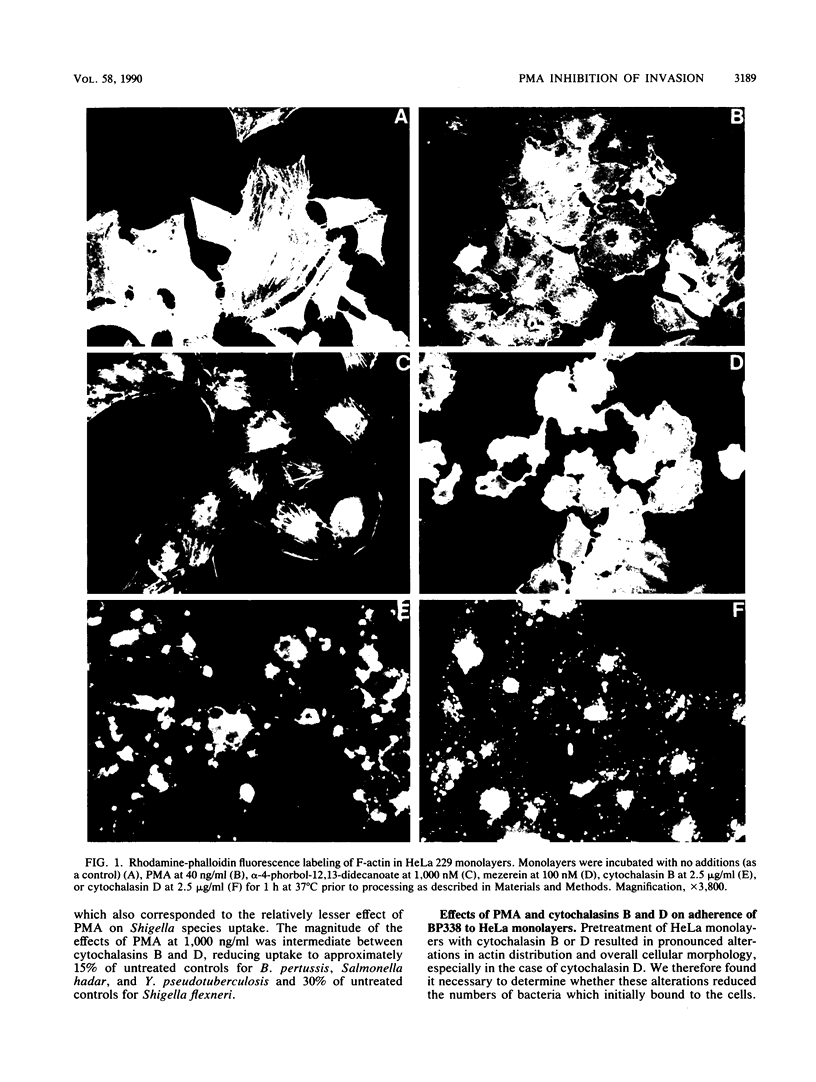
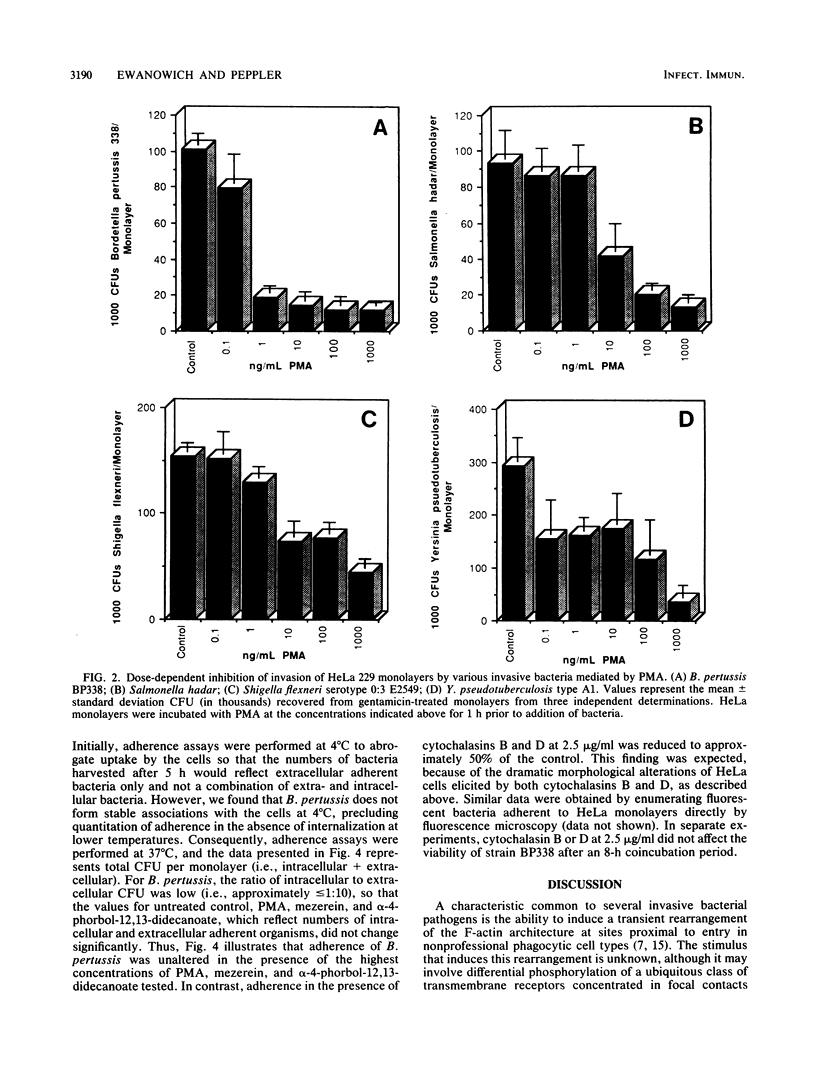
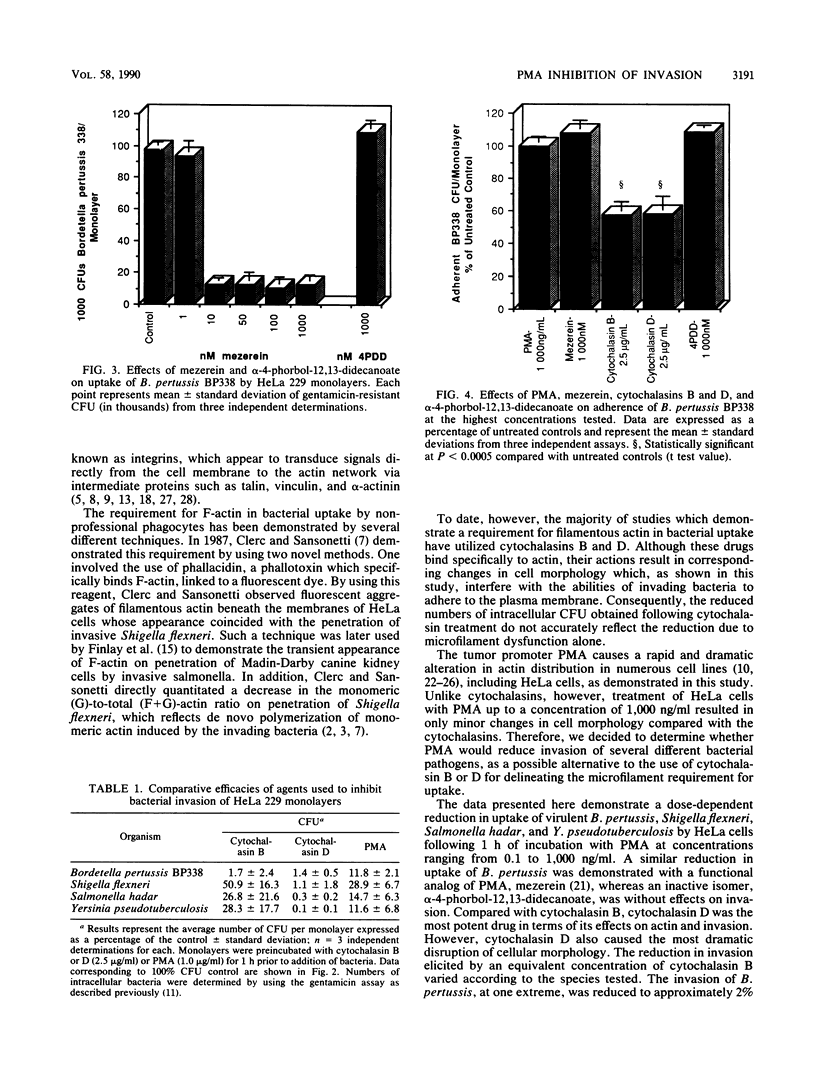
Abstract
The microfilament inhibitors cytochalasins B and D have been traditionally used to indirectly evaluate the requirement for actin in the uptake of invasive bacterial pathogens by nonprofessional phagocytes. Through their effects on microfilaments, both cytochalasins also impart profound alterations in cellular morphology and surface topology, which likely interfere with adherence. Alterations affecting adherence would complicate interpretation of the effect of cytochalasins on entry alone. As an alternative to cytochalasins, the effect of the tumor promoter phorbol myristate acetate (PMA) was examined for its effects on uptake of several invasive bacterial pathogens by HeLa 229 cells. In this communication, PMA was shown to induce a similar change in HeLa cell actin distribution, but, in contrast to cytochalasins B and D, PMA had no significant effect on gross cell morphology. The modified actin distribution was shown to reduce internalization of Bordetella pertussis, Yersinia pseudotuberculosis, Shigella flexneri, and Salmonella hadar in a dose-dependent manner at concentrations ranging from 1 to 1,000 ng/ml. The magnitude of reduction at a PMA concentration of 1,000 ng/ml was greater than the reduction elicited by cytochalasin B at 2.5 micrograms/ml but was less than that elicited by cytochalasin D at 2.5 micrograms/ml. Mezerein, a functional analog of PMA, caused a similar dose-dependent reduction in uptake of B. pertussis, whereas an inactive analog of PMA, alpha-4-phorbol-12,13-didecanoate was without effect on invasion. Binding studies further reveal that pretreatment of HeLa cells with PMA or mezerein did not significantly impair the ability of B. pertussis to adhere, in contrast to cytochalasins B and D, which caused a marked reduction in adherence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Carlsson L. On the dynamics of the microfilament system in HeLa cells. J Cell Biol. 1982 Apr;93(1):122–128. doi: 10.1083/jcb.93.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bukholm G. Effect of cytochalasin B and dihydrocytochalasin B on invasiveness of entero-invasive bacteria in HEp-2 cell cultures. Acta Pathol Microbiol Immunol Scand B. 1984 Jun;92(3):145–149. doi: 10.1111/j.1699-0463.1984.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S. J. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl S. C., Grabel L. B. Integrin phosphorylation is modulated during the differentiation of F-9 teratocarcinoma stem cells. J Cell Biol. 1989 Jan;108(1):183–190. doi: 10.1083/jcb.108.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilov Y. N., Juliano R. L. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989 May;108(5):1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewanowich C. A., Sherburne R. K., Man S. F., Peppler M. S. Bordetella parapertussis invasion of HeLa 229 cells and human respiratory epithelial cells in primary culture. Infect Immun. 1989 Apr;57(4):1240–1247. doi: 10.1128/iai.57.4.1240-1247.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Tyrosine-specific protein phosphorylation is regulated by glycoprotein IIb-IIIa in platelets. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2234–2238. doi: 10.1073/pnas.86.7.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Fry J., Rock E. P., Falkow S. Passage of Salmonella through polarized epithelial cells: role of the host and bacterium. J Cell Sci Suppl. 1989;11:99–107. doi: 10.1242/jcs.1989.supplement_11.8. [DOI] [PubMed] [Google Scholar]
- Godman G. C., Miranda A. F., Deitch A. D., Tanenbaum S. W. Action of cytochalasin D on cells of established lines. III. Zeiosis and movements at the cell surface. J Cell Biol. 1975 Mar;64(3):644–667. doi: 10.1083/jcb.64.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Morris R. E., Bonventre P. F. Shigella infection of henle intestinal epithelial cells: role of the host cell. Infect Immun. 1979 Jun;24(3):887–894. doi: 10.1128/iai.24.3.887-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Kellie S., Holme T. C., Bissell M. J. Interaction of tumour promoters with epithelial cells in culture. An immunofluorescence study. Exp Cell Res. 1985 Oct;160(2):259–274. doi: 10.1016/0014-4827(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Walsh R. C., Jr Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell. 1982 Aug;30(1):253–262. doi: 10.1016/0092-8674(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Miyake R., Tanaka Y., Tsuda T., Kaibuchi K., Kikkawa U., Nishizuka Y. Activation of protein kinase C by non-phorbol tumor promoter, mezerein. Biochem Biophys Res Commun. 1984 Jun 15;121(2):649–656. doi: 10.1016/0006-291x(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Rickaert F., Lamy F., Authelet M., Dumont J. E. Actin stress fiber disruption and tropomyosin isoform switching in normal thyroid epithelial cells stimulated by thyrotropin and phorbol esters. Exp Cell Res. 1989 May;182(1):1–13. doi: 10.1016/0014-4827(89)90274-7. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Sasaki Y., Kanno Y. 12-O-tetradecanoylphorbol-13-acetate disrupts actin filaments and focal contacts and enhances binding of fibronectin-coated latex beads to 3T3-L1 cells. Exp Cell Res. 1988 Oct;178(2):233–241. doi: 10.1016/0014-4827(88)90394-1. [DOI] [PubMed] [Google Scholar]
- Sobue K., Fujio Y., Kanda K. Tumor promoter induces reorganization of actin filaments and calspectin (fodrin or nonerythroid spectrin) in 3T3 cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):482–486. doi: 10.1073/pnas.85.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickel S. K., Wang Y. L. Synthetic peptide GRGDS induces dissociation of alpha-actinin and vinculin from the sites of focal contacts. J Cell Biol. 1988 Sep;107(3):1231–1239. doi: 10.1083/jcb.107.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Urbanik E., Ware B. R. Actin filament capping and cleaving activity of cytochalasins B, D, E, and H. Arch Biochem Biophys. 1989 Feb 15;269(1):181–187. doi: 10.1016/0003-9861(89)90098-2. [DOI] [PubMed] [Google Scholar]