Abstract
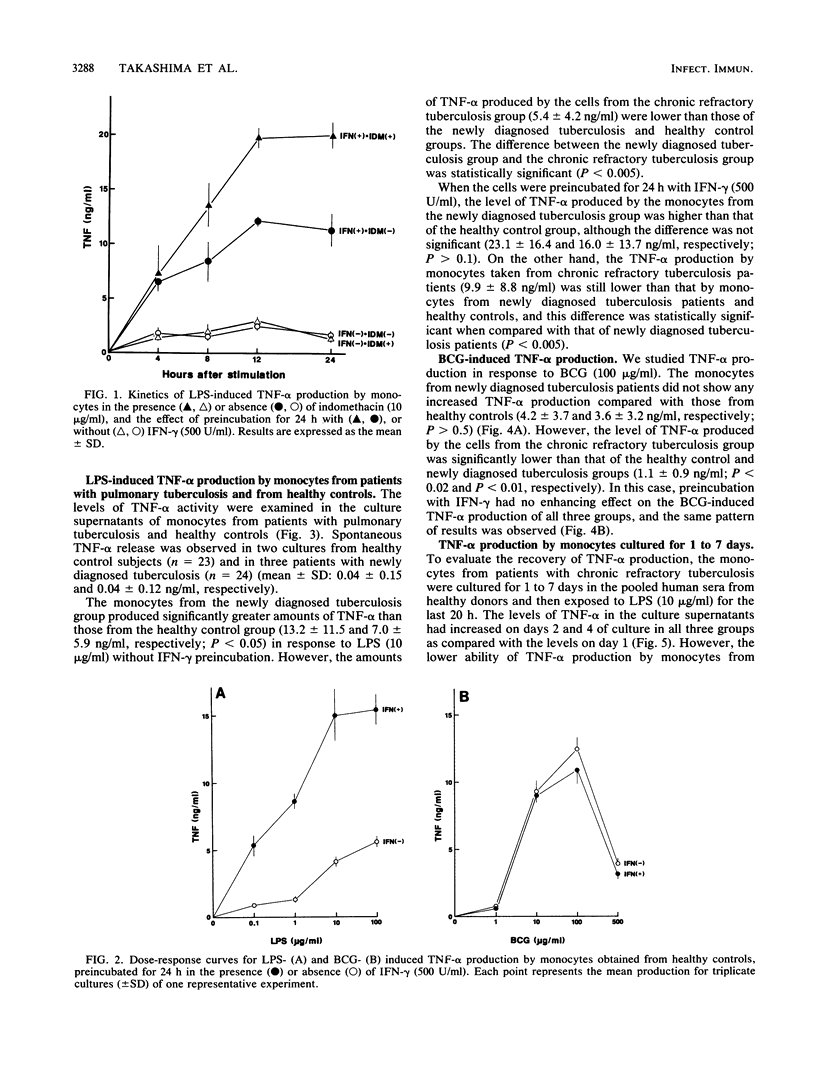
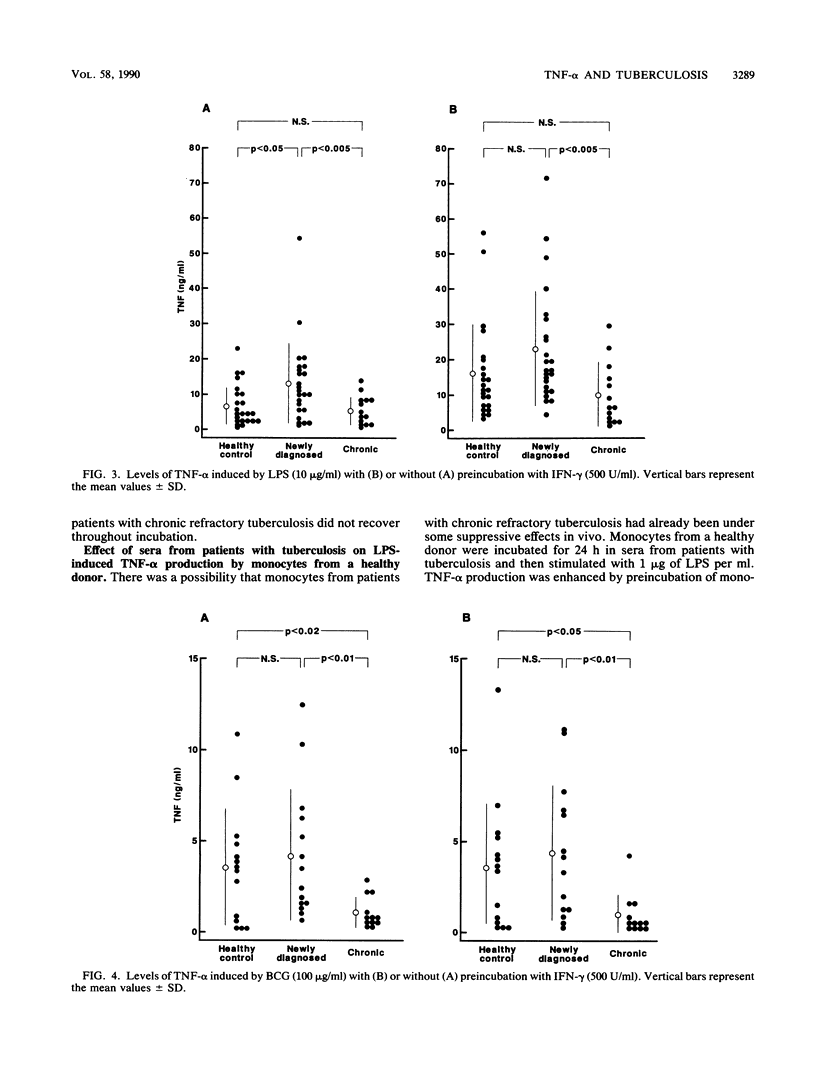
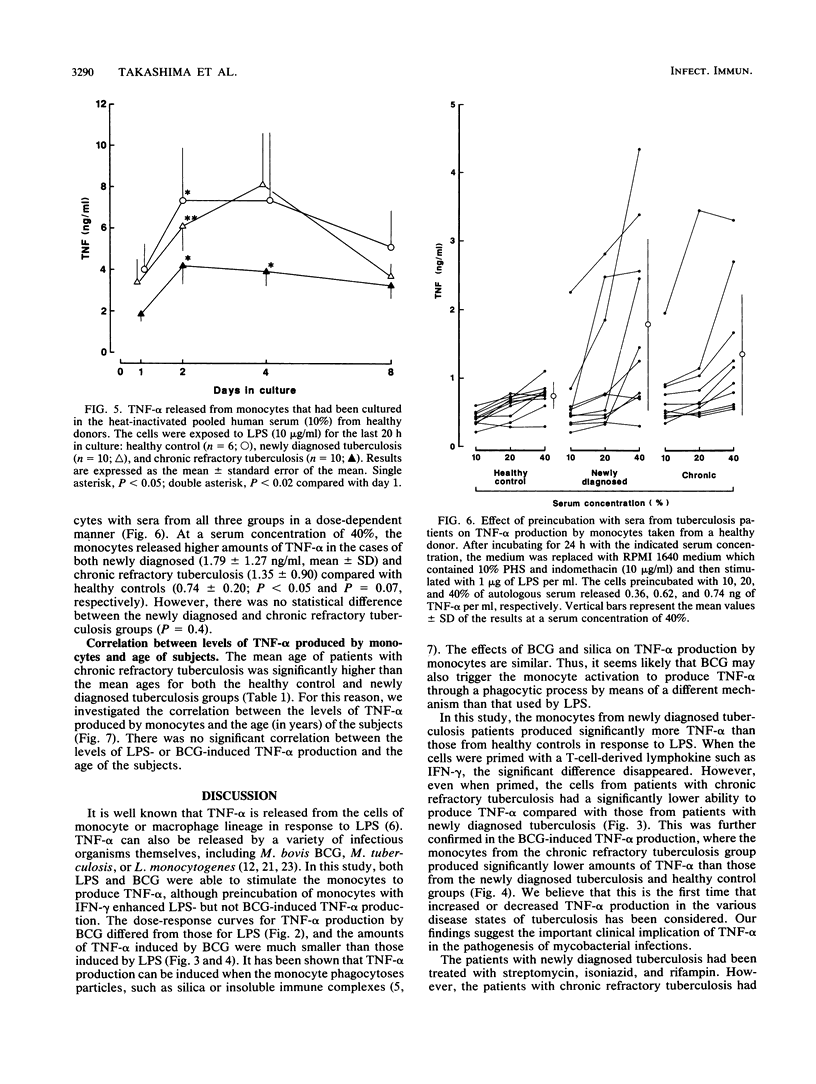
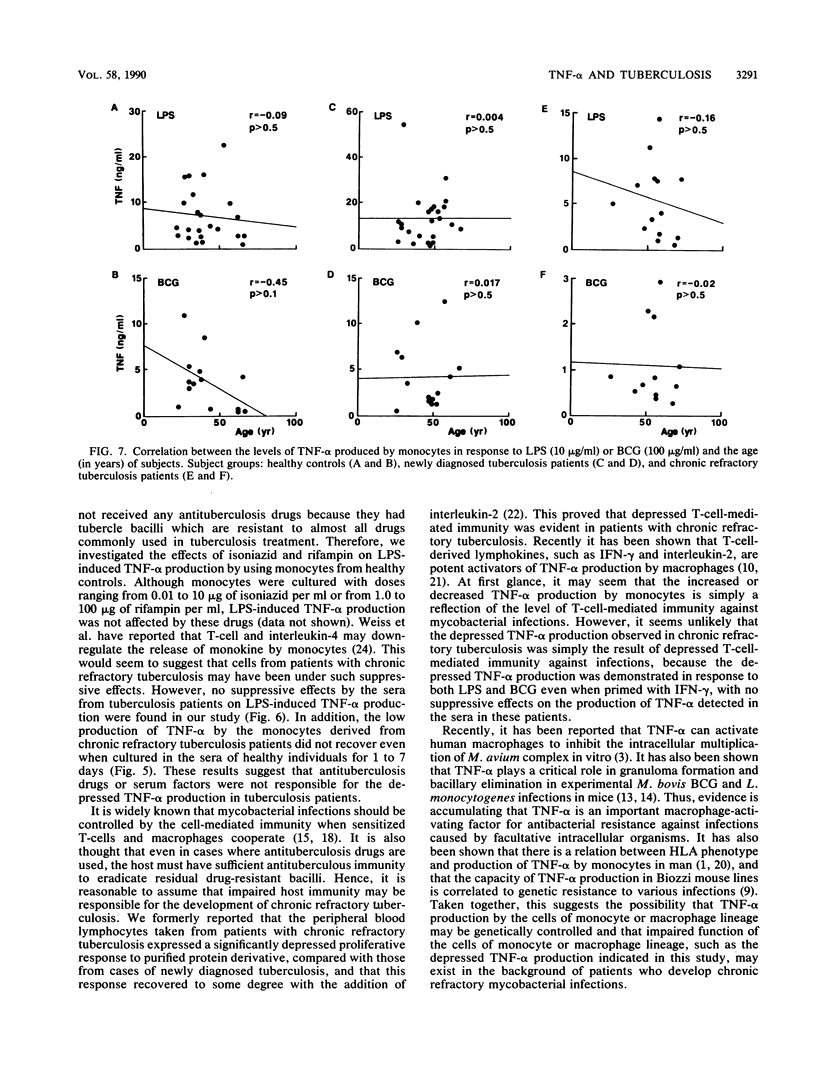
We studied the production of tumor necrosis factor alpha (TNF-alpha) by peripheral blood monocytes taken from patients with pulmonary tuberculosis and from healthy controls. It was found that the monocytes from patients with newly diagnosed tuberculosis released significantly greater amounts of TNF-alpha in vitro in response to lipopolysaccharide than did those from healthy controls (P less than 0.05). However, the monocytes from patients with chronic refractory tuberculosis released significantly lower amounts of TNF-alpha than did those from patients with newly diagnosed tuberculosis (P less than 0.005). Even when the cells were primed for 24 h with 500 U of recombinant interferon gamma per ml, the same pattern of results was observed. The depressed TNF-alpha production by the monocytes from patients with chronic refractory tuberculosis was also shown in response to Mycobacterium bovis BCG. This depressed TNF-alpha production did not recover, even when cultured for 1 to 7 days in the sera of healthy individuals. The sera from patients with chronic refractory tuberculosis did not have any suppressive effect on the lipopolysaccharide-induced TNF-alpha production. Thus, it was demonstrated that the levels of TNF-alpha produced by monocytes were related to the disease states of pulmonary tuberculosis and that the depressed TNF-alpha production by monocytes in patients with chronic refractory tuberculosis might not be acquired.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendtzen K., Morling N., Fomsgaard A., Svenson M., Jakobsen B., Odum N., Svejgaard A. Association between HLA-DR2 and production of tumour necrosis factor alpha and interleukin 1 by mononuclear cells activated by lipopolysaccharide. Scand J Immunol. 1988 Nov;28(5):599–606. doi: 10.1111/j.1365-3083.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Borm P. J., Palmen N., Engelen J. J., Buurman W. A. Spontaneous and stimulated release of tumor necrosis factor-alpha (TNF) from blood monocytes of miners with coal workers' pneumoconiosis. Am Rev Respir Dis. 1988 Dec;138(6):1589–1594. doi: 10.1164/ajrccm/138.6.1589. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Titto E. H., Catterall J. R., Remington J. S. Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol. 1986 Aug 15;137(4):1342–1345. [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J Immunol. 1988 Aug 15;141(4):1197–1201. [PubMed] [Google Scholar]
- Dockrell H. M., Taverne J., Lelchuk R., Depledge P., Brown I. N., Playfair J. H. Macrophage functions in Biozzi mice. Immunology. 1985 Jul;55(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Economou J. S., McBride W. H., Essner R., Rhoades K., Golub S., Holmes E. C., Morton D. L. Tumour necrosis factor production by IL-2-activated macrophages in vitro and in vivo. Immunology. 1989 Aug;67(4):514–519. [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H., Gong J. H., Schmidt A., Nain M., Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct 1;141(7):2388–2393. [PubMed] [Google Scholar]
- Repo H., Jättelä M., Leirisalo-Repo M., Hurme M. Production of tumour necrosis factor and interleukin 1 by monocytes of patients with previous Yersinia arthritis. Clin Exp Immunol. 1988 Jun;72(3):410–414. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Okuda Y., Tsuyuguchi I. Recombinant human interleukin-2 reverses in vitro-deficient cell-mediated immune responses to tuberculin purified protein derivative by lymphocytes of tuberculous patients. Infect Immun. 1987 Sep;55(9):2126–2131. doi: 10.1128/iai.55.9.2126-2131.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Haeffner-Cavaillon N., Laude M., Cavaillon J. M., Kazatchkine M. D. Human T cells and interleukin 4 inhibit the release of interleukin 1 induced by lipopolysaccharide in serum-free cultures of autologous monocytes. Eur J Immunol. 1989 Jul;19(7):1347–1350. doi: 10.1002/eji.1830190731. [DOI] [PubMed] [Google Scholar]


