Abstract
FoxO transcription factors play a conserved role in longevity and act as tissue-specific tumor suppressors in mammals. Several nodes of interaction have been identified between FoxO transcription factors and p53, a major tumor suppressor in humans and mice. However, the extent and importance of the functional interaction between FoxO and p53 have not been fully explored. Here, we show that p53 transactivates the expression of FoxO3, one of the four mammalian FoxO genes, in response to DNA damaging agents in both mouse embryonic fibroblasts and in thymocytes. We show that p53 transactivates FoxO3 in cells by binding to a site in the second intron of the FoxO3 gene, a genomic region recently found to be associated with extreme longevity in humans. While FoxO3 is not necessary for p53-dependent cell cycle arrest, FoxO3 appears to modulate p53-dependent apoptosis. We also find that FoxO3 loss does not interact with p53 loss for tumor development in vivo, although the tumor spectrum of p53 deficient mice may be affected by FoxO3 loss. Our findings indicate that FoxO3 is a p53 target gene, and suggest that FoxO3 and p53 are part of a regulatory transcriptional network that may play an important role during aging and cancer.
Introduction
Aging and cancer are intimately linked. Many cancers have a striking age-dependent onset. Interventions that extend lifespan, such as dietary restriction, decrease the incidence of tumors (Hursting et al., 2003; Masoro, 2005). The connection between aging and cancer raises the possibility that genes that extend lifespan may also be part of a molecular network that suppresses tumorigenesis. An example for such genes is provided by FoxO transcription factors, which play a pivotal role at the interface between longevity and tumor suppression (Greer and Brunet, 2005). In invertebrates, FoxO factors are necessary to extend lifespan downstream of the insulin pathway (Kenyon, 2005). In mammals, the four FoxO family members (FoxO1, FoxO3, FoxO4, and FoxO6) also function downstream of the insulin-signaling pathway (Greer and Brunet, 2005). Single nucleotide polymorphisms (SNPs) in the FoxO3 gene have recently been found to be associated with extreme longevity in humans, suggesting a conserved function for FoxO3 in longevity (Anselmi et al., 2009; Flachsbart et al., 2009; Pawlikowska et al., 2009; Willcox et al., 2008). Interestingly, the FoxO family has also been found to act as a lineage-specific tumor suppressor in mammals (Paik et al., 2007). Combined somatic deletion of FoxO1, FoxO3, and FoxO4 in mice leads to the development of tumors, particularly thymic lymphomas and hemangiomas (Paik et al., 2007). FoxO3−/− mice can also develop cancer, but at a lesser frequency and later in life than FoxO1/FoxO3/FoxO4 compound mutant mice (Paik et al., 2007). In humans, FoxO3 inactivation is correlated with poor prognosis of breast cancers (Hu et al., 2004). Conversely, ectopic expression of FoxO3 in human cells is sufficient to delay tumor development in xenograft models (Hu et al., 2004; Seoane et al., 2004). Thus, FoxO3 may be an important part of a regulatory network that controls both aging and cancer.
FoxO3 is a potent transcriptional activator that triggers the expression of a program of genes involved in cell cycle arrest, DNA repair, hypoxia, and apoptosis (Bakker et al., 2007; Brunet et al., 1999; Medema et al., 2000; Paik et al., 2009; Renault et al., 2009; Seoane et al., 2004; Tothova et al., 2007; Tran et al., 2002; You et al., 2006a). FoxO3 transcriptional activity is inhibited in response to insulin and growth factors via phosphorylation-dependent nuclear export (Brunet et al., 1999; Brunet et al., 2001; Brunet et al., 2002). While the regulation of FoxO3 activity by post-translational modifications, such as phosphorylation, has been well studied (Calnan and Brunet, 2008; Van Der Heide et al., 2004), the molecular mechanisms that regulate the expression of the FoxO3 gene remain mostly unclear.
Given the connection between aging and cancer, it is interesting to note that there are a number of parallels between FoxO3 and the tumor suppressor protein p53. Like FoxO3, p53 induces cell cycle arrest, apoptosis, and DNA repair (Vousden and Lu, 2002). Several FoxO3 target genes such as Gadd45, Wip1, p21Cip1, Puma, and Sestrin1/PA26 are also regulated by p53 (el-Deiry et al., 1993; Fiscella et al., 1997; Kastan et al., 1992; Nakano and Vousden, 2001; Velasco-Miguel et al., 1999; Yu et al., 2001). FoxO3 and p53 are extensively modified in response to stress stimuli, via phosphorylation and acetylation (Calnan and Brunet, 2008; Vousden and Prives, 2009), and both p53 and FoxO3 bind to and are deacetylated by the Sirt1 deacetylase (Brunet et al., 2004; Luo et al., 2001; Motta et al., 2004; Vaziri et al., 2001). These extensive similarities between FoxO3 and p53 suggest that both transcription factors may be part of a common regulatory complex.
A number of direct and indirect links between FoxO3 and p53 have already been uncovered. First, FoxO3 directly binds to p53, at least in the context of overexpression (Nemoto et al., 2004; Wang et al., 2008). Second, FoxO3 leads to stabilization of the p53 protein and activation of p53-dependent apoptosis (You et al., 2006b). FoxO3 also upregulates p19ARF, a positive ups tream regulator of p53 (Bouchard et al., 2007). Conversely, p53 has been reported to inhibit FoxO3 function indirectly by inducing the protein kinase SGK, thereby resulting in the phosphorylation of FoxO3 and its sequestration in the cytoplasm (You et al., 2004). In addition, p53 has been found to inhibit FoxO3 transcriptional activity under conditions of oxidative stress (Miyaguchi et al., 2009) and to induce FoxO3 degradation (Fu et al., 2009). Whether FoxO3 and p53 intersect in other ways, for example by regulating each other’s transcription, remains largely unknown.
p53 is a potent tumor suppressor in humans, as underscored by the fact that nearly all human tumors have mutations or deletions in the p53 gene itself or in the p53 pathway (Vogelstein et al., 2000). Mutations in p53 have been linked to poor prognosis in a variety of human cancers, including lung (Quinlan et al., 1992), breast (Deng et al., 1994), and gastric cancers (Scott et al., 1991), as well as lymphomas (Gaidano et al., 1991; Lo Coco et al., 1993). Consistent with the prevalence of p53 loss in human tumors, p53−/− mice are highly prone to cancer early in life (Donehower et al., 1992; Harvey et al., 1993a; Harvey et al., 1993b; Jacks et al., 1994). p53+/− mice also develop tumors with high frequency (Donehower et al., 1992; Harvey et al., 1993a; Harvey et al., 1993b; Jacks et al., 1994). The connection between FoxO3 and p53 in cells raises the possibility that FoxO3 functionally interacts with p53 for tumor suppression. A dominant-negative form of FoxO factors has been shown to accelerate Myc-driven tumorigenesis by blocking p53-dependent apoptosis (Bouchard et al., 2007). However, the genetic interaction between FoxO3 and p53 loss in cancer progression in the absence of oncogenic stimulation has never been tested.
Here, we explore the connections between FoxO3, a ubiquitously expressed FoxO family member, and p53 in cells and in mice. We find that p53 acts as a direct upstream transcriptional activator of the FoxO3 gene in response to DNA damage in mouse embryonic fibroblasts and in lymphocytes. We show that p53 regulates the transcription of the FoxO3 gene by binding to a site in the second intron of the FoxO3 gene. Although FoxO3 is not necessary for p53-dependent cell cycle arrest, FoxO3 appears to play a role in p53-dependent apoptosis. We also find that while FoxO3 loss does not synergize with p53 loss for tumor development in vivo, tumor spectrum in p53-deficient mice may be affected by the loss of one or both FoxO3 alleles. These results reveal a regulatory mechanism linking FoxO3 and p53, two critical molecules involved in the control of longevity and tumor suppression.
Results
DNA damage and Nutlin treatment increase FoxO3 protein levels in a p53-dependent manner in fibroblasts
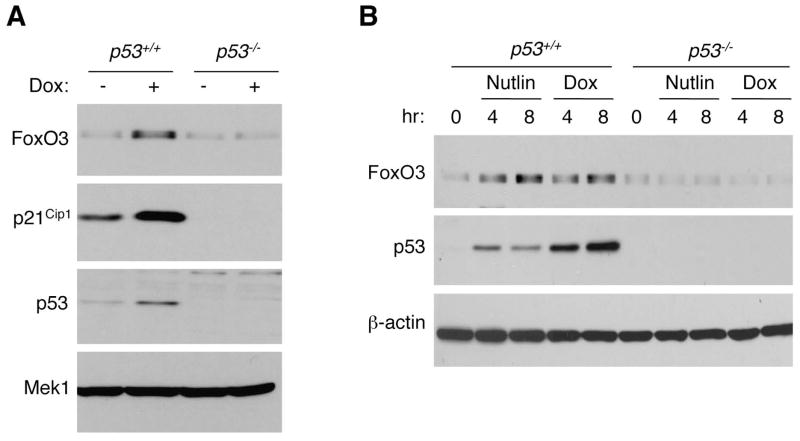
To test if p53 regulates FoxO3 expression in mammalian cells, we compared FoxO3 protein levels in p53+/+ and p53−/− primary mouse embryonic fibroblasts (MEFs) in the absence or presence of doxorubicin, a DNA damaging agent that activates endogenous p53. We found that doxorubicin treatment increased FoxO3 protein expression in p53+/+ MEFs, but not in p53−/− MEFs (Figure 1A). Changes in FoxO3 protein levels were similar to those of p21Cip1, a well-known target of p53 (Figure 1A). To activate p53 in a more specific manner, we used Nutlin, a chemical compound that inhibits binding of p53 to Mdm2, a ubiquitin ligase critical for p53 degradation (Vassilev et al., 2004). Similar to what we observed for doxorubicin, Nutlin treatment increased FoxO3 protein in p53+/+ MEFs, but not in p53−/− MEFs (Figure 1B). Together, these results indicate that p53 is necessary for FoxO3 protein accumulation in MEFs in response to DNA damage and Nutlin.
Figure 1. Doxorubicin and Nutlin elicit an increase in FoxO3 protein expression that is p53-dependent in MEFs.
(A) Western-blot of protein extracts from p53+/+ and p53−/− MEFs incubated in the absence (−) or presence (+) of doxorubicin (Dox, 0.2 μg/ml) for 8 hours, using antibodies to FoxO3, p21Cip1 (a well-known target of p53), p53, and Mek1 (loading control). (B) Western-blot of protein extracts from p53+/+ and p53−/− MEFs incubated with Nutlin (10 μM), a p53 activator, or Doxorubicin (Dox, 0.2 μg/ml) for 0, 4, and 8 hours, using antibodies to FoxO3, p53, and β-actin (loading control). Western-blots are representative of at least two independent experiments, conducted on independent cultures of MEFs.
p53 is necessary for FoxO3 mRNA upregulation in response to DNA damage or Nutlin treatment in fibroblasts
To determine if the p53-dependent accumulation of FoxO3 protein is due to transcriptional or post-transcriptional changes, we compared FoxO3 mRNA levels in p53+/+ and p53−/− MEFs in response to Nutlin or to doxorubicin (Figure 2A). We found that Nutlin or doxorubicin led to an upregulation of FoxO3 mRNA that was significantly attenuated in p53−/− MEFs (Figure 2A), similar to two known p53 targets, p21Cip1 and Mdm2 (Figure 2B–C). We noted that FoxO3 mRNA expression at basal levels is lower in p53−/− MEFs than in p53+/+ MEFs, whereas FoxO3 protein expression is similar in MEFs of both genotypes (see Figure 1), suggesting that there are additional levels of regulation of the FoxO3 protein by p53. In contrast to FoxO3, other FoxO family members (FoxO1, FoxO4, and FoxO6) did not show a p53-dependent increased mRNA expression in response to Nutlin and doxorubicin (Figure 2D–F). FoxO6 mRNA was induced in response to doxorubicin, but in a p53-independent manner (Figure 2F), raising the possibility that other members of the p53 family (e.g. p73) may be responsible for FoxO6 regulation in response to DNA damage. Collectively, these observations indicate that p53 is necessary for the upregulation of FoxO3 mRNA in MEFs in response to signals that activate p53.
Figure 2. p53 is necessary for FoxO3 mRNA upregulation in response to doxorubicin or Nutlin in MEFs.
Real time quantitative PCR analysis of FoxO3 (A), p21Cip1 (B), Mdm2 (C), FoxO1 (D), FoxO4 (E), and FoxO6 (F) mRNA levels in p53+/+ and p53−/− MEFs in response to 4 and 8 hours of treatment with Nutlin (10 μM) or Doxorubicin (Dox, 0.2 μg/ml). Mean +/− SEM of two independent experiments conducted in triplicate. * p<0.05, ** p<0.01, ***p<0.001 between p53+/+ and p53−/− MEFs at a given time point, two-way ANOVA with Bonferroni post-test.
p53 is necessary for FoxO3 mRNA upregulation in response to DNA damage in lymphocytes
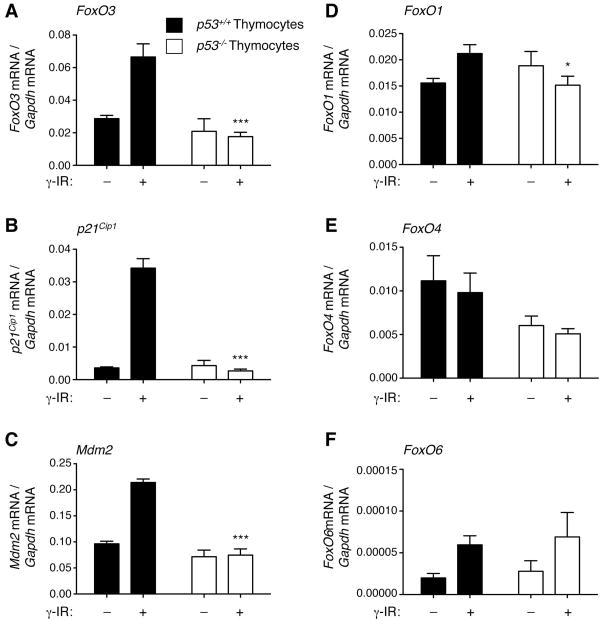
We next asked if the induction of FoxO3 mRNA by p53 was also observed in other cell types. We found that FoxO3 mRNA was upregulated in response to γ irradiation in mouse thymocytes, but that this upregulation was no longer observed in p53−/− thymocytes (Figure 3A). The changes in FoxO3 mRNA levels in thymocytes were similar to the changes observed for two well-known targets of p53, p21Cip1 (Figure 3B) and Mdm2 (Figure 3C). The expression of other FoxO family members (FoxO1, FoxO4, and FoxO6) was not strongly upregulated in response to γ irradiation in a p53-dependent manner (Figure 3D–F), although FoxO1 mRNA was slightly affected by p53 (Figure 3D). These findings indicate that the p53-dependent regulation of FoxO3 mRNA by DNA damaging agents is relatively specific to this FoxO isoform, and is observed in multiple cell types.
Figure 3. p53 is necessary for FoxO3 mRNA upregulation in response to γ irradiation in thymocytes.
Real time quantitative PCR analysis of FoxO3 (A), p21Cip1 (B), Mdm2 (C), FoxO1 (D), FoxO4 (E), and FoxO6 (F) mRNA levels in p53+/+ and p53−/− thymocytes, 3 hours after γ irradiation (γ IR, 10 Gy). Mean +/− SEM of two independent experiments conducted in triplicate on samples from 3–5 mice per genotype. * p<0.05, ***p<0.001 between p53+/+ and p53−/− thymocytes at a given time point, two-way ANOVA with Bonferroni post-test.
p53 transcriptional activity is necessary and sufficient to regulate FoxO3 mRNA in fibroblasts
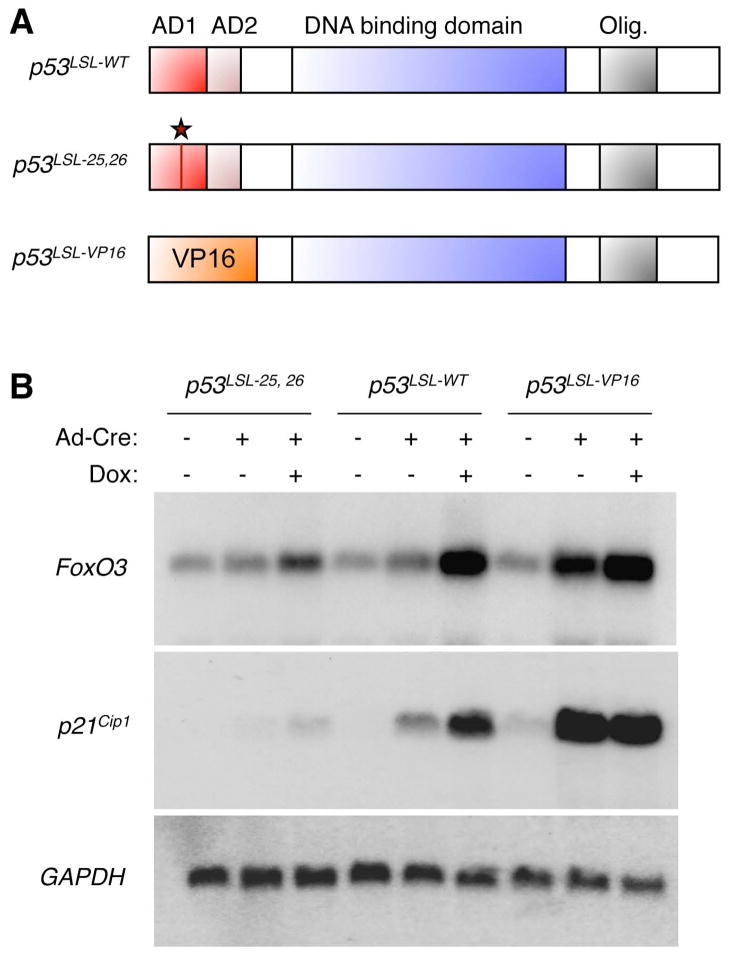
To determine whether FoxO3 mRNA upregulation in response to doxorubicin is mediated by p53’s ability to act as a transcriptional activator, we examined FoxO3 mRNA levels in knock-in MEFs expressing a transcriptionally-impaired mutant of p53 under the control of p53 endogenous promoter (Figure 4A). This mutant p53 is transcriptionally deficient because leucine 25 and tryptophan 26, two key residues for p53 transcriptional activity, are replaced by glutamine and serine, respectively (Johnson et al., 2005). In addition, the transcriptionally-impaired p53 allele, as well as a control WT allele of p53, were rendered inducible by the presence of a transcriptional/translational STOP cassette flanked by LoxP sites upstream of the p53 coding region (p53LSL-WT and p53LSL-25,26) (Figure 4A) (Johnson et al., 2005). In p53LSL-WT and p53LSL-25,26 knock-in MEFs, introduction of Cre recombinase allows expression of the WT or transcriptionally-impaired p53 alleles (Johnson et al., 2005). Northern-blot experiments revealed that the expression of FoxO3 mRNA was higher in p53LSL-WT MEFs in the presence of Cre (i.e. expressing WT p53) than in p53LSL-WT MEFs in the absence of Cre (i.e. not expressing p53), similar to p21Cip1 mRNA, a classical p53 target. These results confirm that p53 is necessary for expression of the FoxO3 mRNA (Figure 4B). In the presence of Cre, doxorubicin elicited a significant increase in FoxO3 and p21Cip1 mRNA in p53LSL-WT MEFs but not in p53LSL-25,26 MEFs (Figure 4B). There was a slight upregulation of FoxO3 and p21Cip1 mRNA in p53LSL-25,26 MEFs in the presence of Cre and doxorubicin (Figure 4B), consistent with the fact that the p5325,26 mutant is not completely transcriptionally inactive (Johnson et al., 2005). Together, these data indicate that p53 transcriptional activity is necessary for the DNA damage-dependent increase in FoxO3 mRNA.
Figure 4. p53 transcriptional activity is necessary and sufficient for FoxO3 mRNA upregulation.
(A) Schematic of the p53 knock-in alleles used. p53LSL-WT: inducible allele encoding a form of wildtype p53. p53LSL-25,26: inducible allele encoding a transcriptionally-impaired mutant of p53 in which leucine 25 is replaced by a glutamine and tryptophan 26 is replaced by a serine. p53LSL-VP16: inducible allele encoding a mutant of p53 in which the transactivation domains (AD1 and AD2) are replaced by the transactivation domain of VP16. AD: activation domain; Olig.: oligomerization domain. The star indicates the location of the 25,26 mutation. (B) Northern-blot analysis of MEFs in which the endogenous allele of p53 has been replaced by an allele encoding inducible forms of WT p53 (p53LSL-WT), a transcription-deficient mutant (p53LSL-25,26), or a mutant of p53 in which the transactivation domains of p53 were replaced by that of VP16 (p53LSL-VP16). The addition of an adenovirus containing Cre recombinase (Ad-Cre) allows the deletion of the Lox-STOP-Lox (LSL) cassette upstream of each allele and allows the expression of each p53 variant. Cells were exposed to 8 hours of doxorubicin (Dox, 0.2 μg/ml). Northern-blots were analyzed with a probe to FoxO3, p21Cip1 (a known target of p53), and GAPDH (loading control).
To test whether p53 transcriptional activation is sufficient to induce FoxO3 mRNA, we used MEFs with a knock-in mutation of p53 fused to the transactivation domain of the Herpes virus transactivator VP16 (p53LSL-VP16, Figure 4A), thus rendering p53 maximally active (Johnson et al., 2005; Johnson et al., 2008). We observed that similar to p21Cip1 mRNA, FoxO3 mRNA was potently induced in Cre-treated p53LSL-VP16 MEFs, even in the absence of doxorubicin (Figure 4B). These findings indicate that p53 transactivation activity is sufficient for FoxO3 mRNA upregulation. Taken together, these results indicate that FoxO3 gene expression is transcriptionally regulated by p53.
p53 directly binds and transactivates regulatory regions in the FoxO3 gene
To determine if FoxO3 is a direct target gene of p53, we searched the 5kb upstream regulatory region as well as all introns of the mouse FoxO3 gene for the presence of putative p53 binding sites, based on the known p53 consensus binding site (el-Deiry et al., 1992). This analysis identified four potential p53 binding elements: three (p53-1, p53-2, and p53-3) in the FoxO3 promoter; and one (p53-4) in the second intron of the FoxO3 gene (Figure 5A). p53-4 has a near perfect match to the p53 consensus binding site and only one base pair of spacing between the two half-sites (Figure 5A), which is a characteristic of optimal p53 binding sites (el-Deiry et al., 1992). In addition, while the region containing p53-4 is not perfectly conserved in human FoxO3, there is also an optimal p53 binding site in the second intron of the human FoxO3 gene, suggesting that the presence of p53 binding site in the second intron may be a conserved feature of the FoxO3 gene. Interestingly, the second intron of the human FoxO3 gene contains SNPs associated with extreme human longevity (Anselmi et al., 2009; Flachsbart et al., 2009; Willcox et al., 2008).
Figure 5. p53 is recruited to a binding site in the second intron of the FoxO3 gene.
(A) Location and sequence of the p53 binding sites (p53-1, p53-2, p53-3, and p53-4) in the promoter and second intron of the mouse FoxO3 gene. R: G or A; W: T or A; Y: C or T; E: exon; I: intron. Also depicted are the consensus for p53 binding sites, the p53 binding site in p21Cip1 promoter, and the mutant of critical bases in p53-4 (p53-4m). (B) ChIP on MEFs treated with Doxorubicin (Dox, 0.2 μg/ml) for 16–20 hours, using antibodies to p53 (colored bars) or control IgG (white bars). The chromatin bound to p53 or to the control IgG was analyzed by quantitative-PCR with primers surrounding a region that did not contain p53 binding sites (−), the distal p53 binding site in FoxO3 promoter (p53-1), the p53-4 binding site in FoxO3 intron 2 (p53-4), and the p53 binding site in the p21Cip1 promoter (p53 p21Cip1). The fold enrichment over the IgG control is represented. Mean +/− SEM of three independent experiments. **: p<0.01, one-way ANOVA. (C) ChIP on p53+/+ and p53−/− MEFs in the absence or presence of Doxorubicin (Dox, 0.2 μg/ml) for 6 hours, using antibodies to p53 or control IgG. The chromatin bound to p53 or to the control IgG was analyzed by quantitative-PCR with primers surrounding a region that did not contain p53 binding sites (−), the distal p53 binding site in FoxO3 promoter (p53-1), the p53-4 binding site in FoxO3 intron 2 (p53-4) and the p53 binding site in the p21Cip1 promoter (p53 p21Cip1). The fold enrichment over the IgG control is represented. Mean +/− SD from triplicates of one experiment. (D) Normalized activity of luciferase reporter constructs driven by 500bp surrounding the p53 binding sites p53-1 or p53-4 in p53+/+ (black) and p53−/− (white) MEFs. Mean +/− SEM of four independent experiments conducted in triplicate. *: p<0.05 between p53-4 and control in p53+/+ MEFs, **: p<0.01 between p53+/+ and p53−/− MEFs for p53-4, one-way ANOVA. (E) Normalized activity of luciferase reporter constructs driven by the region surrounding the p53-4 binding site or by the region surrounding the p53-4 binding site in which the p53 binding site was mutated (p53-4m) in p53+/+ (black) and p53−/− (white) MEFs. Mean +/− SEM of three independent experiments conducted in triplicate. **: p<0.01 between p53-4m and p53-4 in p53+/+ MEFs, one-way ANOVA.
To assess if p53 binds to these two p53 binding sites in MEFs, we performed chromatin immunoprecipitation (ChIP) assays with antibodies to p53 in chromatin extracts from MEFs that had been treated with doxorubicin. The recruitment of p53 to the regulatory regions of the FoxO3 gene was assessed by quantitative PCR using primers surrounding the p53-1 or p53-4 binding sites (Figure 5B). We found that endogenous p53 occupied the p53-4 binding site in the intron of the FoxO3 gene, but not the p53-1 binding site in the FoxO3 promoter, in doxorubicin-treated MEFs (Figure 5B). Endogenous p53 was bound to the p53-4 binding site in the intron of the FoxO3 gene even in the absence of doxorubicin, but the recruitment of p53 to that site was slightly increased in response to a short treatment with doxorubicin (Figure 5C). p53 occupancy at the p53 binding site in FoxO3 second intron was similar to that in the p21Cip1 promoter, although p53 recruitment to the p21Cip1 promoter in response to short treatment with doxorubicin was more robust (Figure 5C). Taken together, these results indicate that p53 directly binds to a site within the second intron of the mouse FoxO3 gene in vivo.
p53 is necessary for FoxO3 transactivation
To determine if p53 can transactivate the FoxO3 gene by binding to the p53-4 binding site, we generated reporter constructs in which luciferase expression is driven by an SV40 minimal promoter fused to a 500 bp region surrounding either p53-1, p53-2, p53-3, or p53-4 and analyzed the luciferase activity of these reporter constructs in p53+/+ and p53−/− MEFs (Figure 5D and data not shown). The region surrounding the p53-4 site in the FoxO3 second intron induced transcriptional activation of the luciferase reporter gene in p53+/+ MEFs, but not in p53−/− MEFs (Figure 5D). In contrast, the transcriptional activity of the p53-1, p53-2, and p53-3 regions was low, regardless of the presence of p53 (Figure 5D, data not shown). Note that region surrounding the p53-4 binding site induced transcriptional activation of the luciferase reporter gene in a p53-dependent manner even in the absence of doxorubicin, perhaps because transfection itself triggered a stress to the cells or because basal FoxO3 mRNA levels are also regulated by p53 (see Figure 2A). Together, these findings indicate that the region surrounding p53-4 in the second intron of the FoxO3 gene can be transactivated by p53.
To examine if the transcriptional activity of the region containing p53-4 was indeed due to this p53 binding site, we created a luciferase reporter construct with a mutated version of p53-4 (p53-4m) that can no longer be bound by p53 because it is missing the critical bases necessary for p53 binding (Figure 5A). Mutating the p53-4 site abolished the transcriptional activity of the luciferase reporter gene (Figure 5E). These experiments indicate that p53 binding to p53-4 in FoxO3 second intron is pivotal for the regulation of the FoxO3 gene by p53.
p53-induced FoxO3 does not play a role in cell cycle arrest in MEFs
p53 is necessary for cell cycle arrest in response to double-strand breaks in MEFs (Kastan et al., 1992). FoxO3 can also promote cell cycle arrest when overexpressed in cells (Medema et al., 2000), although FoxO3 is not necessary for cell cycle arrest in response to DNA damage in MEFs (Castrillon et al., 2003). To test if the upregulation of FoxO3 by p53 mediates part of the cell cycle arrest controlled by p53, we isolated MEFs from FoxO3+/+p53−/− (+/+), FoxO3−/−, and p53−/− mice and assessed BrdU incorporation in these cells in response to doxorubicin (Figure 6A) and Nutlin (Figure 6B). We found that FoxO3−/− MEFs underwent cell cycle arrest to the same extent as +/+ MEFs in response to doxorubicin (Figure 6A) and Nutlin (Figure 6B). In contrast, p53−/− MEFs were partially resistant to cell cycle arrest caused by doxorubicin (Figure 6A) and completely resistant to cell cycle arrest induced by Nutlin (Figure 6B). These results indicate that unlike p53, FoxO3 is not necessary for DNA damage- and p53-dependent cell cycle arrest in MEFs or that there is compensation by another factor, perhaps another FoxO isoform (see below). Figure 6C shows that FoxO3 was dispensable for cell cycle arrest induced by long-term treatment by chronic oxidative stress (H2O2) and by hydroxyurea (HU), which both lead to p53-dependent cellular senescence (Marusyk et al., 2007) (T.M.J. and L.D.A., unpublished). However, FoxO3−/− MEFs displayed significant cell cycle arrest compared to +/+ MEFs in basal long-term culture conditions (Figure 6C), suggesting that FoxO3 loss may itself induce cell cycle arrest over several cellular passages. Consistent with the absence of role for FoxO3 in p53-dependent cell cycle arrest, the expression of p27Kip1, a well-known target of FoxO3 involved in cell cycle arrest (Kops et al., 2002), did not change in response to Nutlin or doxorubicin in p53+/+ MEFs and was even upregulated in p53−/− MEFs (Figure 6D). These observations indicate that the p53-dependent upregulation of FoxO3 mRNA and protein is not accompanied by an increase in FoxO3 transcriptional activity toward p27Kip1, which is likely due to the fact that the FoxO3 protein is known to be exported to the cytoplasm in response to DNA damage in different cell types (A.B., data not shown) (You et al., 2004).
Figure 6. FoxO3 is not necessary for p53-dependent cell cycle arrest in MEFs, but other FoxO family members may compensate for FoxO3 loss.
(A) Percent BrdU-positive cells in FoxO3+/+p53+/+ (+/+), FoxO3−/− and p53−/− MEFs in the presence or absence of doxorubicin (Dox, 0.2 μg/ml) for 24 hours. Mean +/− SEM of three independent experiments, two of which were conducted with independent MEF cultures from distinct animals. ***p<0.001 between +/+ and p53−/− MEFs for the same treatment; ns: non significant between +/+ and FoxO3−/− MEFs for the same treatment, two-way ANOVA with Bonferroni post-test. (B) Percent BrdU-positive cells in FoxO3+/+p53+/+ (+/+), FoxO3−/− and p53−/− MEFs in the presence or absence of Nutlin (10 μM) for 24-36 hours. Mean +/− SEM of three independent experiments. *p<0.05, ***p<0.001 between +/+ and p53−/− MEFs for the same treatment; ns: non significant between +/+ and FoxO3−/− MEFs for the same treatment, two-way ANOVA with Bonferroni post-test. (C) Percent BrdU-positive cells in FoxO3+/+p53+/+ (+/+), FoxO3−/− and p53−/− MEFs in the presence or absence of chronic treatment of H2O2 or hydroxyurea (HU). Mean +/− SEM of two independent experiments with different lines of MEFs. **p<0.01, ***p<0.001 between +/+ and FoxO3−/− or p53−/− MEFs for the same treatment; ns: non significant between +/+ and FoxO3−/− MEFs for the same treatment, two-way ANOVA with Bonferroni post-test. (D) Real time quantitative PCR analysis of p27Kip1 mRNA levels in p53+/+ and p53−/− MEFs in response to 4 and 8 hours of treatment with Nutlin (10 μM) or Doxorubicin (Dox, 0.2 μg/ml). Mean +/− SEM of two independent experiments conducted in triplicate. ***p<0.001 between p53+/+ and p53−/− MEFs at a given time point, two-way ANOVA with Bonferroni post-test. (E) Percent BrdU-positive cells in MEFs infected with control lentiviruses (PSR) or with lentiviruses expressing an shRNA to FoxO family members (PSR “pan FoxO”) in the presence or absence of Nutlin (Nutlin, 10 μM) for 24 hours. The data are expressed as fold decrease, respective to the value obtained in the absence of Nutlin. Mean +/− SEM of two independent experiments. ** p<0.01, between PSR and PSR “pan” FoxO-infected MEFs in the presence of Nutlin, two-way ANOVA with Bonferroni post-test. (F) Western-blot of protein extracts from MEFs infected with control lentiviruses (PSR) or with lentiviruses expressing an shRNA to FoxO family members (PSR “pan FoxO”), using antibodies to FoxO1, FoxO3, FoxO4, FoxO6, and β actin.
The role of FoxO3 in p53-dependent cell cycle arrest may be masked by compensation by other FoxO family members
FoxO3 loss has been found to be compensated by other FoxO family members (Bouchard et al., 2007; Paik et al., 2007). Even though other FoxO family members are not regulated by p53 to the same extent as FoxO3 in MEFs and thymocytes (see Figures 2 and 3), we determined whether interfering with more than one FoxO family member had a more pronounced impact on cell cycle arrest than interfering with FoxO3 alone. We found that Nutlin-induced cell cycle arrest was attenuated in MEFs infected with lentiviruses expressing an shRNA hairpin directed to several FoxO family members (“pan FoxO”) (Hribal et al., 2003) (Figure 6E–F), although Nutlin still caused some cell cycle arrest in these cells. These findings suggest that the FoxO family partially contributes to p53-dependent cell cycle arrest and that the role of FoxO3 in p53-dependent cell cycle arrest may be masked by compensation by other FoxO isoforms.
p53-induced FoxO3 appears to play some role in apoptosis
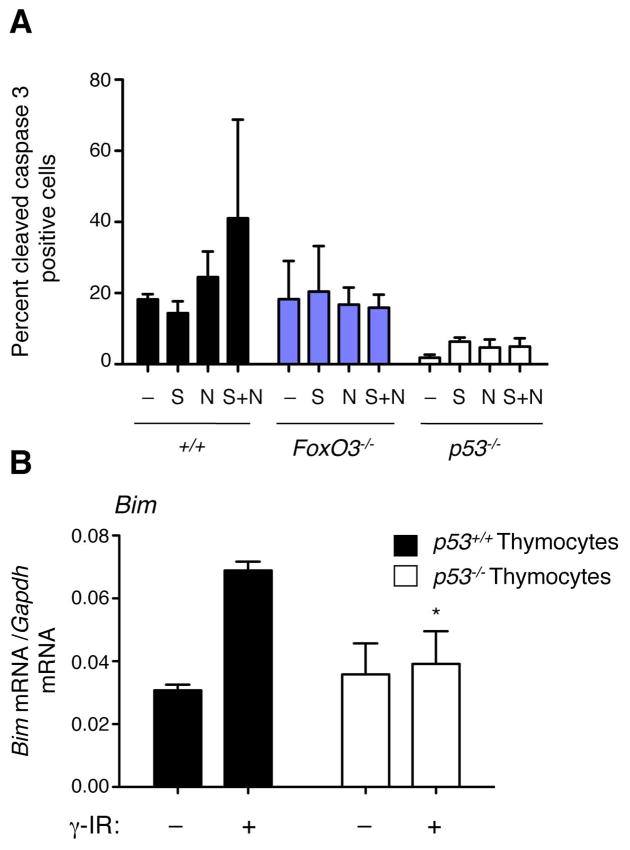
FoxO has been shown to be important for p53-dependent apoptosis in the context of Myc oncogene-transformed cells (Bouchard et al., 2007). We asked if FoxO3 played a role in p53-dependent apoptosis in MEFs. MEFs do not usually respond to p53 activation by undergoing apoptosis, unless they have been transformed with an oncogene, including the E1A protein of the virus (Lowe et al., 1993). We found that Nutlin treatment combined with serum starvation slightly enhanced the percent of cells undergoing apoptosis in E1A-transformed wildtype MEFs (Figure 7A). FoxO3−/− E1A-transformed MEFs appeared to be slightly impaired in their ability to undergo apoptosis in response to Nutlin and serum starvation, although this effect was more modest than that observed in p53−/− E1A-transformed MEFs (Figure 7A). We also found that Bim, a well-known FoxO3 target involved in apoptosis (Dijkers et al., 2000), was upregulated in response to γ-irradiation in thymocytes and that this upregulation was dependent on p53 (Figure 7B). Taken together, these results are consistent with the notion that FoxO3 contributes, at least in part, to p53-mediated apoptosis.
Figure 7. FoxO3 plays a role in p53-dependent apoptosis.
(A) Percent cleaved caspase 3-positive cells in E1A-transformed MEFs (FoxO3+/+p53+/+ (+/+), FoxO3−/−, and p53−/−) in the absence of treatment (−) or in response to Nutlin (N), serum starvation (S), and Nutlin + serum starvation (N+S). Mean +/− SEM of three independent experiments. (B) Real time quantitative PCR analysis of Bim mRNA levels in p53+/+ and p53−/− thymocytes, 3 hours after γ irradiation (γ IR, 10 Gy). Mean +/− SEM of two independent experiments conducted in triplicate on samples from 3–5 mice per genotype. * p<0.05 between p53+/+ and p53−/− thymocytes at a given time point, two-way ANOVA with Bonferroni post-test.
FoxO3 loss does not cooperate with p53 loss for tumor suppression in mice, but may have an impact on tumor spectrum
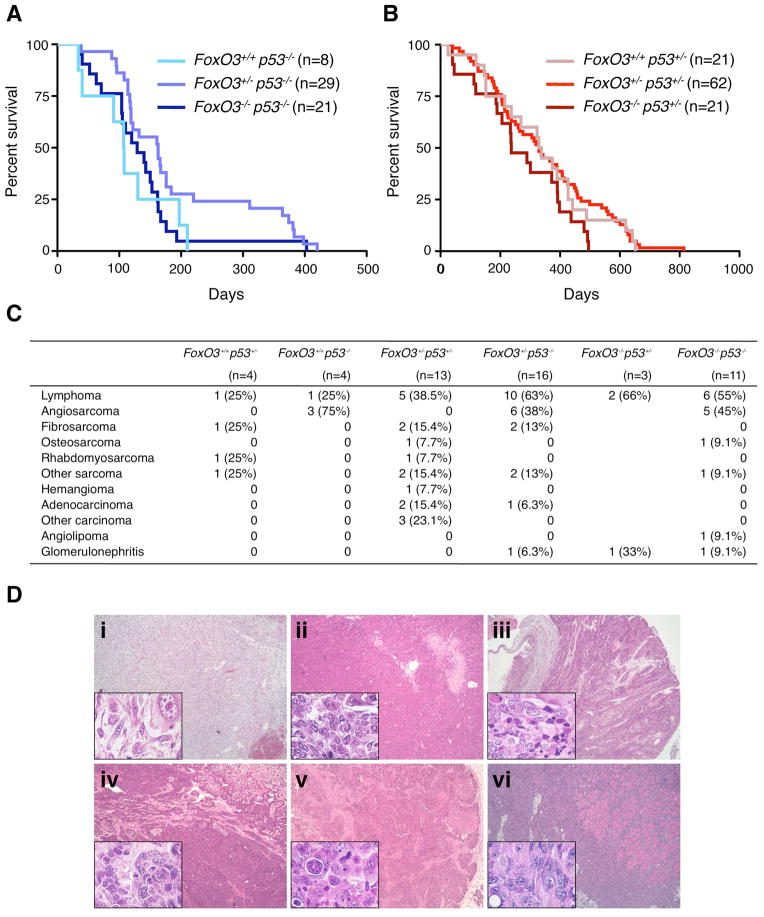
Our study and published findings indicate that FoxO3 and p53 interact in many different ways in cells. While the relevance of the interaction between FoxO3 and p53 in vivo has been tested in a mouse lymphoma model (Bouchard et al., 2007), it has not been assessed in the absence of oncogenic stimulation. Loss of one or both p53 alleles in mice results in predisposition to cancer and death at an early age (Donehower et al., 1992; Jacks et al., 1994). In contrast, FoxO3−/− mice only develop tumors at a very low frequency, and tumor development in FoxO3−/− mice manifests late in life (Paik et al., 2007), probably due to the redundancy of FoxO family members. To test if FoxO3 and p53 interacted in vivo for overall survival and tumor survival, we generated a cohort of compound FoxO3/p53 mutant mice in a mixed FVB/N-129Sv/J background and monitored overall survival in these mice (Figure 8). Loss of one or both alleles of FoxO3 did not accelerate the mortality rate of p53−/− mice in this genetic background (Figure 8A). Similarly, loss of one or both alleles of FoxO3 did not significantly affect the survival rate of p53+/− mice (Figure 8B), indicating that FoxO3 and p53 loss do not cooperate to diminish overall survival in vivo. This result further suggests that a model lacking an activated oncogene may not be sufficient to reveal FoxO3 interaction with p53 for tumor suppression. Alternatively, the absence of synergy between FoxO3 and p53 loss for tumor development could be due to the fact that both molecules are the in same genetic pathway. Histopathological analysis on a subset of FoxO3/p53 compound mutant mice revealed that the loss of FoxO3 in the context of p53−/− mice resulted in the appearance of tumors such as adenocarcinomas and angiolipomas (Figure 8C–D). As these tumor types are rarely seen in p53−/− mice in the same genetic background (Sharpless et al., 2002), this result is consistent with the notion that FoxO3 loss may impact the tumor spectrum of p53-deficient mice. Collectively, these findings suggest that FoxO3 and p53 are part of a common transcriptional network that may affect cellular and organismal responses that are important to counter aging and cancer.
Figure 8. FoxO3 loss does not affect survival in mice that are lacking one or both p53 alleles, but may alter tumor spectrum.
(A). Percent survival of mice with different alleles of FoxO3 in the p53−/− background as a function of time. Kaplan-Meier survival curves with the number of mice indicated for each genotype. p = 0.10, logrank test. (B) Percent survival of mice with different alleles of FoxO3 in the p53+/− background as a function of time. Kaplan-Meier survival curves with the number of mice indicated for each genotype. p = 0.13, logrank test. (C) Tumor types and glomerulonephritis in mice with different alleles of FoxO3 in the p53+/− and p53−/− background. The number of mice is indicated for each genotype. (D) Examples of sarcomas and carcinomas in compound FoxO3/p53 mutant mice. Main panels: 50x. Insets: 630x. i: subcutaneous fibrosarcoma in a FoxO3+/−p53−/− mouse; ii: osteosarcoma in the leg of a FoxO3−/−p53−/− mouse; iii: colon carcinoma in a FoxO3+/−p53−/− mouse; iv: uterine carcinoma in a FoxO3+/−p53+/− mouse; v: breast carcinoma in a FoxO3+/−p53+/− mouse; vi: muscle carcinoma in the arm of a FoxO3+/−p53+/− mouse.
Discussion
Our results indicate that p53 transactivates the expression of the FoxO3 gene by binding to a site located in the second intron of the FoxO3 gene. The regulation of FoxO3 gene expression is likely an important mechanism for the generation of a pool of FoxO3 molecules that could be made active or inactive by post-translational modifications. Our data indicates that DNA damage upregulates the expression of FoxO3 mRNA. FoxO3 gene expression has been shown to be upregulated in response to a number of other environmental stimuli, including nutrient deprivation, growth factor deprivation, and hypoxia (Bakker et al., 2007; Essaghir et al., 2009; Furuyama et al., 2002; Imae et al., 2003), raising the possibility that p53 might transduce the expression of FoxO3 in response to some of these stimuli. The induction of FoxO3 mRNA by hypoxia in MEFs is dependent on hypoxia inducible factor 1 (HIF1 alpha) (Bakker et al., 2007), although whether HIF1 directly binds to FoxO3 regulatory regions is not known. In addition, E2F1, a transcription factor involved in cell cycle progression and apoptosis, has been shown to upregulate FoxO3 mRNA in human neuroblastoma cell lines by binding to two conserved sites in the promoter of the human FoxO3 gene (Nowak et al., 2007). These observations raise the possibility that p53, HIF1, and E2F1 may all interact in controlling the expression of the FoxO3 gene.
The regulation of FoxO3 gene expression by p53 may be specific to a subset of tissues or cell types. FoxO3 and p53 are both expressed relatively ubiquitously, but may function more prominently in some tissues/cells versus others. A cell-type specific regulation has indeed been observed for the FoxO3 gene. For example, FoxO3 mRNA is upregulated by E2F1 in neuroblastoma cell lines and in U2OS cells, but not in HeLa cells, human diploid foreskin fibroblasts, or PC12 cells (Nowak et al., 2007). Similarly, FoxO3 mRNA expression is downregulated by growth factors in human AG01518 fibroblasts, but not in BJ-hTert fibroblasts (Essaghir et al., 2009). Whether the regulation of FoxO3 by p53 that we have identified in MEFs and thymocytes is observed in all cell types will be interesting to test. While our study was being completed, another study reported that in normal adult mouse liver, p53 could bind a response element 4 kb upstream of the FoxO3 transcriptional start site and transactivate FoxO3 mRNA expression (Kurinna et al., 2010). During hepatic regeneration, the binding of p53 to the binding site upstream of the FoxO3 gene was disrupted, leading to a decrease in FoxO3 mRNA expression. Consistent with our findings, p53-dependent activation of FoxO3 was also observed in MEFs and in mouse hepatoma cells overexpressing p53 (Kurinna et al., 2010). Interestingly, another member of the p53 family, p73, also binds to the same regulatory region in the promoter of the FoxO3 gene (Kurinna et al., 2010). Together with our study, these findings indicate that the p53 family of transcription factors regulates FoxO3 in a number of different cell types, by binding to at least two different binding sites in the FoxO3 gene, one 4kb upstream of FoxO3 transcriptional start site and one in the second intron of the FoxO3 gene (our study). It is possible that p53 occupancy at different sites is dependent on cell-type or specific environmental conditions.
p53 and FoxO3 interact at many levels: 1) p53 and FoxO3 proteins physically interact (Nemoto et al., 2004; Wang et al., 2008); 2) p53 and FoxO3 share common target genes (Jacobs et al., 2007; Riley et al., 2008; Tran et al., 2002; Vousden and Lu, 2002; Zhao et al., 2000); 3) FoxO3 stabilizes p53 protein (You et al., 2006b); 4) FoxO3 indirectly activates p53 by upregulating p19ARF, which inhibits the p53 ubiquitin ligase Mdm2 (Bouchard et al., 2007); 5) p53 indirectly inhibits FoxO3 activity by inducing SGK (You et al., 2004), or more directly, by inhibiting FoxO3 transcriptional activity (Miyaguchi et al., 2009) and by inducing FoxO3 degradation via Mdm2 (Fu et al., 2009); and 6) FoxO3 is a p53 target gene (this study and (Kurinna et al., 2010)). Thus, p53 and FoxO3 likely form a regulatory network that elicits appropriate cellular responses in response to stress stimuli. Negative and positive feedback loops within this network could be beneficial for triggering a finely tuned response to cellular stresses. The observation that p53 upregulates FoxO3 mRNA but also indirectly inhibits FoxO3 protein activity (Fu et al., 2009; Miyaguchi et al., 2009; You et al., 2004) suggests that co-incident signals may be needed to activate the FoxO3 molecules generated by p53-dependent transcription. Careful analysis of the kinetics of FoxO3 mRNA upregulation and FoxO3 protein activation in response to co-occurring signals will be required to tease apart the molecular links between p53 and FoxO3.
The presence of a binding site for p53 in the second intron of the FoxO3 gene is a feature that is conserved in the human genome. Mining the genome-wide chromatin immunoprecipitation data available for p53 (Wei et al., 2006) revealed that p53 binds to FoxO3 second intron in the human genome in human HCT116 cells. These observations suggest that the binding of p53 in the second intron of the FoxO3 gene may be crucial for the regulation of FoxO3 gene expression in mammals. Binding sites in introns have been reported previously for a number of p53 target genes, including TRAIL (Takimoto and El-Deiry, 2000), GADD45 (Kastan et al., 1992; Smith et al., 1994) and Mdm2 (Juven et al., 1993; Wu et al., 1993). Thus, p53 binding sites in introns may act as enhancers. Particularly notable are the recent findings that the second intron of the human FoxO3 gene contains single nucleotide polymorphisms (SNPs) associated with extreme longevity in human centenarians from Japanese, German, and Italian descent (Anselmi et al., 2009; Flachsbart et al., 2009; Willcox et al., 2008). Although the causative SNP associated with longevity in the FoxO3 gene has not been located yet, these observations raise the intriguing possibility that sequence variations in the second intron of the FoxO3 gene may be important for human longevity, perhaps by leading to subtle differences in transcription factor binding in this region, ultimately affecting FoxO3 mRNA levels.
Given that FoxO3 and p53 share so many common functions, it is surprising to note that these two molecules have been reported to have antagonistic roles on organismal lifespan. Indeed, FoxO factors extend lifespan in invertebrates (Giannakou et al., 2004; Henderson and Johnson, 2001; Hwangbo et al., 2004), and mutants of the insulin/IGF-1 receptor – which lead to FoxO activation – also display an extended lifespan in mice (Bluher et al., 2003; Holzenberger et al., 2003). In contrast, p53 activity appears to promote aging in worms and flies (Arum and Johnson, 2007; Bauer et al., 2005). Interestingly, ectopic expression of p53 decreased lifespan in male flies, but increased lifespan in female flies (Shen and Tower, 2010). In a FoxO-null background, p53 no longer shortened the lifespan of males, but still extended the lifespan of females, suggesting that FoxO plays a differential role in males and females downstream of p53 (Shen and Tower, 2010). Increased p53 activity has also been shown to elicit premature aging in mice (Maier et al., 2004; Tyner et al., 2002), although p53 can actually prolong mouse lifespan in the context of p19ARF expression (normally regulated by p53?) (Matheu et al., 2007). The molecular bases for the differences between FoxO3 and p53 in regulating lifespan are not known, but understanding of the connections between these two molecules should give crucial insights into the mechanisms that regulate longevity.
Materials and methods
Constructs
A bacterial artificial chromosome (BAC) containing the full-length mouse FoxO3 gene was purchased from BACPAC. The 500 bp regions surrounding the four putative p53 binding sites in the FoxO3 regulatory region were amplified by PCR and were sub-cloned into the pGL3 vector with the minimal SV40 promoter (SV40-pGL3). The mutated p53-4 binding site was generated by site-directed mutagenesis (Quickchange) using the following primers: Forward 5′-GGAGGGTCCTGGGTAAATTTTGGGTATACCCAGATGAGTAG-3′ and Reverse 5′-CTACTCATCTGGGTATACCCAAAATTTACCCAGGACCCTCC-3′. The mutated fragment was entirely sequenced and sub-cloned into SV40-pGL3. The E1A plasmid was described previously (Lowe and Ruley, 1993). The “Pan” FoxO shRNA construct was generated by subcloning the following primers (Hribal et al., 2003) into the pSicoR (PSR) lentiviral expression vector between the HpaI and XhoI sites (Ventura et al., 2004): Forward 5′-TGGATAAGGGCGACAGCAACTTCAAGAGAGTTGCTGTCGCCCTTATCCTTTT TTC-3′ and Reverse 5′-TCGAGAAAAAAGGATAAGGGCGACAGCAACTCTCTTGAAGTTGCTGTCGCC CTTATCCA-3′
Antibodies
Antibodies to human FoxO1, FoxO4, FoxO4, and mouse FoxO6 were generated by immunizing rabbits with a fusion protein between GST and each FoxO family member and purified by affinity (Quality Controlled Biochemicals). The FoxO3 antibody was used previously (Greer et al., 2007; Renault et al., 2009). The FoxO6 antibody was used previously (de la Torre-Ubieta et al., 2010). The antibody to Mek1 was described previously (Lenormand et al., 1993). Antibodies to p53 were obtained from Oncogene Science (Ab1), from Novocastra Laboratories or from Vector Labs (CM5), and from Santa Cruz (DO-1, SC-126X; pAb 1801, S-98X). Antibodies to p21Cip1 and β-actin were purchased from Santa Cruz and Novus Biological, respectively. Antibodies to cleaved caspase 3 and to BrdU were purchased from Cell Signaling and Technology and from AbD Serotec, respectively.
Thymocyte extraction
Thymocytes were extracted from 6–10 week-old mice of pure 129/Sv or mixed 129/Sv x C57/Bl6 background as described (Ihrie et al., 2003). The following genotypes were compared: p53+/+ versus littermate p53−/− or p53LSL-WT mice, which result in complete p53-deficiency. Briefly, mice were euthanized and the thymi of the mice were removed and placed in PBS on ice. Each thymus was passed through a 40 μm nylon cell strainer (BD Falcon) and divided into 2 plates (1 treated and 1 untreated). Thymocytes were then γ-irradiated with 10 Gy using a 137Cs irradiator and RNA was isolated 3 hours later using Trizol (Invitrogen).
Real-time PCR
One μg of total RNA was reverse transcribed with Random hexamers using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol or using Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen). Real-time PCR was performed on a Bio-rad iCycler using iQ SYBR green (Bio-rad) with the following forward and reverse primers:
-
FoxO3 F: AGT GGA TGG TGC GCT GTG T
R: CTG TGC AGG GAC AGG TTG T
-
Mdm2 F: AGC GCA AAA CGA CAC TTA CA
R: ACA CAA TGT GCT GCT GCT TC
-
p21Cip1 F: CAC AGC TCA GTG GAC TGG AA
R: ACC CTA GAC CCA CAA TGC AG
-
FoxO1 F: ACG AGT GGA TGG TGA AGA GC
R: TGC TGT GAA GGG ACA GAT TG
-
FoxO4 F: GGT GCC CTA CTT CAA GGA CA
R: GGT TCA GCA TCC ACC AAG AG
-
FoxO6 F: TGC CCT ACT TCA AGG ATA AAG G
R: CAG CTG CTT CTT GCT CG
-
p27Kip1 F: GGA CCA AAT GCC TGA CTC GT
R: CGC TTC CTC ATC CCT GGA C
-
Bim F: TCC TGT GCA ATC CGT ATC TCC
R: CGC AAG CTT CCA TAC GAC AGT
-
Gapdh F: TGT GTC CGT CGT GGA TCT GA
R: TTG CTG TTG AAG TCG CAG GAG
The experiments were conducted in triplicate and the results were expressed as 2−(Gene of interest number of cycles−β actin number of cycles).
Northern-blot
MEFs were isolated at embryonic day 13.5 from mice of different genotypes (p53LSL-WT, p53LSL-25,26, and p53LSL-VP16). MEFs were infected by adenovirus expressing the Cre recombinase (University of Iowa) at a multiplicity of infection (MOI) of 100 for 24 hours, as described (Johnson et al., 2005). RNA was isolated using the TRIZOL protocol. For Northern-blot experiments, 15 μg of RNA was resolved on a denaturing agarose gel and transferred onto a nylon membrane. Prehybridization and hybridization were performed using ExpressHyb hybridization solution (Clontech). Probes were prepared using a Prime-It II Random Primer Labeling Kit (Stratagene). A KpnI-NotI fragment of the mouse FoxO3 cDNA was used as a probe. The probe for p21Cip1 was described (Attardi et al., 2000).
Western-blot
Cells were lysed in lysis buffer (Tris HCl pH 8.0 (50 mM), NaCl (100 mM), EGTA (2 mM), NaF (10 mM), β-glycerophosphate (40 mM), Triton X-100 (0.4%), aprotinin (10 μg/ml), phenylmethylsulfonyl fluoride (PMSF, 1 mM)). Fifty μg of protein extract was resolved by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies and the primary antibody was visualized using HRP-conjugated anti-mouse or anti-rabbit secondary antibodies and enhanced chemiluminescence (ECL, Amersham).
Chromatin immunoprecipitation with p53 antibodies
MEFs were seeded in 15-cm dishes at a density of 105 to 4×105 cells per ml. Twelve to twenty-four hours after plating, cells were stimulated with doxorubicin (0.2 μg/ml) for 18–20 hours (Figure 4B) or 6 hours (Figure 4C). Cells were cross-linked with formaldehyde (1%) for 10 minutes and incubated with glycine (0.125M) for 5 minutes. Cells were washed with PBS and lysed in the Farnham lysis buffer (5 mM PIPES, 85 mM KCl, 0.5% NP-40, protease inhibitor cocktail (#11697498001, Roche Laboratory)). Nuclear extracts were collected by centrifuging at 2000 rpm for 5 minutes. The cell nuclei were resuspended in RIPA buffer (1 x PBS, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS supplemented with Roche protease inhibitor cocktail). Chromatin was sonicated 5–8 times, for 30 s each time, with a Sonics VirCell 130 sonicator equipped with a stepped microtip. The chromatin was flash-frozen in liquid nitrogen and an aliquot was used to verify that sonication was effective. Antibodies to p53 (Figure 4B: DO-1 and pAb 1801 from Santa Cruz, 2.5 μg each; Figure 4C: CM5 from Novocastra, 25 μl per reaction) or IgG control (Zymed Laboratory or Sigma) were coupled to rabbit secondary antibody coated Dynal Magnetic beads (Invitrogen) in PBS + 5 mg/ml BSA overnight at 4°C. Chromatin extracts were pre-cleared on beads and then incubated with the beads coupled to the p53 antibody overnight at 4°C. The beads were washed twice in 1 ml low-salt ChIP buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 150 mM NaCl), three times in 1 ml high-salt ChIP buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, 500 mM NaCl), four times with 1 ml LiCl ChIP buffer (0.25M LiCl, 1% IGEPAL CA630, 1% deoxycholic acid (sodium salt), 1 mM EDTA, 10 mM Tris, pH 8.1), and once or twice in 1 ml TE buffer. The chromatin complex was eluted in 200–300 μl of IP elution buffer (1% SDS, 0.1 M NaHCO3) at 65oC overnight. The DNA was purified by phenol/chloroform/isoamylalcohol and by PCR purification columns (Qiagen). Quantitative PCR was performed in triplicate using SYBR green (Bio-rad or SA-Biosciences) and a 7900HT Fast Real-Time PCR machine (Applied Biosystems) or a Bio-rad iCycler on 2.5 μl of eluted DNA using the following sets of primers:
Mouse negative control forward primer: GGG GGA TAA TGA TTG CAA AA
Mouse negative control reverse primer: GCG TGG ACA GAG ATC TAG GC
p53-1 forward primer: CCT AAT GCC ACA GCA GAA CTC ATC
p53-1 reverse primer: TGG GAA TGG AAC TCA GTC AGT GC
p53-4 forward primer: GGG TGG GGG ATT CTT TTC ACT C
p53-4 reverse primer: CGA GGT AAG CCA GCA CAT ACA AAT G
p21 forward primer: GAGACCAGCAGCAAAATCG
p21 reverse primer: CAGCCCCACCTCTTCAATTC
For each primer set, a standard curve was established using a 5 to 10-fold dilution series.
Luciferase assays
MEFs were cultivated in DMEM supplemented with 10% FBS (Invitrogen) and antibiotics (50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM glutamine). For luciferase assays, MEFs were seeded at 35,000 cells/well in 24 well plates. One hundred and twenty-five ng of vector encoding the luciferase reporter under different promoters was transfected together with 62.5 ng of a vector expressing Renilla Luciferase to control for variation in transfection efficiency. Two days after transfection, cells were lysed and firefly luciferase and Renilla luciferase were measured according to the Promega protocol.
Lentiviral infection of MEFs
Lentiviruses were produced by transfection of 293T cells with the PSR lentiviral vectors and the helper plasmids 8.2 and VSVg. MEFs were plated at 105 cells/ml in 10 cm plates and 0.45 μm filtered 293T supernatant was applied 24 hours later and the infection was repeated 2–3 times in the presence of Polybrene (8 μg/ml). Puromycin (5 μg/ml) was added to select MEFs that were infected with the virus. On day 5 post-infection, MEFs were used for BrdU or western-blot assays.
BrdU incorporation assays
FoxO3+/+p53+/+, p53−/−, or FoxO3−/− MEFs were seeded in coverchambers at 300,000 cells/ml. Cells were stimulated with doxorubicin (0.2 μg/ml) or Nutlin (10 μM) for 24 hours, unless otherwise noted. Bromodeoxyuridine (BrdU) was added for the last 16 hours and the cells were fixed in 4% formaldehyde for 10 minutes and permeabilized in 0.4 % Triton for 30 minutes. Coverslips were incubated with 2 N HCl for 30 minutes, and washed extensively with PBS. Non-specific antibody binding sites were blocked by incubation with PBS containing 10% goat serum and 7.5% BSA. Coverslips were then incubated with primary antibodies (rat anti-BrdU, 1:500) for 2 hours, and washed five times with PBS. Cells were then incubated for 1 hour with a secondary antibody (goat anti-rat Alexa 488, 1:400). Coverslips were mounted in Vectashield containing DAPI and examined under epifluorescent illumination using a Zeiss microscope digital camera with AxioVision 4 software. For quantification, at least 250–400 cells per coverslip were counted in a blinded manner. The ratio of BrdU-positive nuclei over the total number of nuclei was calculated.
Apoptosis assays in E1A-transformed MEFs
Phoenix cells were plated at 200,000 cells per ml in 10 cm plates and transfected with 10 μg of the E1A-encoding plasmid with the calcium phosphate method. Phoenix cell supernatant was filtered through 0.45 μm filters onto MEFs (70,000 cells per ml in 10 cm plates) in the presence of Polybrene (8 μg/ml) and the infection was repeated once. Infected MEFs were selected 24 hours after the last infection with selection media containing 250 μg/ml Hygromycin. E1A-transformed MEFs were seeded on coverslips at 200,000 cells per well. 24 hours later, MEFs were stimulated with Nutlin (10 μM) for 8 hours in the presence or absence of serum in the medium. Cells were fixed in 4% formaldehyde for 10 minutes and permeabilized in 0.4 % Triton for 30 minutes. Non-specific antibody binding sites were blocked by incubation with PBS containing 10% goat serum and 7.5% BSA. Coverslips were then incubated with primary antibodies (rat anti-cleaved caspase 3, 1:500) for 2 hours, and washed five times with PBS. Cells were then incubated for 1 hour with a secondary antibody (goat anti rat Alexa 555, 1:400). Coverslips were mounted in Vectashield containing DAPI and examined under epifluorescent illumination using a Zeiss microscope digital camera with AxioVision 4 software. For quantification, at least 250–400 cells per coverslip were counted in a blinded manner. The proportion of cleaved caspase 3-positive nuclei over the total number of nuclei was calculated.
Mouse crosses and survival curves
Aging cohorts were produced by three different mating strategies: i) FoxO3−/−p53+/− (male) and FoxO3+/−p53−/− (female), ii) FoxO3+/−p53+/− and FoxO3+/−p53+/− and iii) FoxO3+/−p53+/+ and FoxO3+/+p53+/−. Twenty-one FoxO3−/−p53−/−, 29 FoxO3+/−p53−/− and 8 FoxO3+/+p53−/− mice were generated. Twenty-one FoxO3−/−p53+/−, 62 FoxO3+/−p53+/− and 21 FoxO3+/+p53+/− mice were generated. The cohorts were on a mixed FVB/N and 129Sv/J background. Animals were genotyped by PCR and allowed to age at a maximum of 5 mice per cage with standard chow and water ad libitum in a standard light-day cycle. Mice were monitored once to twice a week and were sacrificed by CO2 asphyxiation and scored as a death in survival analysis when moribund or if external tumors exceeded 1 cm in diameter. Survival analysis was performed using Kaplan-Meier curves with the logrank test.
Histopathology
All tissues, except skin and bone marrow, were fixed in 10% formaldehyde for 24 to 48 hours. Bones from the head, legs and rib cage were fixed in Bouin’s fixative for 1 week before being decalcified for 48 hours. Tissues were embedded in paraffin, sectioned in 5-μm sections, dewaxed and stained with hematoxylin and eosin at the Comparative Medicine Department, Stanford Medical School. Tumors were identified in a blinded manner.
Acknowledgments
We thank Dr. Ron DePinho for his generous gift of the FoxO3−/− mice. We thank Julien Sage for critical discussion, reading of the manuscript, help with tumor analysis, and for taking the pictures for Figure 8D. We thank Jamie Brett for reading the manuscript. We thank Pauline Chu (Comparative Medicine Department, Stanford Medical School) for her help in processing the histopathology samples. We thank Dan Calnan and Phil Oskoui for participating in earlier aspects of this work. This work was supported by NIH R01 AG026648 and a McCormick Award for Women in Science to A.B. V.M.R. received support from the Dean’s Fellowship at Stanford University.
Footnotes
Authors’ contributions
V.M.R. designed, performed, and analyzed the lifespan and tumor spectrum of compound FoxO3/p53 mice (Figure 8). P.U.T completed Figure 1B, Figure 5B, Figure 6B, Figure 6E, Figure 7A and helped with Figure 2 and Figure 6D. K.L.H. completed Figure 5D, Figure 5E, Figure 6A, Figure 6B, and Figure 6C. J.L.W. completed Figure 2, Figure 6D, and helped with Figure 3 and Figure 7B. C.A.B. completed Figure 3 and Figure 7B. D.K.B. completed Figure 5C. O.S.V. initiated this project as her Honors Thesis and completed Figure 1A. T.M.J. completed Figure 4B. P.R.O. completed Figure 6F. Z.X. and E.E.S. identified p53 binding sites in FoxO3 regulatory regions (Figure 5A) under the supervision of M.Q.Z. H.V. performed the tumor identification (Figure 8B). L.D.A. supervised C.A.B., D.K.B. and T.M.J. and provided ideas. A.B. supervised V.M.R., P.U.T., K.L.H., J.L.W. and O.S.V. The manuscript was written by A.B., V.M.R., and O.S.V., with input from L.D.A.
Conflict of interest
We declare that there are no competing financial interests in relation to the work described.
References
- Anselmi CV, Malovini A, Roncarati R, Novelli V, Villa F, Condorelli G, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- Arum O, Johnson TE. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J Gerontol A Biol Sci Med Sci. 2007;62:951–9. doi: 10.1093/gerona/62.9.951. [DOI] [PubMed] [Google Scholar]
- Attardi LD, Reczek EE, Cosmas C, Demicco EG, McCurrach ME, Lowe SW, et al. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000;14:704–18. [PMC free article] [PubMed] [Google Scholar]
- Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–53. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Bauer JH, Poon PC, Glatt-Deeley H, Abrams JM, Helfand SL. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr Biol. 2005;15:2063–8. doi: 10.1016/j.cub.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–4. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Lee S, Paulus-Hock V, Loddenkemper C, Eilers M, Schmitt CA. FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 2007;21:2775–87. doi: 10.1101/gad.453107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol. 2002;156:817–28. doi: 10.1083/jcb.200112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- de la Torre-Ubieta L, Gaudilliere B, Yang Y, Ikeuchi Y, Yamada T, DiBacco S, et al. A FOXO-Pak1 transcriptional pathway controls neuronal polarity. Genes Dev. 2010;24:799–813. doi: 10.1101/gad.1880510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G, Chen LC, Schott DR, Thor A, Bhargava V, Ljung BM, et al. Loss of heterozygosity and p53 gene mutations in breast cancer. Cancer Res. 1994;54:499–505. [PubMed] [Google Scholar]
- Dijkers PF, Medemadagger RH, Lammers JJ, Koenderman L, Coffer PJ. Expression of the pro-apoptotic bcl-2 family member bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–21. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1:45–9. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284:10334–42. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci U S A. 1997;94:6048–53. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A. 2009;106:2700–5. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–4000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–4. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, et al. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991;88:5413–7. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey M, McArthur MJ, Montgomery CA, Jr, Bradley A, Donehower LA. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 1993a;7:938–43. doi: 10.1096/fasebj.7.10.8344491. [DOI] [PubMed] [Google Scholar]
- Harvey M, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993b;5:225–9. doi: 10.1038/ng1193-225. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–80. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–7. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hribal ML, Nakae J, Kitamura T, Shutter JR, Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol. 2003;162:535–41. doi: 10.1083/jcb.200212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–37. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gersham B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–6. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Reczek E, Horner JS, Khachatrian L, Sage J, Jacks T, et al. Perp is a mediator of p53-dependent apoptosis in diverse cell types. Curr Biol. 2003;13:1985–90. doi: 10.1016/j.cub.2003.10.055. [DOI] [PubMed] [Google Scholar]
- Imae M, Fu Z, Yoshida A, Noguchi T, Kato H. Nutritional and hormonal factors control the gene expression of FoxOs, the mammalian homologues of DAF-16. J Mol Endocrinol. 2003;30:253–62. doi: 10.1677/jme.0.0300253. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jacobs SB, Basak S, Murray JI, Pathak N, Attardi LD. Siva is an apoptosis-selective p53 target gene important for neuronal cell death. Cell Death Differ. 2007;14:1374–85. doi: 10.1038/sj.cdd.4402128. [DOI] [PubMed] [Google Scholar]
- Johnson TM, Hammond EM, Giaccia A, Attardi LD. The p53QS transactivation-deficient mutant shows stress-specific apoptotic activity and induces embryonic lethality. Nat Genet. 2005;37:145–52. doi: 10.1038/ng1498. [DOI] [PubMed] [Google Scholar]
- Johnson TM, Meade K, Pathak N, Marques MR, Attardi LD. Knockin mice expressing a chimeric p53 protein reveal mechanistic differences in how p53 triggers apoptosis and senescence. Proc Natl Acad Sci U S A. 2008;105:1215–20. doi: 10.1073/pnas.0706764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juven T, Barak Y, Zauberman A, George DL, Oren M. Wild type p53 can mediate sequence-specific transactivation of an internal promoter within the mdm2 gene. Oncogene. 1993;8:3411–6. [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–60. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Kurinna S, Stratton SA, Tsai WW, Akdemir KC, Gu W, Singh P, et al. Direct activation of forkhead box O3 by tumor suppressors p53 and p73 is disrupted during liver regeneration in mice. Hepatology. 2010;52:1023–32. doi: 10.1002/hep.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pagès G, L’Allemain G, Brunet A, Pouysségur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45 mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Coco F, Gaidano G, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. p53 mutations are associated with histologic transformation of follicular lymphoma. Blood. 1993;82:2289–95. [PubMed] [Google Scholar]
- Lowe SW, Ruley HE. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–45. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–48. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–19. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Wheeler LJ, Mathews CK, DeGregori J. p53 mediates senescence-like arrest induced by chronic replicational stress. Mol Cell Biol. 2007;27:5336–51. doi: 10.1128/MCB.01316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, et al. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–9. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Miyaguchi Y, Tsuchiya K, Sakamoto K. P53 negatively regulates the transcriptional activity of FOXO3a under oxidative stress. Cell Biol Int. 2009;33:853–60. doi: 10.1016/j.cellbi.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–8. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769:244–52. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–53. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009 doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan DC, Davidson AG, Summers CL, Warden HE, Doshi HM. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992;52:4828–31. [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–39. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Scott N, Sagar P, Stewart J, Blair GE, Dixon MF, Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991;63:317–9. doi: 10.1038/bjc.1991.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–23. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Alson S, Chan S, Silver DP, Castrillon DH, DePinho RA. p16(INK4a) and p53 deficiency cooperate in tumorigenesis. Cancer Res. 2002;62:2761–5. [PubMed] [Google Scholar]
- Shen J, Tower J. Drosophila foxo acts in males to cause sexual-dimorphism in tissue-specific p53 life span effects. Exp Gerontol. 2010;45:97–105. doi: 10.1016/j.exger.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, et al. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–80. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–43. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ, Jr, DiStefano PS, et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 2002;296:530–4. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;(Pt) doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, et al. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–37. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- Ventura A, Meissner A, Dillon CP, McManus M, Sharp PA, Van Parijs L, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101:10380–5. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Wang F, Marshall CB, Yamamoto K, Li GY, Plevin MJ, You H, et al. Biochemical and structural characterization of an intramolecular interaction in FOXO3a and its binding with p53. J Mol Biol. 2008;384:590–603. doi: 10.1016/j.jmb.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–19. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, et al. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105:13987–92. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–32. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- You H, Jang Y, You-Ten AI, Okada H, Liepa J, Wakeham A, et al. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc Natl Acad Sci U S A. 2004;101:14057–62. doi: 10.1073/pnas.0406286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006a;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc Natl Acad Sci U S A. 2006b;103:9051–6. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–82. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gish K, Murphy M, Yin Y, Notterman D, Hoffman WH, et al. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 2000;14:981–93. [PMC free article] [PubMed] [Google Scholar]