Abstract
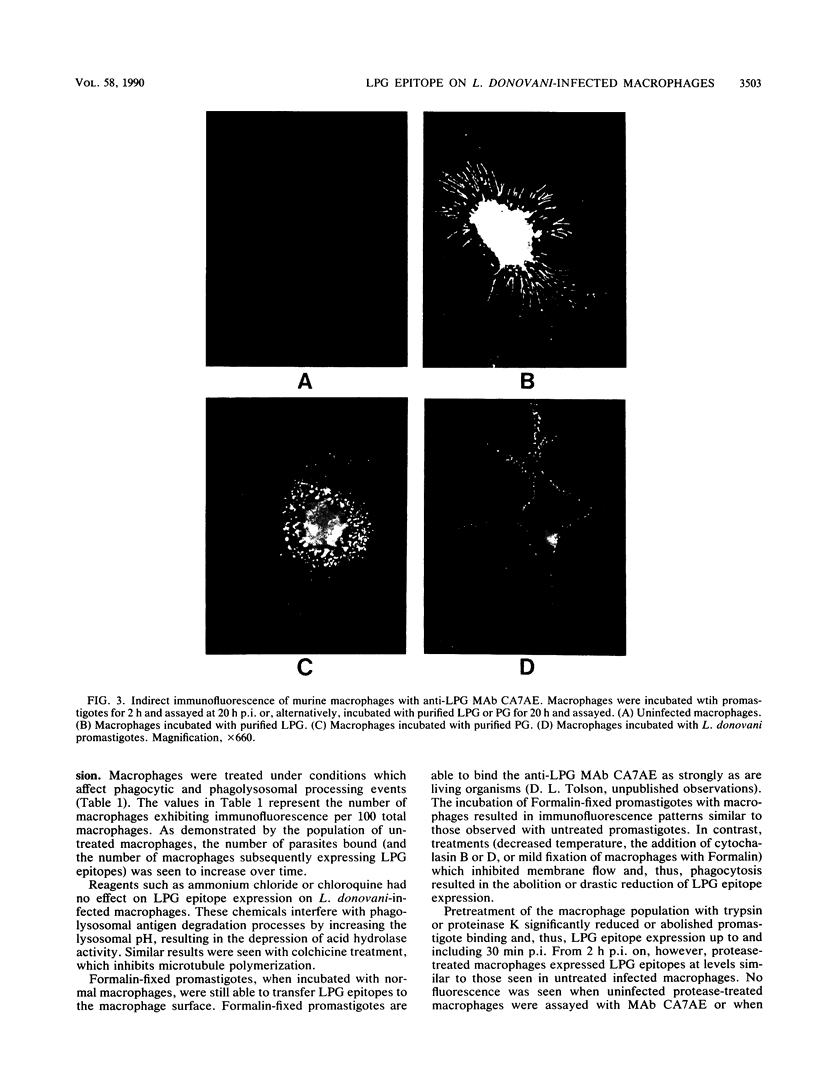
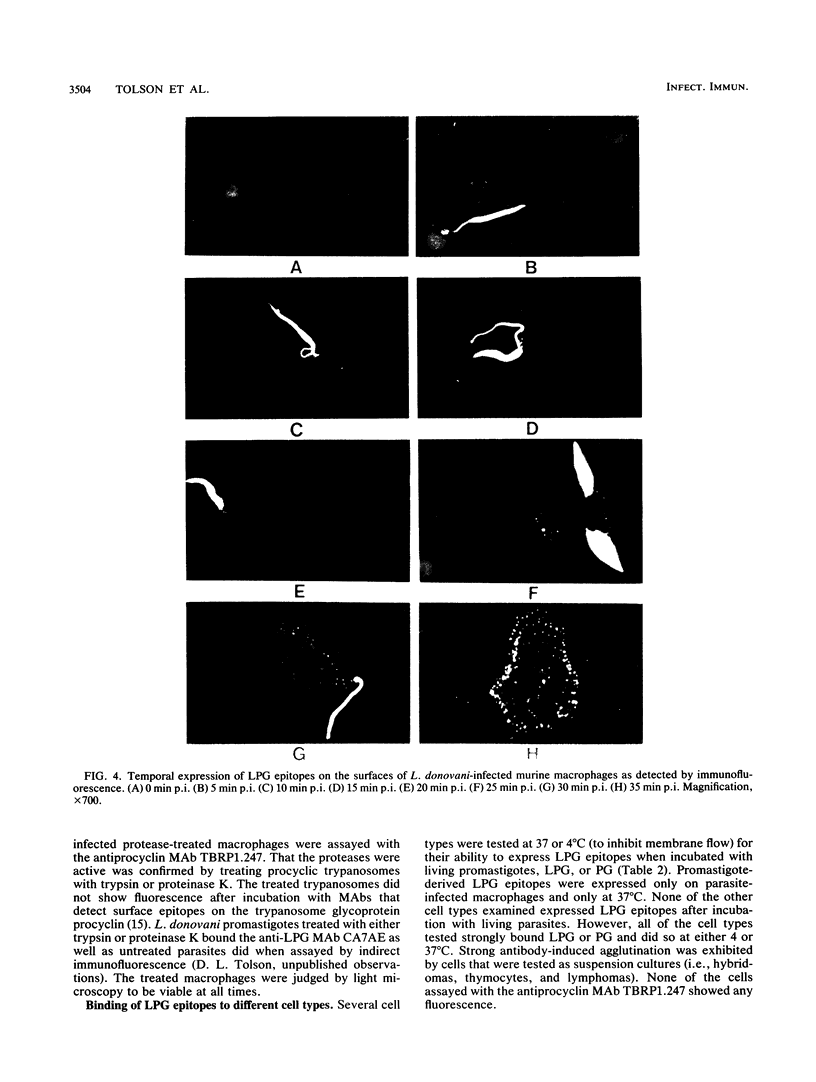
Murine peritoneal macrophages were infected with living, virulent Leishmania donovani promastigotes. At intervals after infection, the macrophage surfaces were probed for the expression of lipophosphoglycan (LPG) epitopes by immunofluorescence with anti-LPG monoclonal antibodies. A repeating phosphorylated disaccharide epitope of LPG was detected as early as 5 to 10 min postinfection and was initially localized to the immediate area of internalization of the promastigote into the macrophage. The epitopes were evenly distributed over the entire macrophage surface by 25 min postinfection. Treatments which inhibited macrophage phagolysosomal degradation processes had no effect on epitope expression, whereas reagents that affected macrophage membrane flow and, thus, phagocytosis drastically reduced or abolished expression. Purified LPG or phosphoglycan, the delipidated form of the LPG molecule, was also shown to bind to a variety of different cell types in a temperature-independent manner. Since LPG has been implicated as having an immunoprotective role in leishmaniasis, these results suggest a further mechanism(s) by which Leishmania LPG might be involved in parasite pathogenicity and virulence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. D., Dwyer D. M. Expression of Leishmania antigen on the surface membrane of infected human macrophages in vitro. Clin Exp Immunol. 1981 May;44(2):342–348. [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani: promastigote--macrophage surface interactions in vitro. Exp Parasitol. 1979 Oct;48(2):175–189. doi: 10.1016/0014-4894(79)90097-3. [DOI] [PubMed] [Google Scholar]
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool. 1977 May;24(2):325–329. doi: 10.1111/j.1550-7408.1977.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Dwyer D. M. Leishmania donovani: surface membrane carbohydrates of promastigotes. Exp Parasitol. 1977 Apr;41(2):341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- Freer S. M. A permanent wet-mount for fluorescent microscopy of surface stained lymphoid cells. J Immunol Methods. 1984 Jan 20;66(1):187–188. doi: 10.1016/0022-1759(84)90261-8. [DOI] [PubMed] [Google Scholar]
- Handman E., Goding J. W. The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J. 1985 Feb;4(2):329–336. doi: 10.1002/j.1460-2075.1985.tb03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Karimi S. T., Schloemer R. H., Wilde C. E., 3rd Accumulation of chlamydial lipopolysaccharide antigen in the plasma membranes of infected cells. Infect Immun. 1989 Jun;57(6):1780–1785. doi: 10.1128/iai.57.6.1780-1785.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M. Antigen presentation and the response to parasitic infection. Parasitol Today. 1987 Oct;3(10):293–299. doi: 10.1016/0169-4758(87)90186-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. T-lymphocytes recognise Leishmania glycoconjugates. Parasitol Today. 1985 Aug;1(2):61–63. doi: 10.1016/0169-4758(85)90117-6. [DOI] [PubMed] [Google Scholar]
- Moll H., Mitchell G. F., McConville M. J., Handman E. Evidence of T-cell recognition in mice of a purified lipophosphoglycan from Leishmania major. Infect Immun. 1989 Nov;57(11):3349–3356. doi: 10.1128/iai.57.11.3349-3356.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P. A., Jr, Turco S. J. Structure of the lipid moiety of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1987 Jul 25;262(21):10384–10391. [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Richardson J. P., Beecroft R. P., Tolson D. L., Liu M. K., Pearson T. W. Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol. 1988 Dec;31(3):203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Jenni L., Beecroft R. P., Pearson T. W. Procyclic tsetse fly midgut forms and culture forms of African trypanosomes share stage- and species-specific surface antigens identified by monoclonal antibodies. J Immunol. 1986 Mar 15;136(6):2259–2264. [PubMed] [Google Scholar]
- Rifkin M. R., Landsberger F. R. Trypanosome variant surface glycoprotein transfer to target membranes: a model for the pathogenesis of trypanosomiasis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):801–805. doi: 10.1073/pnas.87.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P. Leishmania and the macrophage: a marriage of inconvenience. Immunol Today. 1989 Oct;10(10):328–333. doi: 10.1016/0167-5699(89)90188-6. [DOI] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Tolson D. L., Turco S. J., Beecroft R. P., Pearson T. W. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol Biochem Parasitol. 1989 Jun 15;35(2):109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Hull S. R., Orlandi P. A., Jr, Shepherd S. D., Homans S. W., Dwek R. A., Rademacher T. W. Structure of the major carbohydrate fragment of the Leishmania donovani lipophosphoglycan. Biochemistry. 1987 Sep 22;26(19):6233–6238. doi: 10.1021/bi00393a042. [DOI] [PubMed] [Google Scholar]
- Turco S. J., Orlandi P. A., Jr, Homans S. W., Ferguson M. A., Dwek R. A., Rademacher T. W. Structure of the phosphosaccharide-inositol core of the Leishmania donovani lipophosphoglycan. J Biol Chem. 1989 Apr 25;264(12):6711–6715. [PubMed] [Google Scholar]
- Turco S. J. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990 Feb;70(2):241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- Turco S. J. The lipophosphoglycan of Leishmania. Parasitol Today. 1988 Sep;4(9):255–257. doi: 10.1016/0169-4758(88)90144-5. [DOI] [PubMed] [Google Scholar]
- Williams K. M., Sacci J. B., Anthony R. L. Identification and recovery of Leishmania antigen displayed on the surface membrane of mouse peritoneal macrophages infected in vitro. J Immunol. 1986 Mar 1;136(5):1853–1858. [PubMed] [Google Scholar]
- Zenian A., Rowles P., Gingell D. Scanning electron-microscopic study of the uptake of Leishmania parasites by macrophages. J Cell Sci. 1979 Oct;39:187–199. doi: 10.1242/jcs.39.1.187. [DOI] [PubMed] [Google Scholar]