/FGF2) further reduced the number of RCFG cells to 0.27 ± 0.05 × 105 cells. These results indicate that proliferation of RCFG cells largely depends on GDNF/GFRA1, as the doubling time for RCFG cells in GDNF/GFRA1/FGF2 or GDNF/GFRA1 culture conditions was 10.8 or 11.0 d, respectively. As was observed during initial development of the cultures (Fig. 2B), even after long-term culture, RCFG cell proliferation was dependent on C166 feeders, since germ cells could not form clumps or proliferate on STO feeders (Fig. 6C).
Figure 6.
Proliferation requirements of RCFG cells. A) Phase-culture images of RCFG cells cultured in the presence of GDNF, GFRA1, and FGF2 and then subcultured under the 4 culture conditions for 15 d: GDNF/GFRA1/FGF2, no FGF2 (GDNF/GFRA1), no GDNF/GFRA1 (FGF2), and no GDNF/GFRA1/FGF2 (no factor). RCFG cells proliferated in the absence of FGF2, while they did not proliferate in the absence of GDNF/GFRA1. B) Proliferation rate of RCFG cells was investigated. RCFG cells (2×105) were cultured for 15 d under the 4 conditions described in panel A. Cell numbers were counted at d 10 and 15. RCFG cells proliferated in the presence of GDNF/GFRA1 or GDNF/GFRA1/FGF2 at a similar rate. Solid circles, GDNF/GFRA1/FGF2; open circles, GDNF/GFRA1; triangles, FGF2; squares, no factor. Values are means ± se; n=3 separate cultures. C) Effect of feeder cells on RCFG cells. Phase contrast images at 7 d after subculture onto C166 or STO feeder cells. RCFG cells did not form clumps on STO feeders. Scale bars = 100 μm.
Transplantation of genetically modified RCFG cells
The in vitro characteristics of RCFG cells suggest they contain rabbit SSCs. To confirm the presence of SSCs in established cultures, RCFG cells were transplanted into immunodeficient mouse testes to investigate their fate in vivo. If RCFG cells possess SSC potential, they will colonize the seminiferous tubules of recipient mice and be maintained on the basement membrane for long periods. Before transplantation, the reporter gene for lacZ was introduced into the RCFG cells using a lentiviral vector to facilitate identification of donor RCFG cells in recipient mouse seminiferous tubules. Following transduction of the lentiviral vector and antibiotic selection, the RCFG cells were successfully labeled with the lacZ reporter gene (Fig. 7A). RCFG cells expressing lacZ were then transplanted into 16 testes of busulfan-treated nude mice (8 recipients). When one recipient was analyzed 7 wk after transplantation, a few lacZ+ cells were found to have colonized the seminiferous tubules of the recipient mouse (Fig. 7B, C). The remaining 14 testes were periodically analyzed up to 23 wk after transplantation. In all recipient testes, transplanted lacZ+ cells were identified (Fig. 7B–E). Occasionally, dividing lacZ+ cells were observed (Fig. 7C). These results indicate that transplanted RCFG cells proliferated in the mouse seminiferous tubules during the course of several months. Histological analysis indicated that lacZ+ cells were found distributed on the basement membrane of the seminiferous tubules in recipient testes (Fig. 7F). No teratomas were identified in any of the recipient testes.
Figure 7.
Transplantation of RCFG cells to immunodeficient recipients. A) A lacZ reporter gene construct was introduced into RCFG cells using a lentiviral vector. After antibiotic selection, lacZ expression in RCFG cells was confirmed with X-gal staining. B–E). Seminiferous tubules of recipient testes transplanted with the lacZ-expressing RCFG cells. Recipient testes were analyzed with X-gal staining at 7, 16, and 23 wk after transplantation. Donor RCFG cells were stained blue. B, C) At 7 wk after transplantation, blue cells were found, including doublets. D) At 16 wk after transplantation, small clusters of blue cells were found. E) At 23 wk after transplantation, blue cell clusters were spread. F) Histological examination of a recipient testis at 23 wk. Recipient testis was stained with X-gal, sectioned, and counterstained with nuclear fast red. Blue donor cells were found on the basement membrane (arrows). Scale bars = 50 μm (A); 100 μm (B); 25 μm (C–F).
Phenotypic characteristics of RCFG cells in vivo
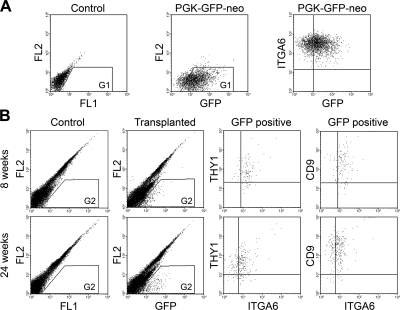
There have been no studies characterizing putative nonrodent SSCs colonizing the mouse testes. Therefore, we investigated the surface characteristics of the transplanted donor cells following colonization to provide additional evidence that they are similar to the transplanted cells and, therefore, likely SSCs or early spermatogonial stages in immunodeficient recipient mouse testes. To identify RCFG cells unequivocally in the recipient testes, a GFP reporter gene was introduced into RCFG cells by a lentiviral vector. The transduction efficiency of the lentivirus for GFP expression at a MOI of 400 was 19% by flow cytometric analysis. After antibiotic selection, GFP-expressing RCFG cells were established in vitro (Fig. 8A), and then transplanted into 6 testes of 3 recipient nude mice treated with busulfan. At 8 wk after transplantation, 1 recipient animal was sacrificed, and a cell suspension from the 2 testes was prepared for flow cytometric analysis. Compared with untransplanted busulfan testes as a control, GFP+ cells were clearly identified in the recipient testes, and their antigenic profile indicated that the GFP+ RCFG cells colonizing the mouse testis were THY1+ ITGA6+ CD9+ (Fig. 8B). To further investigate the surface phenotype of RCFG cells maintained in the xenogeneic environment for a longer period, testis cell suspensions were prepared from the remaining 2 recipient mice 24 wk after transplantation. In this experiment, the testis cell suspension was prepared from each testis (n=4), and analyzed for THY1, ITGA6, and CD9 expression by flow cytometry. Again, the surface phenotype of GFP+ cells was THY1+ ITGA6+ CD9+, which was identical to that from the 8-wk recipient (Fig. 8B). In each case, ITGA6 expression of the GFP+ cells in the recipient testes (Fig. 8B) appeared weaker than that of the GFP-expressing RCFG cells in culture before transplantation (Fig. 8A). The expression level of ITGA6 on RCFG cells was unchanged before or after lentiviral transduction (Figs. 5B and 8A), but was lower in vivo (Fig. 8B). In fact, the level of ITGA6 expression of THY1+ CD9+ cells in the donor rabbit testis (Fig. 1C) was similar to RCFG cells in the recipient mouse testis (Fig. 8B).
Figure 8.
Phenotypic characterization of RCFG cells transplanted into mouse testes. A) A GFP reporter gene expression construct was introduced into RCFG cells using a lentiviral vector. After antibiotic selection, GFP expression in RCFG cells was confirmed using flow cytometry. Left panel: data from nontransduced control cells. Middle panel: data from lentivirus-transduced RCFG cells (PGK-GFP-neo). GFP signal was detected by 2-dimensional dot-plot analyses using FL1 (x axis) and FL2 (y axis), which is representative of the 530/30 and 585/42 bandpass filters, respectively, on the fluorescence detector. Proportion of G1 cells is 83%. Right panel: ITGA6 expression pattern of GFP-expressing RCFG cells. Lentivirus-transduced RCFG cells were stained with a PE-conjugated anti-ITGA6 antibody. B) GFP-expressing RCFG cells were transplanted into busulfan-treated immunodeficient mice. A single-cell suspension was prepared from recipient testes at the indicated time points after transplantation and stained with anti-ITGA6 and anti-THY1 or anti-CD9 antibodies for flow cytometric analyses. GFP+ donor RCFG cells were identified in the recipient testicular cell suspension (transplanted, G2). GFP signal was detected as in A. Data from busulfan-treated nontransplanted testicular cells are shown (control, G2). Proportion of G2 in transplanted testes at 8 and 24 wk after transplantation was 1.0 and 1.1%, respectively. GFP+ donor RCFG cells identified in the recipient testes were THY1+ ITGA6+ CD9+ (GFP+). The x and y axes depict the relative fluorescence intensity of individual cells.
To investigate whether RCFG cells expanded in the recipients, the number of GFP+ cells in the testes was calculated. The percentage of GFP+ cells in total testis cells was 0.83 ± 0.15% (n=4), and the number of GFP+ cells per testis was 5.0 ± 1.4 × 104 (n=4), with each testis initially injected with 3 × 105 RCFG cells. Since the colonization efficiency of mouse SSCs in the mouse seminiferous tubules is ∼5% (40), and if the colonization efficiency of the RCFG cells in nude mouse testes is similar to that of transplanted mouse SSCs, then 1.5 × 104 RCFG cells are expected to have colonized following injection of 3 × 105 (5% of 3×105) RCFG cells. Although the colonization efficiency of a xenogeneic combination (rabbit to mouse) may be lower than that of a syngeneic transplantation (mouse to mouse), the assumption of a similar efficiency of colonization would suggest that RCFG cell number increased more than 3-fold (5.0×104/1.5×104) during 24 wk in the recipient mouse testes. Taken together, these results demonstrate that RCFG cells colonizing the seminiferous tubules of recipient mouse testes, increased in number, while still retaining their original marker antigens and maintaining stem cell characteristics.
DISCUSSION
Although our knowledge regarding mouse and rat SSCs has increased greatly since development of a functional transplantation assay and in vitro culture methods (4, 5, 7, 12, 13, 41, 42), little is known about SSCs of nonrodent mammals, including humans. In this study, we demonstrated, for the first time, that the GDNF/GFRA1/RET system plays a central role in promoting the self-renewal of nonrodent SSCs. Although the GDNF/GFRA1/RET system has been shown to be essential for mouse and rat self-renewal, it was not clear that the self-renewal of nonrodent SSCs also requires GDNF. Several reports suggest that GDNF might be a mitogenic factor for SSCs of nonrodent species, because immunohistochemisitry showed that spermatogonia, located on the basement membrane in the seminiferous tubules, express GFRA1 (23, 24, 43, 44). Furthermore, in vitro culture experiments of nonrodent spermatogonia have used medium containing GDNF, but other growth factors, such as FGF2, EGF, and serum were also included (22, 23, 45). Thus, there are no studies that demonstrate that GDNF itself promotes the self-renewal of those SSCs. In one study, human testicular cells were cultured with a growth factor cocktail containing GDNF and transplanted into immunodeficient mice (22). However, neither growth factor requirement in vitro nor characterization of the donor cells that colonized the recipients was performed. Therefore, it was not clear whether colonized cells were SSCs, differentiated germ cells, or testicular somatic cells. In the present study, we demonstrate that rabbit germ cells formed clumps and proliferated in the presence of GDNF/GFRA1; however, they did not replicate in the absence of GDNF/GFRA1. This result clearly demonstrates that the GDNF/GFRA1/RET system plays a central role for self-renewal replication of rabbit germ cell clumps, as it does for rodent SSCs. Although the doubling time of RCFG cells in vitro is longer than that of mouse SSCs in culture (11.0 vs. 5.6 d), which is similar to the ∼11.0 d observed in rat SSCs in culture (7, 12). The overall success of establishing RCFG cells in vitro (4 of 7 attempts) is comparable to that of generating SSC cultures from rats.
On the basis of the morphological appearance of rabbit germ cells in the culture system developed, we designated them RCFG cells, and they are likely enriched for rabbit SSCs for several reasons. First, the RCFG cells expressed SSC marker molecules, including THY1, GFRA1, RET, POU5F1, and ZBTB16, which have been shown to be characteristic of mouse and rat SSCs (7, 12, 26). These marker proteins have also been identified in putative SSC subpopulations of nonrodent mammals, including humans (23, 24, 43, 44, 46). Furthermore, RCFG cells are AP+, as has been shown in primitive germ cell populations, including SSCs (7, 12, 39). Second, as discussed above, the RCFG cells continuously proliferate in the presence of GDNF, retaining the characteristic phenotype of SSCs. Third, the morphological appearance of the RCFG cells was similar to that of rodent SSCs. Clump formation in culture is typical of proliferating mouse and rat SSCs (7, 12, 13, 42). Fourth, the RCFG cells were able to colonize mouse seminiferous tubules and proliferate for several months. Unequivocal identification of SSCs depends on the existence of a functional in vivo assay, but an assay to evaluate rabbit SSC activity that includes both self-renewal and differentiation to spermatozoa has not been established. Therefore, the ability of RCFG cells to form small colonies following transplantation into the seminiferous tubules of immunodeficient mouse testes currently represents the most reliable indication of SSC potential.
Putative SSCs of nonrodent mammalian species have been previously evaluated by the transplantation approach (17–20, 43–46). However, a definitive characterization of the antigenic profile of the colonizing cells has never been determined. In this study, the antigenic profile of the colonizing RCFG cells was clearly demonstrated to be the same as the transplanted RCFG cells in culture, as well as the original spermatogonial population isolated from the donor rabbit testes. The ability to continuously proliferate on the basement membrane in the recipient mouse seminiferous tubules, likely in the SSC niche, and still retain the antigenic profile of the RCFG cells in vivo is the strongest evidence provided that the RCFG cells contain SSCs, that they have indeed been cultured and expanded in vitro, and that they have a specific requirement for GDNF for self-renewal. However, to definitively prove that the RCFG cells contain SSCs, future studies will need to show that they can make spermatozoa with normal fertilizing ability.
Although several similarities exist between RCFG cells and rodent SSCs, such as GDNF-dependent self-renewal and in vitro phenotypic characteristics (e.g., clump formation), the present study identified an important difference between them. While rodent SSCs continuously replicated on STO feeder cells, a mouse embryonic fibroblast cell line, RCFG cells cannot be maintained on STO feeders. The rabbit cells require a yolk sac-derived endothelial cell line (C166) for their proliferation in culture. Although the exact reason C166 cells facilitated proliferation and clump formation of rabbit SSCs is not known, the cellular origin may be an important factor. Recently, a tentative location of the mouse SSC niche in the testis was identified and is associated with the vascular system in the testes (47). Thus, endothelial cells may secrete paracrine signals to support SSC self-renewal in the mouse testis, and C166 cells may produce similar factors to support clump formation of RCFG cells in culture. This finding may be a critical factor in developing long-term nonrodent SSC culture systems, including those for humans. Although the GDNF/GFRA1/RET system appears to be conserved as a positive regulator of SSC self-renewal for many mammalian species, reconstitution of microenvironments to support continuous self-renewal and proliferation of SSCs in culture will likely require specific types of feeder cells for different species. Thus, the culture conditions for RCFG cell cultures developed in this study may yet be improved, for example, by the use of other growth factors or different feeder cells.
Development of germline modification techniques in animals is important in agricultural and medical sciences. Generation of transgenic animals with targeted gene modifications using genetically modified embryonic stem cells has been extremely valuable in mice. Although similar techniques have not been possible in nonrodent experimental animals or primates, nuclear transfer or cloning technology with genetically modified somatic cells has resulted in a few animals with mutated genes. Because the efficiency of producing cloned large animals is still extremely low, development of alternative approaches is critical. Generation of transgenic animals with new genes or mutated genes using SSCs is an alternate approach, and its success has been reported using mouse and rat SSCs to produce both gain-of-function and loss-of-function transgenic animals (48–50). In the present study, RCFG cells were labeled with two reporter genes, lacZ and GFP, using lentiviral vectors. The lentiviral vector system is one of the most efficient methods for introduction of exogenous genes into SSCs, but establishing lentivirally labeled RCFG cells was more difficult than for mouse SSCs. Both labeling efficiency and reporter gene expression appeared to be lower in RCFG cells than in mouse SSCs (unpublished results). Nevertheless, genetically modified RCFG cells could be established, and they were able to colonize the SSC niche in mouse seminiferous tubules, demonstrating the potential to generate transgenic rabbits and likely other nonrodent animals using cultured SSCs.
To our knowledge, this is the first report demonstrating the growth requirements of nonrodent SSCs that resulted in establishment of a long-term culture system. An important aspect of the studies was the maintenance of well-established rodent SSC marker proteins in the RCFG cells in long-term in vitro culture and long-term in vivo proliferation following transplantation into recipient mice. While our study clearly suggests that there is significant similarity between rodent and rabbit SSCs, some differences, such as dependency on endothelial cell feeders, were identified. Subsequent elucidation of the cellular and molecular mechanisms supporting SSC self-renewal in various animal species, especially primates, will facilitate development of novel approaches to the treatment of male infertility and applications in regenerative medicine.
Supplementary Material
Acknowledgments
The authors thank Dr. Zhiyv Niu for helpful comments on the manuscript, Drs. L. Wang and J. Johnston for construction and production of lentivirus vectors at the Penn Vector Core, C. Freeman and R. Naroznowski for assistance with animal maintenance and experimentation, and J. Hayden for photography.
Financial support for the research was from the U.S. National Institute of Child Health and Human Development (HD 052728) and the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Russell L. D., Ettlin R. A., Sinha-Hikim Amiya P., Clegg E. D. (1990) Histological and Histopathological Evaluation of the Testis, Cache River Press, Clearwater, FL, USA [Google Scholar]
- 2. De Rooij D. G., Russell L. D. (2000) All you wanted to know about spermatogonia but were afraid to ask. J. Androl. 21, 776–798 [PubMed] [Google Scholar]
- 3. Nakagawa T., Sharma M., Nabeshima Y. I., Braun R. E., Yoshida S. (2010) Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinster R. L., Zimmermann J. W. (1994) Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. U. S. A. 91, 11298–11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinster R. L., Avarbock M. R. (1994) Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. U. S. A. 91, 11303–11307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng X., Lindahl M., Hyvonen M. E., Parvinen M., de Rooij D. G., Hess M. W., Raatikainen-Ahokas A., Sainio K., Rauvala H., Lakso M., Pichel J. G., Westphal H., Saarma M., Sariola H. (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287, 1489–1493 [DOI] [PubMed] [Google Scholar]
- 7. Kubota H., Avarbock M. R., Brinster R. L. (2004) Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. U. S. A. 101, 16489–16494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Naughton C. K., Jain S., Strickland A. M., Gupta A., Milbrandt J. (2006) Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol. Reprod. 74, 314–321 [DOI] [PubMed] [Google Scholar]
- 9. Viglietto G., Dolci S., Bruni P., Baldassarre G., Chiariotti L., Melillo R. M., Salvatore G., Chiappetta G., Sferratore F., Fusco A., Santoro M. (2000) Glial cell line-derived neutrotrophic factor and neurturin can act as paracrine growth factors stimulating DNA synthesis of Ret-expressing spermatogonia. Int. J. Oncol. 16, 689–694 [DOI] [PubMed] [Google Scholar]
- 10. Sariola H., Saarma M. (2003) Novel functions and signalling pathways for GDNF. J. Cell Sci. 116, 3855–3862 [DOI] [PubMed] [Google Scholar]
- 11. Kubota H., Avarbock M. R., Brinster R. L. (2004) Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol. Reprod. 71, 722–731 [DOI] [PubMed] [Google Scholar]
- 12. Ryu B. Y., Kubota H., Avarbock M. R., Brinster R. L. (2005) Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc. Natl. Acad. Sci. U. S. A. 102, 14302–14307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamra F. K., Chapman K. M., Nguyen D. M., Williams-Stephens A. A., Hammer R. E., Garbers D. L. (2005) Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc. Natl. Acad. Sci. U. S. A. 102, 17430–17435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanatsu-Shinohara M., Muneto T., Lee J., Takenaka M., Chuma S., Nakatsuji N., Horiuchi T., Shinohara T. (2008) Long-term culture of male germline stem cells from hamster testes. Biol. Reprod. 78, 611–617 [DOI] [PubMed] [Google Scholar]
- 15. Brinster R. L. (2002) Germline stem cell transplantation and transgenesis. Science 296, 2174–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brinster R. L. (2007) Male germline stem cells: from mice to men. Science 316, 404–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobrinski I., Avarbock M. R., Brinster R. L. (1999) Transplantation of germ cells from rabbits and dogs into mouse testes. Biol. Reprod. 61, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 18. Dobrinski I., Avarbock M. R., Brinster R. L. (2000) Germ cell transplantation from large domestic animals into mouse testes. Mol. Reprod. Dev. 57, 270–279 [DOI] [PubMed] [Google Scholar]
- 19. Nagano M., McCarrey J. R., Brinster R. L. (2001) Primate spermatogonial stem cells colonize mouse testes. Biol. Reprod. 64, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 20. Nagano M., Patrizio P., Brinster R. L. (2002) Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 78, 1225–1233 [DOI] [PubMed] [Google Scholar]
- 21. Kubota H., Brinster R. L. (2006) Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat. Clin. Pract. Endocrinol. Metab. 2, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadri-Ardekani H., Mizrak S. C., van Daalen S. K., Korver C. M., Roepers-Gajadien H. L., Koruji M., Hovingh S., de Reijke T. M., de la Rosette J. J., van der Veen F., de Rooij D. G., Repping S., van Pelt A. M. (2009) Propagation of human spermatogonial stem cells in vitro. JAMA 302, 2127–2134 [DOI] [PubMed] [Google Scholar]
- 23. He Z., Kokkinaki M., Jiang J., Dobrinski I., Dym M. (2010) Isolation, characterization, and culture of human spermatogonia. Biol. Reprod. 82, 363–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spinnler K., Kohn F. M., Schwarzer U., Mayerhofer A. (2010) Glial cell line-derived neurotrophic factor is constitutively produced by human testicular peritubular cells and may contribute to the spermatogonial stem cell niche in man. Hum. Reprod. 25, 2181–2187 [DOI] [PubMed] [Google Scholar]
- 25. McKenna M., Bell S. (1997) Classification of Mammals: Above the Species Level, Columbia University Press, New York [Google Scholar]
- 26. Kubota H., Brinster R. L. (2008) Culture of rodent spermatogonial stem cells, male germline stem cells of the postnatal animal. Methods Cell Biol. 86, 59–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kubota H., Avarbock M. R., Schmidt J. A., Brinster R. L. (2009) Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation. Biol. Reprod. 81, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Friedrich G., Soriano P. (1991) Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5, 1513–1523 [DOI] [PubMed] [Google Scholar]
- 29. Naldini L., Blomer U., Gage F. H., Trono D., Verma I. M. (1996) Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 93, 11382–11388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kobinger G. P., Weiner D. J., Yu Q. C., Wilson J. M. (2001) Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 19, 225–230 [DOI] [PubMed] [Google Scholar]
- 31. Ogawa T., Arechaga J. M., Avarbock M. R., Brinster R. L. (1997) Transplantation of testis germinal cells into mouse seminiferous tubules. Int. J. Dev. Biol. 41, 111–122 [PubMed] [Google Scholar]
- 32. Kubota H., Avarbock M. R., Brinster R. L. (2003) Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc. Natl. Acad. Sci. U. S. A. 100, 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shinohara T., Avarbock M. R., Brinster R. L. (1999) beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. U. S. A. 96, 5504–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kanatsu-Shinohara M., Toyokuni S., Shinohara T. (2004) CD9 is a surface marker on mouse and rat male germline stem cells. Biol. Reprod. 70, 70–75 [DOI] [PubMed] [Google Scholar]
- 35. Lu L. S., Wang S. J., Auerbach R. (1996) In vitro and in vivo differentiation into B cells, T cells, and myeloid cells of primitive yolk sac hematopoietic precursor cells expanded >100-fold by coculture with a clonal yolk sac endothelial cell line. Proc. Natl. Acad. Sci. U. S. A. 93, 14782–14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fujiwara Y., Komiya T., Kawabata H., Sato M., Fujimoto H., Furusawa M., Noce T. (1994) Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. U. S. A. 91, 12258–12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buaas F. W., Kirsh A. L., Sharma M., McLean D. J., Morris J. L., Griswold M. D., de Rooij D. G., Braun R. E. (2004) Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 36, 647–652 [DOI] [PubMed] [Google Scholar]
- 38. Costoya J. A., Hobbs R. M., Barna M., Cattoretti G., Manova K., Sukhwani M., Orwig K. E., Wolgemuth D. J., Pandolfi P. P. (2004) Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659 [DOI] [PubMed] [Google Scholar]
- 39. Matzuk M. M. (2004) Germ-line immortality. Proc. Natl. Acad. Sci. U. S. A. 101, 16395–16396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagano M. C. (2003) Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 69, 701–707 [DOI] [PubMed] [Google Scholar]
- 41. Nagano M., Avarbock M. R., Leonida E. B., Brinster C. J., Brinster R. L. (1998) Culture of mouse spermatogonial stem cells. Tissue Cell 30, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kanatsu-Shinohara M., Ogonuki N., Inoue K., Miki H., Ogura A., Toyokuni S., Shinohara T. (2003) Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 69, 612–616 [DOI] [PubMed] [Google Scholar]
- 43. Hermann B. P., Sukhwani M., Simorangkir D. R., Chu T., Plant T. M., Orwig K. E. (2009) Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Hum. Reprod. 24, 1704–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu X., Schmidt J. A., Avarbock M. R., Tobias J. W., Carlson C. A., Kolon T. F., Ginsberg J. P., Brinster R. L. (2009) Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. U. S. A. 106, 21672–21677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aponte P. M., Soda T., Teerds K. J., Mizrak S. C., van de Kant H. J., de Rooij D. G. (2008) Propagation of bovine spermatogonial stem cells in vitro. Reproduction 136, 543–557 [DOI] [PubMed] [Google Scholar]
- 46. Reding S., Stepnoski A., Cloninger E., Oatley J. (2010) THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction 139, 893–903 [DOI] [PubMed] [Google Scholar]
- 47. Yoshida S., Sukeno M., Nabeshima Y. (2007) A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317, 1722–1726 [DOI] [PubMed] [Google Scholar]
- 48. Nagano M., Brinster C. J., Orwig K. E., Ryu B. Y., Avarbock M. R., Brinster R. L. (2001) Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc. Natl. Acad. Sci. U. S. A. 98, 13090–13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamra F. K., Gatlin J., Chapman K. M., Grellhesl D. M., Garcia J. V., Hammer R. E., Garbers D. L. (2002) Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc. Natl. Acad. Sci. U. S. A. 99, 14931–14936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kanatsu-Shinohara M., Ikawa M., Takehashi M., Ogonuki N., Miki H., Inoue K., Kazuki Y., Lee J., Toyokuni S., Oshimura M., Ogura A., Shinohara T. (2006) Production of knockout mice by random or targeted mutagenesis in spermatogonial stem cells. Proc. Natl. Acad. Sci. U. S. A. 103, 8018–8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.