Abstract
Ear mesenchymal stem cells (EMSCs) represent a readily accessible population of stem-like cells that are adherent, clonogenic, and have the ability to self-renew. Previously, we have demonstrated that they can be induced to differentiate into adipocyte, osteocyte, chondrocyte, and myocyte lineages. The purpose of the current study was to characterize the growth kinetics of the cells and to determine their ability to form colonies of fibroblasts, adipocytes, osteocytes, and chondrocytes. In addition, the immunophenotypes of freshly isolated and culture-expanded cells were evaluated. From 1 g of tissue, we were able to isolate an average of 7.8 × 106 cells exhibiting a cell cycle length of ∼2–3 days. Colony-forming unit (CFU) assays indicated high proliferation potential, and confirmed previously observed multipotentiality of the cells. Fluorescence-activated cell sorting (FACS) showed that EMSCs were negative for hematopoietic markers (CD4, CD45), proving that they did not derive from circulating hematopoietic cells. The FACS analyses also showed high expression of stem cell antigen-1 (Sca-1) with only a minor population of cells expressing CD117, thus identifying Sca-1 as the more robust stem cell biomarker. Additionally, flow cytometry data revealed that the expression patterns of hematopoietic, stromal, and stem cell markers were maintained in the passaged EMSCs, consistent with the persistence of an undifferentiated state. This study indicates that EMSCs provide an alternative model for in vitro analyses of adult mesenchymal stem cells (MSCs). Further studies will be necessary to determine their utility for tissue engineering and regenerative medical applications.
Introduction
Adult mesenchymal stem cells (MSCs) are postnatal stem cells possessing abilities to self-renew and differentiate into multiple tissue phenotypes. The multipotential of these cells, their easy isolation and culture, as well as their high ex vivo expansive potential make them a promising source of cells in the field of regenerative medicine. The growing body of information regarding sources of primary MSCs indicates that MSC-like cells have been identified in a number of different tissues. MSCs have been isolated from adult peripheral blood [1], adipose tissue [2], skin tissue [3], fetal blood, liver and bone marrow [4], lung [5], intestinal tract [6], and kidney [7]. Cells that display morphology and characteristic features of stem cells have also been harvested from both inner [8,9] and outer ears [10,11]. Moreover, it has been shown that human auricular cartilage may be a good source of chondrocytes for in vitro production of cartilage implants [11], and has also been used in the clinical treatment of cartilage defects [10].
Previous studies in our laboratory showed that both the external murine ears [12] and ear punches obtained during standard procedure used for marking live animals [13] are a source of MSCs. Termed ear mesenchymal stem cells (EMSCs), these cells have the characteristics of stem cells including the ability to self-renew and to commit into adipocyte, osteocyte, chondrocyte, and myocyte lineages at the clonal level [12–14]. Moreover, cells isolated from the ears of regenerative (FOXN1-deficient—nude) and non-regenerative (wild-type) mice showed similar in vitro differentiation potentials, suggesting that EMSCs are necessary but not sufficient for the regeneration in ear punched model [12]. Immunophenotype analysis of the cells [13] revealed that EMSCs are positive for stem cell (stem cell antigen-1; Sca-1) and stromal markers (CD90, CD73, CD44), but negative for hematopoietic markers (CD45, CD4); however, the study was limited only to cells cultured at passage 0. The present study was undertaken to extend the characteristics of EMSCs. Growth kinetics, including doubling time and plating density, has been determined. Colony-forming unit (CFU) assays have been used to describe the frequency of cells with the capacity to differentiate along specific lineage pathways. Furthermore, analyses of cell surface protein expression have been done on both freshly isolated and cultured cells from passages 0 through 5.
Materials and Methods
Animals
The animal experimental protocols were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee in accordance with NIH guidelines. All procedures were designed to minimize the suffering of the experimental animals. C57BL/6J mice were housed in a temperature- and humidity-controlled room (22 ± 2°C and 30%–70%, respectively) with a 12-h light/12-h dark cycle (lights on at 6.00 am), and food and water were provided ad libitum. Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation.
Isolation of EMSCs and cell cultures
Primary cultures were prepared from outer ears of 3-to 6-week-old mice and subjected to both mechanical and enzymatic dissociation as described previously [12]. In brief, excised ears were minced and digested with collagenase type I (Worthington Biochemical, Freehold, NJ) for 1 h at 37°C with gentle agitation. The cell suspension was filtered through a 70-μm cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ), centrifuged (360g, 5 min, room temperature), and resuspended for 1 min in 1 mL red blood lysis buffer (Sigma Co., St. Louis, MO). After washing by centrifugation as described earlier, Trypan blue exclusion and Cellometer Auto T4 system (Nexcelom Bioscience LLC, Lawrence, MA) were used to determine the vitality, size, and the total number of nucleated cells. The isolated cells were plated in 100-mm Petri dishes (passage 0; P0) in Dulbecco's modified Eagle medium (DMEM/F12; Invitrogen, Carlsbad, CA) supplemented with 1% antibiotic solution and 15% fetal bovine serum (FBS; Invitrogen). The culture was kept in a humidified 5% CO2 incubator at 37°C, and next day non-adherent cells were removed by changing medium. The growth medium was changed every 2–3 days.
Cell-doubling method
Cell-doubling time was performed on EMSCs from cultured cells from passages 0 through 6. The cells were plated in 24-well plates at a density of 105 nucleated cells/well using the expansion medium described earlier. Fresh medium was supplied every 2–3 days. At 70%–90% confluence, cells were passaged by digestion with 0.05% trypsin/0.53 nM EDTA (Life Technologies, New York), counted with a hemocytometer, and reseeded as passage 1 (P1). Subsequent passages (P1–P6) were allowed to multiply until 70%–90% confluence was reached. Cell-doubling times (DT) were calculated according to the following formula [15]:
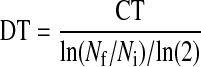 |
where DT is the cell-doubling time, CT the cell culture time, Nf the final number of cells, and Ni the initial number of cells.
Isolation of bone marrow-derived mesenchymal stem cells (BMSCs)
Bone marrow-derived mesenchymal stem cells (BMSCs) were freshly isolated from femurs and tibias by capping the bones. The bone marrow was flushed out using the syringe filled with PBS, filtered through a 70-μm nylon mesh, centrifuged, and resuspended in growth medium (DMEM supplemented with 20% FBS and penicillin/streptomycin). The cells were plated in 75-cm2 flasks, and kept at 37°C in a humidified atmosphere containing 95% air and 5% CO2. About 24 h after plating, supernatant containing nonadherent cells was removed, and fresh medium was added. When primary cultures became ∼70%–80% confluent, BMSCs were harvested for the migration assay.
Plating density
EMSCs at passage 1 were plated in 6-well plates at densities of 0.5 × 103, 1 × 103, 5 × 103, 10 × 103, and 50 × 103. The expansion medium was replaced every 2–3 days until cells reached 80%–90% confluency. The cells were counted in duplicate using a hemocytometer, and a doubling time for each cell density was computed as described earlier.
Colony-forming unit (CFU) assays
EMSCs at passage 1 were used to quantify CFU for fibroblasts (CFU-F), and cells capable of differentiating into adipocytes, osteoblasts, and chodrocytes (CFU-Ad, CFU-Ob, CFU-Ch, respectively). Cells were seeded in T25 flasks at the densities of 101.5, 102, 102.5, 103, and 103.5 per flask. The flasks were incubated at 37°C in a 5% CO2 humidified incubator. The cells were fed the expansion medium for a total of 11 days to establish colonies. Medium was changed every 2–3 days. After colonies were established, 5–6 flasks at each cell density were fixed as outlined below for CFU-F assay, while remaining cells were maintained in culture with adipocyte, osteogenic, or chondogenic differentiation medium for CFU-Ad, CFU-Ob, or CFU-Ch assay, respectively, as described below. At the conclusion of the studies, the number of positive colonies under each cell density was determined. Positive staining was defined as a colony that contained at least 20 positive cells (CFU-F, CFU-Ad) or 1 bone or cartilage nodule (CFU-Ob, CFU-Ch, respectively). These values were used to compute the percentage of CFU progenitors among the originally seeded cells. Images of formed colonies were taken using Nikon CoolSnap camera (Nikon Instruments Inc., New York), and stored with Metamorph imaging software (Molecular Devices Corp, Sunnyvale, CA).
For CFU-F assay, the cells were rinsed twice with pre-warmed phosphate-buffered saline (PBS), fixed in 10% formalin for 20 min at room temperature, followed by 1 h staining with 0.1% Toluidine blue in 1% paraformaldehyde in PBS. Cells were gently rinsed with tap water. Aggregates of >20 Toluidine blue staining cells were counted as a positive CFU-F using a phase contrast microscope.
For CFU-Ad assay, formation of mature adipocytes occurred following treatment of EMSCs with adipogenic induction medium containing DMEM/F12, 5% FBS, 1% antibiotic solution, 0.5 mM isobutylmethylxanthine, 1.7 μM insulin, and 1 μM dexamethasone for 2 days, followed by 7-day exposure to DMEM/F12 medium supplemented with 5% FBS, 1% antibiotic solution, 17 nM insulin, and 2 μM thiazolidinedione. Differentiated cells were fixed for 1 h in 10% formalin at room temperature and later stained for lipid accumulation for 20 min with Oil red O. Cells were washed 3 times with water; colonies containing >20 Oil red O-positive cells were observed and counted under a phase contrast microscope.
For CFU-Ob assay, established colonies were stimulated for 20 days by osteogenic induction medium—DMEM-F12 supplemented with 10% FBS, 200 μM ascorbic acid 2-phosphate, 10 nM dexamethasone, and 7 mM β-glycerophosphate. At the end of mineralization period, flasks were rinsed 3 times with 150 nM NaCl. Cells were fixed in 70% ethanol for 1 h at +4°C, and stained with 2% Alizarin red solution in distilled water for 10 min at room temperature. After washing, aggregates of >20 Alizarin red-positive cells were counted and imaged.
For CFU-Ch assay, EMSCs were induced in vitro by treating a monolayer culture with pro-chondrogenic cocktail as published previously [12]. In brief, cells were exposed to DMEM-F12 medium supplemented with 10% FBS, 200 μM ascorbic acid 2-phosphate, 100 nM dexamethasone, and transforming growth factor β1 (1 ng/mL). Differentiated cells were washed with PBS and stained with aqueous 0.1% Safranin O to identify glycosaminoglycans in the extracellular matrix of differentiated chondrocytes, followed by washing in 95% ethanol. Aggregates of >20 Safranin O-positive cells were counted and imaged.
Flow cytometry
Phenotypic characterization of freshly isolated and cultured EMSCs from passages 0 through 5 was performed by flow cytometry, as previously described [13]. In brief, cells were cryopreserved at concentration of 1–2 × 106 per mL in 20% FBS, 10% dimethylsulfoxide, and 70% DMEM/F-12 medium for period of ∼1 to 4 months before analysis. Two days before flow cytometry analysis, individual vials of cells were thawed in 37°C water bath, resuspended in 10 mL expansion medium, centrifuged, and plated in 100-mm Petri dishes. On the day of analysis, unattached cells in cultures were washed out with PBS, and adherent cells were trypsinized. After washing, cells were suspended in 0.5% BSA in PBS at a concentration of 4 × 104 per mL and incubated in blocking buffer (containing 25 μg/mL IgG) for 10 min followed by 40-min incubation with 10 μL (1 μg/10 μL) of phycoerythrin-conjugated anti-CD4, -CD44, -CD45, -CD90, -CD73, -CD117, and -Sca-1 antibodies (BD Pharmingen, San Diego, CA) on ice. Labeled cells were washed in PBS with 0.5% BSA and fixed in cold Cytofix™ buffer (BD Pharmingen, San Diego, CA). The assay was performed using a flow cytometer (Becton Dickinson, San Jose, CA), and data were analyzed with a Macintosh G5 workstation (Apple Computer, Cupertino, CA), which contains Cellquest graphics software (Becton Dickinson). The total number of cells counted for each sample was ∼10,000. Positive cells were identified by comparison with PE-conjugated mouse immunoglobulin (IgG1) and rat immunoglobulin (IgG2A, IgG2B) as isotype controls.
Migration assay
To investigate migration activity, a modified Boyden chamber assay was performed using a 24-transwell migration system (8 μm pores Falcon HTS 24-Multiwell inserts; BD Biosciences, San Jose, CA). In brief, the EMSCs and BMSCs were resuspended separately at 5 × 105 cells/mL in 300 μL of DMEM/F-12 or DMEM, respectively, supplemented with 0.1% BSA and penicillin/streptomycin, and seeded in the upper chamber. The 700 μL of the final dilution of the recombinant Stromal-Derived Factor-1α (SDF-1α) or Hepatocyte Growth Factor (HGF) (both obtained from Peprotech, Rocky Hill, NJ) were used as a chemoattractant in the lower chamber at concentrations of 1, 25, and 500 ng/mL. The transwells were incubated at 37°C for 3 h or 8 h. Control migration was performed without R-SDF-1α in the lower chamber. Following incubation, the filter was removed, and the cells on the filter were counted manually in random high-power fields (200×) in each well. The migration filters were then solubilized and analyzed using a FLUOstar OPTIMA (BMG Labtechnologies, Offenburg, Germany). The same procedure was repeated using HGF dilutions as the chemoattractant. All groups were studied in triplicate.
Statistical analysis
Values are expressed as mean ± SD. To determine significance among analyzed groups, one-way analysis of variance followed by Tukey's multiple comparison test was employed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). A value of P < 0.05 was considered statistically significant.
Results
Cell yield and growth characteristics of EMSCs
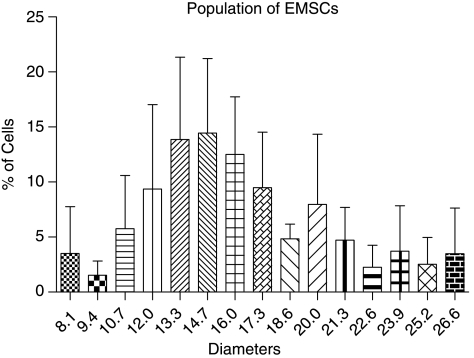
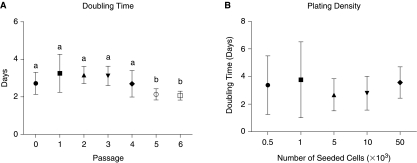
Outer ears obtained from mice were processed by collagenase digestion and differential centrifugation resulted in the harvest of 7.8 ± 0.8 × 106 nucleated cells/g of tissue (n = 3). The size of the cells (analyzed at passage 1) ranged from 8.1 to 26.6 μm in diameter (Fig. 1) with the major population (∼72%) between 12 μm and 20 μm. For the assessment of growth characteristics of EMSCs, the doubling time in culture and the plating density methods were used. For primary cells and subsequent passages up to passage 4, DT ranged between 2.7 ± 0.6 and 3.3 ± 1.0 days; no significant differences were observed (Fig. 2A). However, the DT decreased significantly, as the cells reached passages 5 and 6 (2.1 ± 0.3 and 2.1 ± 0.2, respectively). The growth kinetics was not influenced by plating density (Fig. 2B). A constant expansion rate was observed between densities with regard to time required for population doubling. The mean doubling time for all analyzed cell densities was 3.2 ± 0.2 days.
FIG. 1.
Size characterization of undifferentiated ear mesenchymal stem cells (EMSCs) analyzed at passage 1. All values reflect the mean ± standard deviation of cell diameters.
FIG. 2.
Growth kinetics of undifferentiated ear mesenchymal stem cells. (A) Cell-doubling time of cultured primary and passaged cells. (B) Influence of plating density on proliferative characteristics of the cells. All values reflect the mean ± standard deviation. Passages with different lower case letters are significantly different (P < 0.05).
CFU assays
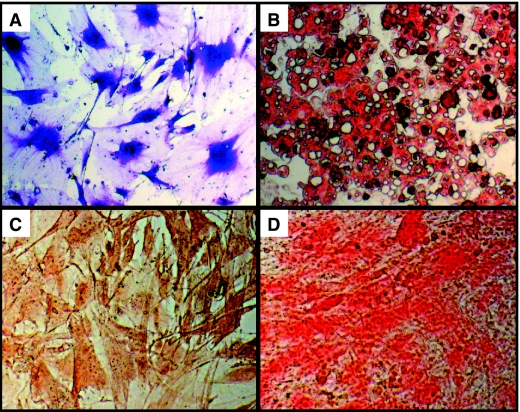
For CFU assays cells were seeded at increasing densities in T25 flasks. An exemplary image representing stained colonies is shown in Figure 3. CFU frequency for specific lineage phenotypes was determined based on histochemical staining characteristics. CFU-F assays (Table 1; Fig. 4A) determined that an adherent fibroblastic phenotype occurred in 11.8% ± 5.2% cells plated at the density of 101.5. With the increasing densities, the frequency of cells that form colonies decreased reaching a significant drop at densities ranged from 102.5 to 103.5. CFU-Ad (Table 1; Fig. 4B) and CFU-O (Table 1; Fig. 4C) colonies were formed only at densities ranged between 102.5 and 103.5. The mean lipid droplets accumulation did not change between analyzed densities, whereas in osteogenic cultures the highest number of nodule-like structures was observed at the density of 102.5. A similar pattern of frequency was determined for CFU-Ch (Table 1; Fig. 4D). In addition, EMSCs were able to form these colonies at the density of 102.
FIG. 3.

Colony-forming unit assay. The panel displays representative photomicrograph of cells seeded, expanded, and stained in T25 flasks.
Table 1.
The Summary of Frequencies of Colony-Forming Units With Fibroblastic Phenotype (CFU-F), Adipogenic (CFU-Ad), Osteogenic (CFU-Ob), and Chondrogenic (CFU-Ch) Capacity
| No. of cells | 101.5 | 102 | 102.5 | 103 | 103.5 |
|---|---|---|---|---|---|
| CFU-F | 11.82 ± 5.20a | 8.63 ± 3.90a,b | 6.12 ± 2.80a | 5.94 ± 2.40b | 4.94 ± 0.40b |
| CFU-Ad | n/d | n/d | 0.15 ± 0.25a | 0.23 ± 0.12a | 0.20 ± 0.06a |
| CFU-O | n/d | n/d | 0.79 ± 0.29a | 0.59 ± 0.13a,b | 0.37 ± 0.13a |
| CFU-Ch | n/d | 0.39 ± 0.41b | 1.49 ± 0.44a | 0.86 ± 0.34b | 0.48 ± 0.16b |
All values reflect the mean of percent ± standard deviation. Values with different superscript letters are significantly different (P < 0.05).
Abbreviation: n/d, non-detectable.
FIG. 4.
Colony-forming unit (CFU) assays and staining. The panels display representative photomicrographs for CFU-fibroblast detected by Toluidine blue staining (A), CFU-adipocyte detected by Oil red O staining (B), CFU-osteoblast detected by Alizarin red staining (C), and CFU-chondrocyte detected by Safranin O (D).
Surface protein expression by EMSCs
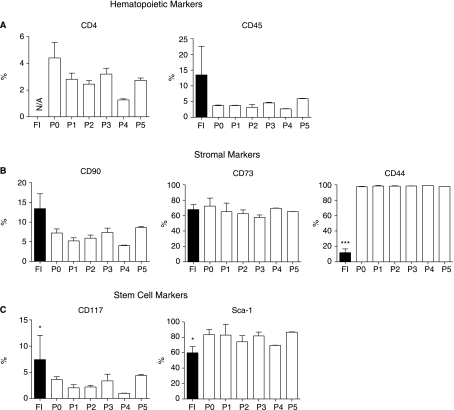
Flow cytometric analysis was performed on freshly isolated and cryopreserved cells after each stage of purification and passage (Fig. 5). All analyzed cells were positive for hematopoietic (Fig. 5A), stromal (Fig. 5B), and stem cell phenotypic markers (Fig. 5C). The EMSCs contained a small population of cells positive for hematopoietic markers (CD4, CD45). The greatest percentage of CD45-positive cells was observed in the freshly isolated fraction, whereas CD4 and CD45 were nearly undetectable in culture-expanded cells. The stromal cell-associated marker—CD90 and CD44—were expressed at low levels in freshly isolated cells (13.4% ± 3.8% and 12.0% ± 4.8%, respectively). However, with successive passages, the percentage of cells staining positive for CD90 decreased, whereas the percentage of CD44-positive cells increased significantly. The presence of CD73-positive cells—other stromal cell markers—did not change significantly through passage 5. Stem cell markers were represented in the study by CD117 and Sca-1. Only 7.4% ± 2.7% of freshly isolated cells expressed CD117 and the number of the positive cells declined with successive passages. In contrast, a large population of initial EMSCs expressed Sca-1 (60.0% ± 4.7%) and the number of Sca-1+ cells significantly increased with the culture.
FIG. 5.
Immunophenotype of freshly isolated (FI) and passaged (P0–P5) EMSCs. (A) Hematopoietic markers CD4 and CD45. (B) Stromal markers CD90, CD73, and CD44. (C) Stem cell markers CD117 and Sca-1. All values reflect the mean ± standard deviation. Asterisks indicate significant differences between freshly isolated and passaged cells (*P < 0.05; ***P < 0.001). Abbreviation: N/A, non-analyzed.
Migration assay
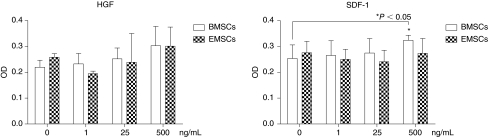
To further explore the characteristics of the EMSCs, their chemotaxis in response to growth factors was evaluated using a modified Boyden chamber migration assay in comparison to primary cultured BMSCs. Cells were seeded in the upper chamber of transwells and migration was monitored in response to increasing concentrations of the chemoattractants HGF or SDF-1α over periods of 3 h and 8 h under serum-free conditions (Fig. 6). After 3 h, the chemoattractants did not stimulate either BMSC or EMSC migration significantly (data not shown). Following an 8-h incubation, only the highest concentration of SDF-1α significantly increased the migration of BMSCs but not EMSCs across the transwell membrane; in contrast, HGF had no significant effect on either BMSC or EMSC migration at any concentration.
FIG. 6.
Migration assays. The chemotaxis of bone marrow derived mesenchymal stem cells (BMSCs; open) and ear mesenchymal stem cell (EMSCs; checkered) in response to increasing concentrations of hepatocyte growth factor (HGF) and stromal-derived factor-1α (SDF-1α) were determined after an 8-h incubation. Values are the mean ± standard deviation of triplicate assays. Asterisks indicate significant differences relative to control (0 ng/mL) concentrations of the growth factor (*P < 0.05).
Discussion
Since MSCs can be purified and expanded while retaining multipotent properties, they have been considered to be a suitable source of stem cells for therapy, regenerative medicine, and transplantation. Previously, we isolated fibroblast-like stromal/stem cells from mouse outer ears and documented their characteristics based on immunophenotype and multilineage differentiation potentials [12–14]. We showed that EMSCs can be isolated from regenerative FOXN1-deficient (nude) and non-regenerative (wild-type) strain of mouse. Morphological, histochemical, and molecular analysis after the induction of EMSC differentiation revealed multiple differentiation potentials in all studied murine strains independent of their ability for regeneration. We concluded that the absence of regeneration in wounded ears of wild-type mice is not related to the absence of MSC differentiation in tissue culture [12]. Like the FOXN1-deficient (nude) murine strain, the MRL/MpJ mice have been found to exhibit a comparable regenerative capacity [16]. Following a surgical wounding, the ears of these mice are able to spontaneously repair holes with a high degree of efficiency, consistent with a regenerative process [16]. Nevertheless, the characteristics of EMSCs in the MRL/MpJ mice have not been investigated to date.
The present study has extended the characterization of EMSCs by documenting their morphology, growth kinetics, and colony-forming unit frequency. Moreover, we have evaluated the expression patterns of hematopoietic, stromal, and stem cell markers in freshly isolated and cultured EMSCs as a function of progressive passage in vitro.
Bone marrow is considered the main source of both hematopoietic and MSCs. Protocols that have been developed for isolation of bone marrow MSCs yield 6.4 ± 3.4 × 106 nucleated cells/mL of aspirate from adult horses [17] and 2.33 ± 0.5 × 108 mononuclear cells per 20 mL aspirate from young adult pigs [18]. In our study, we were able to isolate an average of 7.8 × 106 nucleated cells from 1 g of ear tissue. Thus, the number of isolated cells obtained per unit weight of ear tissue is similar to that obtained from bone marrow; however, it is high in comparison to the yield from other soft tissue. Published data show 10-fold lower number of cells isolated from fat tissue samples of primates and pig [19–21].
Kinetic analyses revealed a stable doubling time in culture through passage 4 (∼3 days) with a significant drop reduction in doubling time at passages 4 and 5 (∼2 days). This time course is consistent with the other studies. Mouse bone marrow and lung mesenchymal progenitor cell populations demonstrate a cell cycle length of ∼2–3 days [22,23], whereas a complete cell cycle for adipose-derived stromal/stem cells takes ∼1.5–2.8 days in humans [2,19] and 3.3 in pigs [20]. Since cell seeding density is also considered as a factor that plays a role in the expansion capacity of MSCs, we determined doubling times of EMSCs in relation to cell plating density. Colter et al. observed that human adult stem cells from bone marrow stroma proliferate most rapidly if plated at low density [24,25]. In contrast to these data, our experiment did not indicate any dependency between seeding density and the length of the cell cycle.
In the present study, we report that ∼5%–12% of EMSCs form colonies of fibroblasts. We observed a reduction in frequency as a function of plating density; an increased density of seeded cells resulted in a reduced number of colonies. Nevertheless, a comparison of frequency between our report and data previously published is striking. The frequency of bone marrow-derived MSCs has been reported to be between 1 per 103 and 1 per 105 nucleated cells [17,26–29]. Similar frequency was also found in adult human liver stem cells [30]. Higher frequency (∼3%), which is similar to that obtained in our study, was found in cells obtained from human subcutaneous adipose tissue aspirates [31]. These variations for primary cell frequencies can result from biological features unique to a particular species and differences in the quantitative methods. Magnitude discrepancy can also be observed between individual subjects [17,28], and influenced by an age of donor [32,33]. Nevertheless, results obtained from CFU-F assay indicate the abundance and high proliferation potential of EMSCs. CFU-adipogenesis, -osteogenesis, and -chondrogenesis assays showed lower, compared to CFU-F frequency, density-related ability of EMSCs to form colonies. In contradiction to studies done on human bone marrow-derived adult stem cells showing that cells plated at lower density yield greater number of adipocytes [34], low density seeding in our study was not associated with improved differentiation potential. Instead, only cultures seeded at higher density were able to form the colonies of differentiated cells.
Flow cytometric analysis revealed changes in surface protein expression between freshly isolated and cultured cells. Previous study from our laboratory had defined the immunophenotype of plastic adherent EMSCs only at passage 0. In the present study, we analyzed cell surface protein expression of stromal, stem, and hematopoietic markers in freshly isolated and cultured cells expanded through passage 5. Our results showed that freshly isolated EMSCs were positive for hemapoietic marker (CD45), whereas passaged cells can be considered as CD45-negative. The higher expression of CD45 in primary cells might result from hematopoietic cell contamination; this finding has been previously observed during the culture and expansion of bone marrow-derived MSCs [35,36]. The lack of hematopoietic stem markers suggests that EMSCs did not derive from circulating hematopoietic cells. The fluorescence-activated cell sorting (FACS) analyses of stem cell markers carried out here demonstrate that EMSCs contain a major population of cells expressing Sca-1 in both freshly isolated and cultured cells, and a minor population of cells expressing CD117. The percentage of CD117-positive cells declined with passage expansion of the cells. Based on this observation, we consider EMSCs as CD117-negative cells, and conclude that Sca-1 will serve as a better “stem cell”-associated antigen in this tissue. A body of earlier studies shows that Sca-1 is expressed in a wide variety of tissues and organs [37–42]. Its expression varies from tissue to tissue and the population of cells expressing Sca-1 is considerably lower comparing to EMSCs. FACS analysis of stem cells in different tissues revealed that only 4.2% Sca-1 positivity was observed in bone marrow-derived cells [38], 15% in prostate [40], and 20% in mammary epithelial cells [37]. Among pancreatic cells only 9% of islet and 15% of ductal cells expressed Sca-1 [39,41]. A higher frequency of Sca-1-expressed cells (∼31%) was observed in murine adipose-derived adult stem cells [42]; however, even this remains lower than that currently identified among EMSCs. Summarizing our flow cytometry analyses, the present data revealed that expressions of hematopoietic, stromal, and stem cell markers remain unchanged in culture; thus taken together, immunophenotype characteristics of EMSCs suggest that passaged cells persist in an undifferentiated state.
Modified Boyden chamber assays revealed that the presence of either HGF or SDF-1α at concentrations of 500 ng/ mL failed to stimulate the chemotaxis and migration of the EMSCs. In contrast, the maximal concentration of SDF-1α induced the migration of BMSCs in parallel experiments. These findings are consistent with published reports documenting the ability of SDF-1 to stimulate migration of human BMSCs by up to 2-fold [43] and of SDF-1 and HGF to stimulate the migration of C2C12 myoblasts by 1.5- to 2-fold [44] relative to baseline levels. The passage of the EMSCs in culture could account for the current observations. Studies of human adipose-derived stromal cells (hADSC) have revealed that the SDF-1 receptor, CXCR4, was down-regulated as a function of successive passage [45]. Subsequent overexpression of CXCR4 increased the migratory response of the human ADSC by 2.5-fold when exposed to 500 ng/mL SDF-1 [45]. Further studies will be necessary to determine if the relative expression levels of the HGF receptor, c-met, and the SDF-1 receptor, CXCR4, on the murine BMSC and EMSC populations exhibit similar changes as a function of passage in vitro.
In conclusion, the current work documents the growth characteristics, lineage-specific frequency, and cell surface protein expression profile of multipotent MSCs isolated from mouse outer ears. Their features, along with availability and relative simple, noninvasive harvesting technique from living animals, make EMSCs an interesting model for studying adult MSC activity and differentiation. Further studies and greater effort must be made to determine whether these cells can be considered as an alternative and potential source of stem cells in regenerative medicine for repairing, replacing, or regenerating diseased tissues or organs.
Acknowledgments
This work was supported in part by National Institutes of Health; Grant 346 No. RO1 1 P20 RR021945 COBRE (all authors) and by a CNRU Center Grant #1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK (J.M.G.), as well as the Pennington Biomedical Research Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zvaifler NJ. Marinova-Mutafchieva L. Adams G. wards CJ. Moss J. Burger JA. Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 3.Chunmeng S. Tianmin C. Effects of plastic-adherent dermal multipotent cells on peripheral blood leukocytes and CFU-GM in rats. Transplant Proc. 2004;36:1578–1581. doi: 10.1016/j.transproceed.2004.05.079. [DOI] [PubMed] [Google Scholar]
- 4.Campagnoli C. Roberts IA. Kumar S. Bennett PR. Bellantuono I. Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 5.in't Anker PS. Noort WA. Scherjon SA. Kleijburg-van der Keur C. Kruisselbrink AB. van Bezooijen RL. Beekhuizen W. Willemze R. Kanhai HH. Fibbe WE. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multi-lineage differentiation potential. Haematologica. 2003;88:845–852. [PubMed] [Google Scholar]
- 6.Leedham SJ. Brittan M. McDonald SA. Wright NA. Intestinal stem cells. J Cell Mol Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekel B. Zangi L. Shezen E. Reich-Zeliger S. Eventov-Friedman S. Katchman H. Jacob-Hirsch J. Amariglio N. Rechavi G. Margalit R. Reisner Y. Isolation and characterization of nontubular sca-1+lin- multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 8.Lang H. Fekete DM. Lineage analysis in the chicken inner ear shows differences in clonal dispersion for epithelial, neuronal, and mesenchymal cells. Dev Biol. 2001;234:120–137. doi: 10.1006/dbio.2001.0248. [DOI] [PubMed] [Google Scholar]
- 9.Li H. Liu H. Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 10.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 11.Quatela VC. Sherris DA. Rosier RN. The human auricular chondrocyte. Responses to growth factors. Arch Otolaryngol Head Neck Surg. 1993;119:32–37. doi: 10.1001/archotol.1993.01880130034004. [DOI] [PubMed] [Google Scholar]
- 12.Gawronska-Kozak B. Regeneration in the ears of immunodeficient mice: identification and lineage analysis of mesenchymal stem cells. Tissue Eng. 2004;10:1251–1265. doi: 10.1089/ten.2004.10.1251. [DOI] [PubMed] [Google Scholar]
- 13.Gawronska-Kozak B. Manuel JA. Prpic V. Ear mesenchymal stem cells (EMSC) can differentiate into spontaneously contracting muscle cells. J Cell Biochem. 2007;102:122–135. doi: 10.1002/jcb.21286. [DOI] [PubMed] [Google Scholar]
- 14.Rim JS. Mynatt RL. Gawronska-Kozak B. Mesenchymal stem cells from the outer ear: a novel adult stem cell model system for the study of adipogenesis. FASEB J. 2005;19:1205–1207. doi: 10.1096/fj.04-3204fje. [DOI] [PubMed] [Google Scholar]
- 15.Rainaldi G. Pinto B. Piras A. Vatteroni L. Simi S. Citti L. Reduction of proliferative heterogeneity of CHEF18 Chinese hamster cell line during the progression toward tumorigenicity. In Vitro Cell Dev Biol. 1991;27A:949–952. doi: 10.1007/BF02631122. [DOI] [PubMed] [Google Scholar]
- 16.McBrearty BA. Clark LD. Zhang XM. Blankenhorn EP. Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci USA. 1998;95:11792–11797. doi: 10.1073/pnas.95.20.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal MA. Kilroy GE. Johnson JR. Lopez MJ. Moore RM. Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Bosch P. Pratt SL. Stice SL. Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol Reprod. 2006;74:46–57. doi: 10.1095/biolreprod.105.045138. [DOI] [PubMed] [Google Scholar]
- 19.Oedayrajsingh-Varma MJ. van Ham SM. Knippenberg M. Helde MN. Klein-Nulend J. Schouten TE. Ritt MJ. van Milligen FJ. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–177. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 20.Williams KJ. Picou AA. Kish SL. Giraldo AM. Godke RA. Bondioli KR. Isolation and characterization of porcine adipose tissue-derived adult stem cells. Cells Tissues Organs. 2008;188:251–258. doi: 10.1159/000121431. [DOI] [PubMed] [Google Scholar]
- 21.Izadpanah R. Trygg C. Patel B. Kriedt C. Dufour J. Gimble JM. Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y. Zhang C. Xiong F. Yu MJ. Peng FL. Shang YC. Zhao CP. Xu YF. Liu ZS. Zhou C. Wu JL. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. 2008;9:24. doi: 10.1186/1471-2121-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summer R. Fitzsimmons K. Dwyer D. Murphy J. Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colter DC. Class R. DiGirolamo CM. Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colter DC. Sekiya I. Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadiyala S. Young RG. Thiede MA. Bruder SP. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 27.Martin DR. Cox NR. Hathcock TL. Niemeyer GP. Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 28.Phinney DG. Kopen G. Isaacson RL. Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 29.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Herrera MB. Bruno S. Buttiglieri S. Tetta C. Gatti S. Deregibus MC. Bussolati B. Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell JB. McIntosh K. Zvonic S. Garrett S. Floyd ZE. Kloster A. Di Halvorsen Y. Storms RW. Goh B. Kilroy G. Wu X. Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 32.Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 33.D'Ippolito G. Schiller PC. Ricordi C. Roos BA. Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 34.Sekiya I. Larson BL. Smith JR. Pochampally R. Cui JG. Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 35.Baddoo M. Hill K. Wilkinson R. Gaupp D. Hughes C. Kopen GC. Phinney DG. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 36.Hisha H. Nishino T. Kawamura M. Adachi S. Ikehara S. Successful bone marrow transplantation by bone grafts in chimeric-resistant combination. Exp Hematol. 1995;23:347–352. [PubMed] [Google Scholar]
- 37.Majka SM. Jackson KA. Kienstra KA. Majesky MW. Goodell MA. Hirschi KK. Distinct progenitor populations in skeletal muscle are bone marrow derived and exhibit different cell fates during vascular regeneration. J Clin Invest. 2003;111:71–79. doi: 10.1172/JCI16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miles C. Sanchez MJ. Sinclair A. Dzierzak E. Expression of the Ly-6E.1 (Sca-1) transgene in adult hematopoietic stem cells and the developing mouse embryo. Development. 1997;124:537–547. doi: 10.1242/dev.124.2.537. [DOI] [PubMed] [Google Scholar]
- 39.Triel C. Vestergaard ME. Bolund L. Jensen TG. Jensen UB. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp Cell Res. 2004;295:79–90. doi: 10.1016/j.yexcr.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Xin L. Lawson DA. Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano S. Ito Y. Fujimoto M. Hamazaki TS. Tamaki K. Okochi H. Characterization and localization of side population cells in mouse skin. Stem Cells. 2005;23:834–841. doi: 10.1634/stemcells.2004-0226. [DOI] [PubMed] [Google Scholar]
- 42.Zheng B. Cao B. Li G. Huard J. Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng. 2006;12:1891–1901. doi: 10.1089/ten.2006.12.1891. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt A. Ladage D. Schinkothe T. Klausmann U. Ulrichs C. Klinz FJ. Brixius K. Arnhold S. Desai B. Mehlhorn U. Schwinger RH. Staib P. Addicks K. Bloch W. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24:1750–1758. doi: 10.1634/stemcells.2005-0191. [DOI] [PubMed] [Google Scholar]
- 44.Odemis V. Boosmann K. Dieterlen MT. Engele J. The chemokine SDF1 controls multiple steps of myogenesis through atypical PKCzeta. J Cell Sci. 2007;120:4050–4059. doi: 10.1242/jcs.010009. [DOI] [PubMed] [Google Scholar]
- 45.Cho HH. Kyoung KM. Seo MJ. Kim YJ. Bae YC. Jung JS. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006;15:853–864. doi: 10.1089/scd.2006.15.853. [DOI] [PubMed] [Google Scholar]