Abstract
Aims and objectives
Describe patterns of morning and evening fatigue in adults with HIV and examine their relationship to demographic and clinical factors and other symptoms.
Background
Most studies of HIV-related fatigue assess average levels of fatigue and do not address its diurnal fluctuations. Patterns of fatigue over the course of the day may have important implications for assessment and treatment.
Design
A cross-sectional, correlational design was used with six repeated measures over 72 hours.
Method
A convenience sample of 318 HIV-infected adults was recruited in San Francisco. Socio-demographic, clinical and symptom data were collected with questionnaires. CD4+ T-cell count and viral load were obtained from medical records. Participants completed a four-item version of the Lee Fatigue Scale each morning and evening for three consecutive days. Participants were grouped based on their diurnal pattern of fatigue (high evening only, high morning only, high morning and evening and low morning and evening). Group comparisons and logistic regression were used to determine the unique predictors of each fatigue pattern.
Results
The high evening fatigue pattern was associated with anxiety and the high morning pattern was associated with anxiety and depression. The morning fatigue pattern showed very little fluctuation between morning and evening, the evening pattern showed the largest fluctuation. The high morning and evening pattern was associated with anxiety, depression and sleep disturbance and this group reported the most fatigue-related distress and interference in functioning.
Conclusions
These results provide initial evidence for the importance of assessing the patient’s daily pattern of fatigue fluctuation, as different patterns were associated with different symptom experiences and perhaps different etiologies.
Relevance to clinical practice
Different fatigue patterns may benefit from tailored intervention strategies. Management of depressive symptoms could be tested in patients who experience high levels of morning fatigue.
Keywords: Fatigue, HIV/AIDS, Symptom Control, Anxiety, Depression, Quality of Life, nurses, nursing
Introduction
Studies have shown that 37–69% of adults living with HIV experience fatigue (Lee et al. 2001, Phillips et al. 2004, Henderson et al. 2005, Sullivan and Dworkin 2003, Grierson et al. 2002). Fatigue can interfere with people’s capacity for self care and daily activities (Jenkin et al. 2006, Barroso 2001, Harmon et al. 2008); therefore, it is important for nurses to understand different types of fatigue so that appropriate intervention strategies can be developed and tested.
Fatigue is a complex multidimensional symptom. It can be defined as a sense of exhaustion, lack of energy, or tiredness distinct from sleepiness, sadness or weakness (Lee et al. 1994, Krupp et al. 1988, Lerdal 1998). However, unlike typical tiredness, clinically significant fatigue is unrelieved by a night of good quality sleep (Lee et al. 1994). Since sleep is normally restorative, individuals generally have more energy in the morning than in the evening. While daily fluctuations in fatigue and energy levels are common (Dimsdale et al. 2003), a general pattern of lower fatigue in the morning and higher fatigue in the evening has been reported in a variety of patient populations, including individuals with HIV infection (Lee et al. 1999, Barroso 2001, Lee et al. 2001), multiple sclerosis (Schwid et al. 2002), cancer (Dhruva et al. 2010, Miaskowski et al. 2008) and other chronic diseases (Lerdal 2002).
Despite the evidence for diurnal fluctuations in fatigue, most prior research on HIV-related fatigue has not examined patterns of fatigue, but has instead assessed average fatigue levels across time, such as the past week or month (i.e., Pence et al. 2008, Henderson et al. 2005) 0. The lack of information about the diurnal pattern of fatigue has implications for assessment, etiology and intervention strategies.
HIV-related fatigue has been associated with socio-demographic and clinical factors, although findings have not been consistent across studies. In some studies, fatigue was associated with gender (Voss 2005), race (Lee et al. 2009, Voss 2005) and employment (Barroso et al. 2010). Lower CD4+ T-cell count has been associated with higher fatigue, particularly in the evening (Lee et al. 1999), but others found no association with CD4+ T-cell count (Barroso et al. 2003). Anti-retroviral use and longer time since diagnosis have been associated with lower fatigue (Pence et al. 2009).
Several co-morbid symptoms have also been associated with fatigue, including disturbed sleep (Lee et al. 2001), depression or anxiety (Barroso et al. 2003, Sewell et al. 2000, Barroso et al. 2010, Jong et al. 2010), pain (Aouizerat et al. 2009, Creavin et al. 2010) and cognitive complaints (Millikin et al. 2003, Woods et al. 2007). These symptoms have also been commonly reported by adults living with HIV/AIDS (Lee et al. 2009). Although relationships between fatigue and other symptoms have been well-documented, research has not addressed diurnal fatigue patterns. It remains unclear if these co-morbid symptoms are associated with specific fatigue patterns. Different types of fatigue (Dimsdale et al. 2003, Dimsdale et al. 2007, Nikolaus et al. 2010) have been described in an effort to better understand this complex multi-dimensional symptom. However, to assess a patient’s fatigue, more knowledge is needed about diurnal patterns and how these patterns relate to other symptoms and clinical factors.
The aims of this study were to:
describe patterns of morning and evening fatigue in adults with HIV/AIDS,
identify socio-demographic and clinical variables and co-morbid symptoms associated with diurnal patterns of fatigue and
determine how diurnal fatigue patterns influence fatigue-related distress and interference in functioning.
Methods
Data were collected as part of a prospective longitudinal study of adults living with HIV in the San Francisco area (Lee et al. 2009). The study was designed to characterise the symptom experience of HIV-positive adults and identify associated biological and genetic markers. This analysis reports on morning and evening fatigue ratings collected at the baseline assessment in relation to demographic and clinical characteristics and measures of symptom experience.
Sample and Procedures
A convenience sample of 350 adults with HIV was enrolled in the study over a 3-year period (April 2005 - December 2007). The participants were recruited using flyers posted at local HIV clinics and community sites. Study visits were conducted at the University of California, San Francisco, Clinical Research Center. Eligible participants were English-speaking, at least 18 years old and diagnosed with HIV at least 30 days before enrollment. Individuals were excluded if they currently used illicit drugs, worked nights, had been pregnant in the previous three months, or reported having a diagnosed sleep disorder, bipolar disorder, schizophrenia, or dementia.
Measures
The questionnaires included demographic information on age, gender, race/ethnicity, level of education, partner status and employment status. Participants also reported whether they had ever received a diagnosis of AIDS. With the patient’s written authorisation, the most recent CD4+ T-cell counts and viral load levels were obtained from the patient’s health care provider. Participants also provided urine samples for toxicology screening using RediCup® (Redwood Toxicology Laboratory, Inc, Santa Rosa CA, USA).
A 4-item version of the Lee Fatigue Scale (LFS) (Lee et al. 1991) was used to assess diurnal variations in fatigue. In a previous sample (Lee et al. 1991), these 4 items were highly correlated with the 13-item fatigue scale (r=0.95 for morning and 0.91 for evening ratings). Participants completed the LFS within 30 minutes of awakening to measure morning fatigue and within 30 minutes prior to going to sleep to measure evening fatigue for three consecutive days. Morning and evening fatigue scores were calculated as the mean of the four items across the three days and could range from 0-10, with higher scores indicating greater fatigue. The LFS has been used to measure fatigue in healthy individuals (Gay et al. 2004, Lee et al. 1991), as well as in patients with cancer (Miaskowski et al. 2008) and HIV (Lee et al. 1999) and has established validity and internal consistency. In this sample the Cronbach α was 0.93 for the morning and 0.88 for the evening ratings.
The Memorial Symptom Assessment Scale (MSAS) was used to assess symptom experience in the past week (Portenoy et al. 1994). It is a reliable and valid self-report measure that has been used in various clinical populations (Blinderman et al. 2008, Sawicki et al. 2008), including patients with HIV (Harding et al. 2006). The MSAS evaluates symptom prevalence, frequency, severity and distress using four or five-point Likert scales. Individual symptom scores were computed for each symptom as the average score on the severity, frequency and distress scales. Fatigue, sleep disturbance, pain, anxiety, depression and cognitive complaints were the most prevalent symptoms in this sample (Lee et al. 2009). For this analysis, only the pain symptom score and the distress rating for the ‘lack of energy’ item were used. If the respondent did not report pain or lack of energy in the past week, the symptom score and distress rating were assigned a value of 0. Symptom scores of 2 or higher indicate moderate to severe pain or fatigue that is somewhat to very distressing.
Fatigue interference during the last week was measured with the FSS-7 (Lerdal et al. 2010a) (in review), a Rasch measure based on five items from the Fatigue Severity Scale (Krupp et al. 1989). Each item was rated on a numeric rating scale from 1 (strongly disagree) - 7 (strongly agree) and the items were averaged to yield a score from 1 (no fatigue interference) - 7 (extreme fatigue interference). The FSS has well-established test-retest reliability and clearly differentiates between patients with chronic disease and healthy adults (Krupp et al. 1989). In this study, Cronbach alpha for the FSS was 0.93. The FSS-7 Rasch measure was used because it was recently found to have better psychometric properties than the original FSS among adults living with (Lerdal et al. 2010a) (in review). The five items retained in the Rasch measure all pertain to how fatigue interferes with functioning (e.g., Fatigue interferes with work, family, or social life).
The 19-item Pittsburgh Sleep Quality Index (PSQI) was used to assess perceived sleep disturbance in the past month (Buysse et al. 1989). The PSQI has demonstrated test-retest reliability and validity (Backhaus et al. 2002, Buysse et al. 1989). It yields seven component scores representing different aspects of sleep and subscale scores are summed to yield a total score ranging 0-21. A total score >5 has been designated as a sensitive and specific cutpoint for distinguishing poor sleepers (Buysse et al. 1989).
The Center for Epidemiological Studies-Depression Scale (CES-D) (Radloff 1977) was used to assess frequency of depressive symptoms in the past week. The CES-D has acceptable reliability and validity (Radloff 1977) and has been frequently used in the HIV population (Cockram et al. 1999, Vosvick et al. 2010). Twenty symptoms are rated on frequency in the past week: 0 (rarely or none of the time, <1 day) - 3 (most or all of the time, 5-7 days in the past week). Item scores are then summed to yield a total score ranging from 0-60, with higher scores indicating more depressive symptoms. A cut-off score of 16 is used to indicate a need for further referral to evaluate depression (Cook et al. 2002). In this study, the Cronbach alpha coefficient for the CES-D was 0.88.
The Profile of Moods State (POMS) Tension-Anxiety subscale (McNair et al. 1971) was used to assess anxiety in the past week. The 9-item subscale scores can range from 0-36, with 8.6 as the mean in HIV-infected outpatients (Illa et al. 2008). The POMS has well-established concurrent and construct validity. In this study, the Cronbach alpha coefficient was 0.86.
Participants completed the six-item cognitive function scale from the Medical Outcome Study (MOS) Health-Related Quality of Life (HRQOL) measure (Stewart et al. 1992). This self-report instrument has been used in previous studies of HIV-infected adults to measure perceived cognitive function and is valid and reliable (Wu et al. 1993, O’Dell et al. 1998). Respondents are asked to indicate how often in the past month they experienced difficulties with concentration, attention, forgetfulness and confusion. Each item was rated from 1 (all of the time) to 6 (none of the time). Scores are transformed to a 0-100 scale, with higher scores indicating better cognitive function. The Cronbach alpha coefficient for this sample was 0.91.
Statistical analysis
All analyses were conducted using SPSS version 18.0 (SPSS, Inc, Chicago IL, USA). Square root transformations were used to normalise skewed distributions of scores on the CES-D and POMS subscale and a logarithmic transformation was used to normalise viral load values. Descriptive statistics were used to summarise mean morning and evening fatigue scores across three days. Because there are no validated cutoff scores indicating clinically significant morning or evening fatigue for adults living with HIV, we used a median split to group participants based on their self-report scores: 1) low morning and evening fatigue, 2) high morning fatigue, 3) high evening fatigue, or 4) high morning and evening fatigue. The fatigue groups were compared on demographic and clinical characteristics and symptom experience measures using chi-square or ANOVA with Scheffe post-hoc testing. ANOVA results were confirmed with the Kruskal-Wallis non-parametric test. Demographic variables were analyzed using levels described in Table 1. When applicable, both continuous variables and clinically meaningful categories were evaluated (e.g., CD4+ T-cell count <200 cells/mm3 and detectable viral load).
Table 1.
Sample demographics, clinical characteristics, and symptom experience by fatigue pattern
| Fatigue Pattern |
||||||
|---|---|---|---|---|---|---|
| Full Sample (N=318) | A Low morning and evening (n=111) | B High evening only (n=46) | C High morning only (n=47) | D High morning and evening (n=114) | Test statistic p-value | |
| DEMOGRAPHIC | ||||||
| Age | 45.1 (8.3) | 45.6 (8.3) | 45.4 (7.1) | 43.6 (9.5) | 45.2 (8.3) | F = 0.62; p = .601 |
| Gender | χ2 = 6.84; p = .336 | |||||
| Male | 68% | 61% | 72% | 66% | 74% | |
| Female | 25% | 28% | 26% | 26% | 21% | |
| Transgender | 7% | 11% | 2% | 8% | 5% | |
| Race | χ2 = 34.7; p < .001 | |||||
| African-American | 39% | 59%D | 33% | 36% | 23%A | |
| Caucasian | 41% | 27%D | 46% | 34% | 54%A | |
| Other | 21% | 14% | 22% | 30% | 23% | |
| Employed/in school | 15% | 14% | 13% | 17% | 18% | χ2 = 1.00; p = .802 |
| Has children | 36% | 43% | 33% | 38% | 29% | χ2 = 5.33; p = .149 |
| CLINICAL VARIABLES | ||||||
| CD4+ T-cells/mm3 (n=303) | ||||||
| Mean count of cells/mm3 | 449 (265) | 416 (257) | 483 (274) | 480 (260) | 455 (272) | F = 0.62; p = .304 |
| % < 200 cells/mm3 | 17% | 19% | 9% | 24% | 18% | χ2 = 3.55; p = .314 |
| Viral load, copies/mL (n=296) | χ2 = 5.07; p = .535 | |||||
| undetectable | 50% | 46% | 52% | 49% | 55% | |
| detectable – 9,999 | 30% | 31% | 36% | 34% | 25% | |
| ≥ 10,000 copies/mL | 20% | 23% | 11% | 17% | 20% | |
| Years since HIV diagnosis | 12.0 (6.7) | 11.7 (6.3) | 12.3 (6.8) | 10.1 (7.6) | 13.0 (7.2) | F = 2.09; p = .102 |
| AIDS diagnosis | 52% | 44% | 59% | 51% | 58% | χ2 = 5.17; p = .160 |
| Taking anti-retroviral therapy | 70% | 63% | 74% | 72% | 75% | χ2 = 4.62; p = .202 |
| Body Mass Index | 27.0 (5.5) | 26.9 (5.2) | 27.1 (7.5) | 28.6 (5.2) | 26.4 (5.0) | F = 1.87; p = .134 |
| RELATED SYMPTOMS | ||||||
| Depression (CES-D) | ||||||
| Mean score (SD) | 16.9 (10.4) | 12.4 (9.4)C,D | 15.5 (10.8)D | 20.0 (8.6)A | 20.5 (10.3)A,B | F = 17.7; p < .001 |
| % ≥ 16 | 50% | 32% | 40% | 72% | 61% | χ2 = 30.1; p < .001 |
| Anxiety (POMS) | ||||||
| Mean score (SD) | 8.7 (7.0) | 5.3 (5.7)B,C,D | 9.2 (7.7)A | 10.6 (5.9)A | 11.0 (7.2)A | F = 20.7; p < .001 |
| % > HIV norm of 8.6 | 42% | 20% | 44% | 58% | 56% | χ2 = 35.3; p < .001 |
| Cognitive Function (MOS) | ||||||
| Mean score (SD) | 70 (24) | 77 (22)D | 70 (25) | 67 (23) | 63 (24)A | F = 0.62; p < .001 |
| % < median of 77 | 48% | 34% | 47% | 55% | 58% | χ2 = 13.9; p < .003 |
| Pain score (MSAS) | ||||||
| Mean score (SD) | 1.4 (1.4) | 1.2 (1.3)D | 1.1 (1.3)D | 1.4 (1.3) | 1.7 (1.5)A,B | F = 4.59; p = .004 |
| % ≥ 2 (moderate pain) | 41% | 33% | 31% | 41% | 53% | χ2 = 11.1; p = .011 |
| Sleep disturbance (PSQI) | ||||||
| Mean score (SD) | 7.6 (3.8) | 6.3 (3.4)C,D | 6.5 (3.3)C,D | 8.7 (4.0)A,B | 8.9 (3.6)A,B | F = 13.5; p < .001 |
| % > 5 | 66% | 54% | 56% | 77% | 78% | χ2 = 19.1; p < .001 |
Note: CES-D=Center for Epidemiologic Studies-Depression Scale; POMS=Profile of Mood States – Tension-Anxiety Subscale; MOS=cognitive subscale from the Medical Outcomes Study; MSAS=Memorial Symptom Assessment Scale; PSQI=Pittsburgh Sleep Quality Index
Exponents indicate groups that differed significantly in post-hoc testing:
differed from Low morning and evening group
differed from High evening-only group
differed from High morning-only group
differed from High morning and evening group
Logistic regression analyses were conducted to identify the unique contributions of demographic and clinical characteristics to each fatigue pattern using the low morning and evening fatigue group as the reference. Race, gender, anti-retroviral therapy and CD4+ T-cell count were included in all three models based on associations with fatigue in prior research. In addition, variables in Table 1 associated with fatigue patterns in bivariate analyses (p< 0.10) were retained for multivariable analysis. Variables that did not have unique contributions (p< 0.20) to any of the three fatigue patterns were dropped from the model. Demographic, clinical and symptom variables were entered in separate steps to evaluate the variance explained by each domain. Potential interactions between race, gender and clinical characteristics were also assessed. For all analyses, p< 0.05 was considered statistically significant.
Ethics
The study was approved by the Committee on Human Research at the University of California, San Francisco. All participants provided written informed consent and written authorisation for their health care providers to release their medical records for research purposes.
Results
Sample characteristics
Of the 350 adults with HIV enrolled in the larger study, one was excluded after being unable to submit a urine sample for drug screening and 31 were excluded after screening positive for illicit drugs (cocaine, amphetamine, ecstasy, methamphetamine, or phencyclidine). Demographic and clinical characteristics for the 318 participants included in the final sample are presented in Table 1. The sample was ethnically diverse and predominantly male, reflecting the local population of adults with HIV. Over half (61%) of the 78 women were African American. Most participants had been living with HIV for many years and were currently on anti-retroviral therapy. They were taking an average of 6.5 (SD4.3) medications (median 6, range 0-25), 51% had been diagnosed with AIDS and 28% with an AIDS diagnosis had a current CD4+ T-cell count of <200 cells/mm3. Employment rates were low (15%) and most (75%) were receiving medical disability assistance.
Fatigue Ratings
The morning and evening fatigue ratings were stable across the three days, with intraclass correlation coefficients of 0.80 for the three morning ratings and 0.81 for the evening ratings. Mean morning and evening fatigue ratings are presented in Table 2. The morning and evening ratings were correlated (r=0.64, p<0.001), but as expected, evening ratings were significantly higher than morning ratings (Paired t[317]=15.3, p<0.001). Those who reported high morning fatigue were also likely to report high evening fatigue (36%), but almost 30% reported only high morning or high evening fatigue. Another 35% reported low levels of both morning and evening fatigue.
Table 2.
Morning and evening fatigue ratings by fatigue category
| Fatigue Category |
||||||
|---|---|---|---|---|---|---|
| Full Sample (N=318) | A Low morning and evening (n=111) | B High evening only (n=46) | C High morning only (n=47) | D High morning and evening (n=114) | Test statistic p-value | |
| FATIGUE RATINGS | N=318 | N=111 | N=46 | N=47 | N=114 | |
| Morning mean (SD) | 3.6 (2.3) | 1.5 (1.0)C,D | 2.1 (1.0)C,D | 4.7 (0.8)A,B,D | 5.8 (1.5)A,B,C | F = 278.2; p < .001 |
| Evening mean (SD) | 5.3 (2.2) | 3.0 (1.5)B,C,D | 6.8 (0.8)A,C | 4.6 (0.8)A,B,D | 7.1 (1.1)A,C | F = 275.6; p < .001 |
Note: Exponents indicate groups that differed significantly in post-hoc testing, as in Table 1.
The morning and evening fatigue ratings across the three days (six ratings total) are illustrated in Figure 1. Each group has a distinct pattern of fatigue and the pattern was consistent across the three days. The low morning and evening pattern had a rhythm of higher fatigue in the evening, with lower fatigue in the morning and all fatigue ratings are relatively low. The high evening fatigue pattern had the most extreme fluctuation between morning and evening and the high morning fatigue pattern had almost no fluctuation. The high morning and evening fatigue pattern had a strong rhythm, but with consistently high fatigue ratings.
Figure 1.
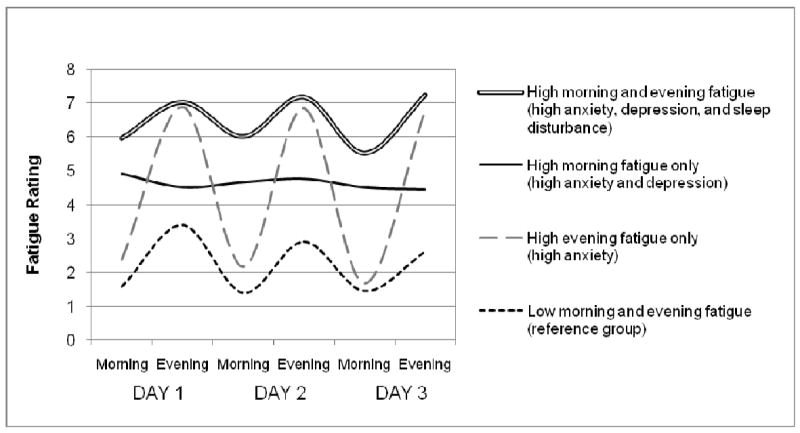
Mean morning and evening fatigue ratings over 3 days for the four fatigue patterns. The symptoms uniquely associated with each pattern are included in the legend.
The four fatigue patterns were compared on demographic and clinical characteristics, as well as symptom measures (Table 1). Race was the only demographic characteristic that differed by fatigue pattern, with African-American participants being over-represented in the low fatigue group and under-represented in the high morning and evening fatigue group compared with Caucasians. There was no gender difference in fatigue and fatigue categories did not differ on any of the clinical characteristics evaluated.
The four fatigue patterns differed on all of the co-morbid symptoms assessed (Table 1). In general, those reporting both morning and evening fatigue experienced the worst symptoms, followed closely by those with morning fatigue only. For most symptoms, the experience of those with low fatigue did not differ from those only reporting evening fatigue.
Multivariate Analyses of Fatigue Patterns
Logistic regression analyses are presented in Table 3. Pain and cognitive complaints were not associated with fatigue patterns when controlling for other factors and were excluded from regression models. Interactions of demographic and clinical variables were evaluated for each fatigue pattern and were only in regression models if p< 0.10.
Table 3.
Multiple logistic regression analysis of fatigue patterns
| Model |
||||||
|---|---|---|---|---|---|---|
| High Evening Only | High Morning Only | High Morning and Evening | ||||
| PREDICTORS | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Step 1 – Demographics | ΔR2 = .048 | .086 | ΔR2 = .051 | .073 | ΔR2 = .169 | <.001 |
| Race (African-American) | 0.49 (0.21, 1.13) | .094 | 0.38 (0.15, 1.01) | .053 | 0.22 (0.11, 0.46) | <.001 |
| Gender (Female) | 0.89 (0.32, 2.45) | .817 | 1.60 (0.57, 4.47) | .371 | 1.61 (0.44, 5.82) | .471 |
| Step 2 – Clinical | ΔR2 = .068 | .025 | ΔR2 = .042 | .109 | ΔR2 = .023 | .114 |
| CD4+ count <200 cells/mm3 | 0.23 (0.05, 1.12) | .069 | 1.87 (0.68, 5.12) | .224 | 0.32 (0.11, 0.91) | .032 |
| Anti-retroviral therapy | 2.36 (0.97, 5.74) | .058 | 2.45 (0.94, 6.35) | .066 | 3.31 (1.39, 7.88) | .007 |
| Step 3 – Interactions* | - | - | - | - | ΔR2 = .047 | .012 |
| Gender by CD4+ | - | - | - | - | 43.1 (3.9, 472.5) | .002 |
| Gender by therapy | - | - | - | - | 0.18 (0.03, 1.00) | .050 |
| Step 4 – Other Symptoms | ΔR2 = .059 | .085 | ΔR2 = .195 | <.001 | ΔR2 = .173 | <.001 |
| Anxiety (POMS ≥ 8.6) | 3.05 (1.13, 8.20) | .027 | 2.91 (1.13, 7.52) | .028 | 2.91 (1.34, 6.32) | .007 |
| Depression (CES-D≥ 16) | 0.97 (0.35, 2.68) | .960 | 2.79 (1.06, 7.38) | .038 | 2.37 (1.09, 5.15) | .030 |
| Sleep disturbance (PSQI>5) | 0.96 (0.42, 2.18) | .918 | 1.66 (0.65, 4.24) | .288 | 2.28 (1.11, 4.68) | .025 |
| OVERALL MODEL FIT | χ2=18.89 | .009 | χ2=32.19 | <.001 | χ2=79.21 | <.001 |
| Pseudo R2 | .175 | .288 | .412 | |||
Note. Low morning and evening fatigue group is the reference group; OR=Odds ratio; POMS=Profile of Mood States – Tension-Anxiety Subscale; CES-D=Center for Epidemiologic Studies-Depression Scale; PSQI=Pittsburgh Sleep Quality Index);
Interactions between demographic and clinical variables were only included in the model if p<.10
Evening fatigue pattern
Anxiety was the only significant predictor of the high evening fatigue pattern (without morning fatigue) when controlling for other factors. Those with high anxiety were 3 times more likely to have the evening fatigue pattern compared with those reporting lower levels of anxiety. Race, anti-retroviral therapy and CD4+ T-cell count made small contributions to the evening fatigue model. African-Americans had slightly lower risk and those taking anti-retroviral therapy had somewhat higher risk of having the evening fatigue pattern. Contrary to expectation, the evening fatigue pattern was slightly more common among those with higher CD4+ T-cell counts (≥200), who were also more likely to be employed than those with lower counts (18% vs 2% χ2=8.82, p=0.003). While the model was statistically significant, it only explained 17.5% of the variance in evening-only fatigue.
Morning fatigue pattern
Predictors of high morning fatigue (without evening fatigue) included elevated symptoms of both depression and anxiety. Clinically significant depressive symptoms (CES-D ≥ 16) and higher anxiety (POMS-anxiety ≥ 8.6) were each associated with nearly 3 times the risk of morning-only fatigue. Like the evening fatigue model, African-Americans had a slightly reduced risk and taking ant-retroviral therapy had a slightly increased risk of morning fatigue, but the unique contributions of these variables were not statistically significant. The overall model was significant and explained about 29% of the variance in morning-only fatigue.
High morning and evening fatigue pattern
This pattern was the only one significantly associated with demographic, clinical and symptom factors. African-Americans had a significantly lower risk of this pattern compared with participants of other races. Although this pattern was generally associated with higher CD4+ T-cell count (≥200) and taking anti-retroviral therapy, these effects were considerably confounded by gender. Interaction effects in this model indicate that among women, this fatigue pattern was associated with lower CD4+ T-cell counts and not taking anti-retroviral therapy. This pattern was also significantly associated with other symptoms. As in the previous model, anxiety and depression were associated with greater risk. This is the only pattern where sleep disturbance emerged as an independent predictor. Anxiety was associated with nearly three times the risk and depression and sleep disturbance were each associated with more than twice the risk of having high morning and evening fatigue. The regression model was significant and explained 41% of the variance.
Fatigue-related distress and interference with functioning
Table 4 compares levels of fatigue-related distress and interference in functioning across the four fatigue patterns. Compared with the other three groups, the group with both morning and evening fatigue experienced more distress and reported the most interference with functioning. Those with low fatigue, or evening-only fatigue, reported the least distress and interference and these two groups did not differ on interference with functioning.
Table 4.
Fatigue-related distress and interference by fatigue category
| Fatigue Category |
||||||
|---|---|---|---|---|---|---|
| Full Sample | A Low morning and evening | B High evening only | C High morning only | D High morning and evening | Statistic p-value | |
| FATIGUE EXPERIENCE | N=318 | N=111 | N=46 | N=47 | N=114 | |
| Distress (MSAS) | 1.55 (1.37) | 0.86 (1.13)C,D | 1.28 (1.45)D | 1.65 (1.27)A,D | 2.29 (1.21)A,B,C | F = 26.0; p < .001 |
| Interference (FSS-7) | 3.76 (1.71) | 2.89 (1.53)C,D | 3.45 (1.71)D | 3.91 (1.55)A | 4.66 (1.49)A,B | F = 25.3; p < .001 |
Note: Exponents indicate groups that differed significantly in post-hoc testing; FSS-7=Fatigue Severity Scale – 7 item version; MSAS=Memorial Symptom Assessment Scale
Discussion
To our knowledge, this is the first study to identify different diurnal patterns of fatigue and explore their relationships with socio-demographic and clinical variables, other symptoms and fatigue-related distress and interference. The patterns of fatigue differed significantly on all of the symptom measures, with the high morning and evening fatigue pattern reporting the highest levels of depression, anxiety, pain, cognitive complaints and sleep disturbance. Because these symptoms are highly correlated with each other, multivariate analyses were conducted to identify the independent contributions of each symptom and each pattern was found to have a unique set of symptom predictors. In addition, each pattern was associated with varying degrees of fatigue-related distress and fatigue-related interference in functioning.
High evening fatigue pattern: Anxiety
Anxiety was the only factor independently associated with the high evening fatigue pattern and those with anxiety were 3 times more likely to have the high evening pattern than the low morning and evening pattern when controlling for other factors. In addition, those with high evening fatigue showed the largest fluctuation between morning and evening compared with other groups (Fig. 1). This large difference may indicate that while much energy is used during the day, sleep is quite effective at relieving their fatigue. The relatively low symptom burden in the high evening fatigue group suggests that fatigue in the evening is not necessarily problematic as long as sleep is restorative and results in substantially lower morning fatigue ratings. Furthermore, this type of fatigue might be considered a more healthy form of tiredness since it is relieved by sleep (Lee et al. 1994).
High morning fatigue pattern: Anxiety and depression
The morning fatigue pattern was not only predicted by anxiety, it was also associated with depressive symptoms, even when controlling for demographic, clinical and other symptoms. Those with clinically significant depressive symptoms (CES-D ≥ 16) were nearly three times more likely to have the morning fatigue pattern, as were those reporting high anxiety. As shown in Fig. 1, this group had a distinct pattern of nearly no fluctuation between morning and evening. This pattern suggests that sleep fails to provide relief from fatigue, but also that little energy is used during the day, since fatigue ratings do not increase later in the day. This pattern was associated with significant symptom burden and the group did not differ from the more debilitating high morning and evening fatigue pattern on any symptom measure. Diagnostic criteria for depression include early morning worsening of symptoms among its melancholic features(American Psychiatric Association 2000), which is consistent with the relatively high level of morning fatigue in this group. A pan-European survey of depression in the community (Tylee et al. 1999) showed that tiredness was recorded by 73.5% of those with depression and that tiredness was the second most prevalent symptom among depressed adults.
High morning and evening fatigue pattern: Anxiety, depression and sleep disturbance
The high morning and evening fatigue pattern was the most debilitating, distressing and clinically complex type of fatigue identified in this study. In addition to anxiety and depression, sleep disturbance was also a significant predictor of this pattern. This group was nearly three times more likely to have anxiety, more than twice as likely to have clinically significant depressive symptoms and more than twice as likely to have disturbed sleep compared with those in the low morning and evening fatigue group when controlling for other factors. Demographic and clinical factors were also significant predictors of this pattern and clinical predictors differed by gender, highlighting the importance of examining interactions to fully understand these complex relationships. Even though the high morning and evening pattern had relatively normal daily fluctuations, fatigue ratings were consistently higher than average. The significance of sleep disturbance in this model suggests that this pattern may be a type of sleep-related fatigue where sleep disturbance plays a key role in the etiology.
Anxiety and fatigue
Anxiety was a consistent predictor of fatigue regardless of fatigue’s diurnal pattern. This finding supports previous studies reporting a relationship between fatigue and anxiety, psychological distress and stress (Paddison et al. 2009, Salahuddin et al. 2009, Phillips et al. 2004, Leserman et al. 2008). In one recent study (Theuninck et al. 2010) HIV diagnosis, treatment and physical symptoms were strongly associated with post-traumatic stress disorder (PTSD), which has an estimated prevalence of 30–60% among adults with HIV (Theuninck et al. 2010, Reisner et al. 2009, Whetten et al. 2008). Studies have also reported an association between PTSD symptoms and fatigue in HIV-infected adults (Barroso et al. 2010) and in the general population (Lerdal et al. 2010b). Our findings indicate that psychological distress (anxiety, stress, or PTSD symptoms) is an important antecedent to fatigue in HIV-infected adults.
Depression and Fatigue
The relationship between depression and fatigue is well-documented (Barroso et al. 2010, Corless et al. 2008, Jong et al. 2010, Phillips et al. 2004, Voss et al. 2007, Millikin et al. 2003, Walker et al. 1997). However, this study clarified that the relationship may be specific to morning fatigue, regardless of evening fatigue levels. In this sample, those reporting evening-only fatigue did not differ from those in the low morning and evening fatigue group on the CES-D. Although depression and fatigue have conceptual overlap (Voss et al. 2007), our study showed that different fluctuating patterns distinguish between the two concepts.
Sleep Disturbance and fatigue
Previous studies have documented a relationship between sleep disturbance and fatigue (Salahuddin et al. 2009, Lee et al. 2001, Lee et al. 1999, Phillips et al. 2004, Pence et al. 2008). In this study, sleep disturbance was associated with all three patterns in bivariate analyses. However, in multivariate analyses controlling for demographic, clinical and other symptom factors, sleep disturbance was only a significant independent predictor of the high morning and evening fatigue pattern. This finding is consistent with previous research showing that poor sleep is associated not only with greater fatigue the next morning, but also with more fatigue the following evening (Lee et al. 1999). A study of healthy adults (Morris et al. 1992) showed a higher level of fatigue in the morning among people who were sleep deprived, while those without sleep deprivation had slightly lower fatigue ratings in the morning than in the evening, indicating that sleep disturbance can influence patterns of fatigue. The finding that sleep disturbance was not independently associated with either evening-only or morning-only fatigue suggests that bivariate relationships between sleep disturbance and these two fatigue patterns may be better explained by psychological symptoms rather than sleep disturbance specifically.
Pain, cognitive complaints and fatigue
Although pain and cognitive complaints differed across fatigue groups in bivariate analyses, neither symptom was uniquely predictive of any fatigue pattern when controlling for other factors. We previously reported that those with pain in this sample had higher levels of fatigue interference than those without pain (Aouizerat et al. 2010). However, in this analysis, pain was only related to fatigue patterns in bivariate analyses and did not contribute to multivariate models. The finding that pain is not a unique predictor of fatigue pattern is consistent with another study in HIV-infected adults (Paddison et al. 2009). Cognitive complaints have also been associated with fatigue (Woods et al. 2007), and while they differed across fatigue patterns in bivariate analyses in this study, their nonsignificance in multivariate models highlights the importance of controlling for other factors when identifying correlates of fatigue.
Demographics and fatigue
In this study, African Americans reported lower morning and evening fatigue than Caucasians and other races. Therefore, African Americans were over-represented in the low morning and evening fatigue group and under-represented in the high morning and evening group. This finding is consistent with other studies reporting lower symptom burden (Phillips et al. 2004, Silverberg et al. 2009) and lower fatigue (Voss 2005, Henderson et al. 2005) among African-Americans with HIV. Additional research is needed to better understand whether this phenomenon is due to under-reporting of symptoms or to better coping and social support among African-Americans compared with others.
Gender was unrelated to fatigue patterns in bivariate analyses, but was an important moderator of clinical factors in the multivariate model of morning and evening fatigue pattern. Although some have found more fatigue among HIV-infected women than men (Voss 2005), most have not identified gender differences in HIV-related fatigue (Henderson et al. 2005). Gender difference in fatigue in the general population has a very small effect size (Lerdal et al. 2010b), indicating a weak relationship. It is likely that the stress of living with HIV would mask any subtle gender difference in fatigue.
Our findings were also consistent with a previous study indicating no relationship between fatigue and age (Voss et al. 2007). Previous studies (Voss 2005, Lee et al. 1999) documented an association between fatigue and employment, but this was not observed in our sample. There were also no differences between those with and without children in this study, in contrast to previous findings (Lee et al. 1999).
Clinical factors and fatigue
Clinical variables had complex relationships with the different fatigue patterns and the relationships were evident in multivariate analyses after controlling for demographic variables. Anti-retroviral therapy was associated with higher risk of the three different fatigue patterns, although among women, it was associated with a lower risk of the morning and evening fatigue pattern. Similarly, low CD4+ T-cell count was associated with lower risk for two of the three fatigue patterns, except that among women it was associated with substantially higher risk for the high morning and evening pattern. Our findings are not surprising given the inconsistent results of prior studies (Henderson et al. 2005, Darko et al. 1992, Lee et al. 1999, Phillips et al. 2004, Barroso et al. 2010). Consistent with other studies, viral load (Henderson et al. 2005, Barroso et al. 2010) was unrelated to fatigue pattern in this sample.
Study limitations
As a cross-sectional and correlational study, causal interpretations need to be made with caution. Longitudinal studies will facilitate greater understanding of how fatigue and other symptoms develop and influence one another over time. In future studies, it would be warranted to include larger samples who report morning-only or evening-only fatigue to have more statistical power for multivariate analyses. This study was limited to fatigue values over three days, thereby restricting the variability of patterns observed. Studies of fatigue throughout the 24-hour cycle and over longer periods of time could determine whether additional patterns exist (Dimsdale et al. 2003).
Implication for research and theory development
Our review of research on HIV-related fatigue showed that diurnal patterns have not been adequately or empirically studied, despite the availability and use of morning and evening fatigue measures and despite fluctuation being described as one characteristic of fatigue in inductive theory development (Lerdal 2002). Further examination is warranted to explore different patterns of fatigue fluctuation in relation to level of physical activity, sleep-wake patterns and circadian rhythms. Since the most clinically problematic fatigue pattern was associated with several symptoms previously identified as part of a recognised symptom cluster, studies should investigate possible symptom clusters and explanatory models for these various fatigue patterns (Dodd et al. 2004). Future research should also evaluate whether available treatments are more effective for some fatigue patterns than others. Randomised clinical trials that test a tailored approach toward reducing fatigue based on its pattern of expressive would be more likely to yield better results than a more generic intervention approach.
Conclusion
This study has shown that clinicians and researchers need to consider patterns of fatigue to distinguish normal fluctuations in energy level from clinically significant fatigue related to other severe symptoms (such as anxiety, depression and sleep disturbance), distress and interference with activity in daily life. Testing or implementing intervention strategies would have better outcomes in reducing fatigue if they are tailored toward the pattern of fatigue experienced by the patient.
Relevance to clinical practice
Fatigue can be a healthy symptom for adults who feel tired at the end of the day. In this case, fatigue serves as a healthy reminder to rest. When fatigue is relieved by sleep or rest, fatigue would be considered a healthy symptom experience. Our findings in a clinical sample of adults with HIV/AIDS demonstrate that fatigue is less likely to be related to their clinical disease and more likely to be related to mental health symptoms of depression and anxiety. Our data indicate that anxiety can be fatiguing and nurses should assess for anxiety when patients who have any type of chronic illness complain of fatigue. These results provide initial evidence for the importance of assessing the patient’s daily fluctuation in fatigue, as different patterns can be associated with different symptom experiences and perhaps different etiologies.
The most severe and debilitating types of fatigue (those with severe morning and evening fatigue and those with morning-only fatigue) should be further evaluated for potential sleep problems that may include non-restorative sleep. A more detailed assessment of the patient’s rest-activity patterns associated with their fatigue pattern can provide important clinical information when planning and prescribing appropriate levels of rest and activity as well as self-care behaviors to cope with excessive fatigue. Nurses should also consider the need for referral to a sleep disorders expert if fatigue is not relieved by a night of sleep (Lee & Ward 2005). Different fatigue patterns may benefit more from tailored intervention strategies to reduce symptoms of anxiety or depression than from prescriptions to ‘get plenty of rest.’ Management strategies for coping with anxiety or depressive symptoms, or strategies for addressing inadequate or disturbed sleep patterns should be targeted for patients who experience high levels of morning fatigue.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (NIMH, 5 R01 MH074358). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health”. Data collection was supported by the General Clinical Research Center in the UCSF CTSA (1 UL RR024131). Dr. Lerdal has received funding from the Research Council of Norway (Grant # 19256), the Norwegian Nurses Organization and the U.S.–Norway Fulbright Foundation. Dr. Aouizerat is supported by an NIH Roadmap K12 (KL2 RR024130). Authors wish to acknowledge the contributions to the study from Traci Coggins, Skip Davis, Ryan Kelly, Yeonsu Song, Kristen Nelson, and Matthew Shullick.
Footnotes
Contributions Study design: BA, CJP, CLG, KAL
Data collection and analysis: AL, BA, CLG, KAL
Manuscript preparation: AL, BA, CJP, CLG, KAL.
Conflict of interest No conflicts of interest to declare
Contributor Information
Anners Lerdal, Lovisenberg Deaconal University College, Oslo, Norway & Oslo University Hospital, Medical Department, Section of Gastroenterology, Oslo Norway: anners.lerdal@ldh.no.
Caryl L. Gay, Department of Family Health Care Nursing, University of California, San Francisco, CA, USA; caryl.gay@ucsf.edu.
Bradley E. Aouizerat, Department of Physiological Nursing and Institute for Human Genetics, University of California, San Francisco, CA, USA; bradley.aouizerat@nursing.ucsf.edu.
Carmen J. Portillo, Community Health Systems, University of California, San Francisco, CA, USA; carmen.portillo@nursing.ucsf.edu.
Kathryn A. Lee, Department of Family Health Care Nursing, University of California, San Francisco, CA, USA; kathryn.lee@nursing.ucsf.edu.
References
- American Psychiatric Association. Text Revision: DSM–IV–TR. Fourth. American Psychiatric Publishing, Inc.; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders; pp. 419–420. [Google Scholar]
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Wara W, Swift P, Dunn LB, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Aouizerat BE, Miaskowski CA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Lee KA. Risk factors and symptoms associated with pain in HIV-infected adults. Journal of the Association of Nurses in AIDS Care. 2010;21:125–133. doi: 10.1016/j.jana.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Barroso J. ‘Just Worn Out’: A Qualitative Study of HIV-related Fatigue. In: Funk SG, Tornquist EM, Leeman J, Miles MS, Harrell JS, editors. Key Aspects of Preventing and Manageing Chronic Illness. Springer Publishing Company; New York: 2001. pp. 183–194. [Google Scholar]
- Barroso J, Carlson JR, Meynell J. Physiological and psychological markers associated with HIV-related fatigue. Clinical Nursing Research. 2003;12:49–68. doi: 10.1177/1054773803238740. [DOI] [PubMed] [Google Scholar]
- Barroso J, Hammill BG, Leserman J, Salahuddin N, Harmon JL, Pence BW. Physiological and Psychosocial Factors that Predict HIV-Related Fatigue. AIDS and Behaviour. 2010 doi: 10.1007/s10461-010-9691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. Journal of Pain abd Symptome Management. 2008;35:594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cockram A, Judd FK, Mijch A, Norman T. The evaluation of depression in inpatients with HIV disease. Australian and New Zealand Journal of Psychiatry. 1999;33:344–352. doi: 10.1046/j.1440-1614.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, Palacio H, Richardson J, Wilson T, Young M. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. Journal of Acquired Immune Deficiency Syndromes. 2002;30:401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- Corless IB, Voss JG, Nicholas PK, Bunch EH, Bain CA, Coleman C, Dole PJ, Eller LS, Hamilton MJ, Holzemer WL, Kemppainen JK, Kirksey KM, Sefcik EF, Nokes KM, Tsais YF, Reynolds NR, Wantland DJ, Mc GC, Davis SM, Mendez MR, Valencia CP. Fatigue in HIV/AIDS patients with comorbidities. Applied Nursing Research. 2008;21:116–122. doi: 10.1016/j.apnr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Creavin ST, Dunn KM, Mallen CD, Nijrolder I, van der Windt DA. Co-occurrence and associations of pain and fatigue in a community sample of Dutch adults. European Journal of Pain. 2010;14:327–334. doi: 10.1016/j.ejpain.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Darko DF, McCutchan JA, Kripke DF, Gillin JC, Golshan S. Fatigue, sleep disturbance, disability and indices of progression of HIV infection. American Journal of Psychiatry. 1992;149:514–520. doi: 10.1176/ajp.149.4.514. [DOI] [PubMed] [Google Scholar]
- Dhruva A, Dodd M, Paul SM, Cooper BA, Lee K, West C, Aouizerat BE, Swift PS, Wara W, Miaskowski C. Trajectories of fatigue in patients with breast cancer before, during and after radiation therapy. Cancer Nursing. 2010;33:201–212. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48:247–252. doi: 10.1176/appi.psy.48.3.247. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Ancoli-Israel S, Elsmore TF, Gruen W. Taking fatigue seriously: I. Variations in fatigue sampled repeatedly in healthy controls. Journal of Medical Engenering &Technology. 2003;27:218–222. doi: 10.1080/0309190031000075354. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Miaskowski C, Lee KA. Occurrence of symptom clusters. Journal of National Cancer Institute Monographs. 2004:76–78. doi: 10.1093/jncimonographs/lgh008. [DOI] [PubMed] [Google Scholar]
- Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biological Research in Nursing. 2004;5:311–318. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson J, Mission S, McDonald K, Pitts M, O’Brien M. The Australian Research Centre in Sex. Health and Society, La Trobe University; Melbourne: 2002. HIV Futures 3: Positive Australians on Services, Health and Well-being; pp. 1–125. [Google Scholar]
- Harding R, Molloy T, Easterbrook P, Frame K, Higginson IJ. Is antiretroviral therapy associated with symptom prevalence and burden? International Journal of STD and AIDS. 2006;17:400–405. doi: 10.1258/095646206777323409. [DOI] [PubMed] [Google Scholar]
- Harmon JL, Barroso J, Pence BW, Leserman J, Salahuddin N. Demographic and illness-related variables associated with HIV-related fatigue. Journal of the Association of Nurses In Aids Care. 2008;19:90–97. doi: 10.1016/j.jana.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M, Safa F, Easterbrook P, Hotopf M. Fatigue among HIV-infected patients in the era of highly active antiretroviral therapy. HIV Medicine. 2005;6:347–352. doi: 10.1111/j.1468-1293.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- Illa L, Brickman A, Saint-Jean G, Echenique M, Metsch L, Eisdorfer C, Bustamante-Avellaneda V, Sanchez-Martinez M. Sexual risk behaviors in late middle age and older HIV seropositive adults. AIDS Behavior. 2008;12:935–942. doi: 10.1007/s10461-008-9370-8. [DOI] [PubMed] [Google Scholar]
- Jenkin P, Koch T, Kralik D. The experience of fatigue for adults living with HIV. Journal of Clinical Nursing. 2006;15:1123–1131. doi: 10.1111/j.1365-2702.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, Wagener MN, van DM, Fischer S, van Gorp EC. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24:1387–1405. doi: 10.1097/QAD.0b013e328339d004. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Archives of Neurology. 1988;45:435–437. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Lee KA, Gay C, Portillo CJ, Coggins T, Davis H, Pullinger CR, Aouizerat BE. Symptom experience in HIV-infected adults: a function of demographic and clinical characteristics. Journal of Pain abd Symptome Management. 2009;38:882–893. doi: 10.1016/j.jpainsymman.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- Lee KA, Lentz MJ, Taylor DL, Mitchell ES, Woods NF. Fatigue as a response to environmental demands in women’s lives. Image – Journal of Nursing Scholarship. 1994;26:149–154. doi: 10.1111/j.1547-5069.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. Journal of Obstetric, Gynecologic and Neonatal Nursing. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The influence of sleep and activity patterns on fatigue in women with HIV/AIDS. Journal of the Association of Nurses In Aids Care. 2001;12(Suppl):19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- Lee KA, Ward TM. Critical components of a sleep assessment for clinical practice settings. Issues in Mental Health Nursing. 2005;26:739–750. doi: 10.1080/01612840591008320. [DOI] [PubMed] [Google Scholar]
- Lerdal A. A concept analysis of energy. Its meaning in the lives of three individuals with chronic illness. Scandinavian Journal of Caring Science. 1998;12:3–10. doi: 10.1080/02839319850163075. [DOI] [PubMed] [Google Scholar]
- Lerdal A. A theoretical extension of the concept of energy through an empirical study. Scandinavian Journal of Caring Science. 2002;16:197–206. doi: 10.1046/j.1471-6712.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- Lerdal A, Kottorp A, Gay C, Aouizerat BE, Portillo C, Lee KA. A 7-item version of the Fatigue Severity Scale has better psychometric properties among HIV-infected adults: An application of a Rasch model. Quality of Life Research. 2010a doi: 10.1007/s11136-011-9877-8. In Press. [DOI] [PubMed] [Google Scholar]
- Lerdal A, Lee KA, Rokne B, Knudsen O, Wahl AK, Dahl AA. A Population-Based Study of Associations Between Current Posttraumatic Stress Symptoms and Current Fatigue. Journal of Traumatic Stress. 2010b;23:606–614. doi: 10.1002/jts.20562. [DOI] [PubMed] [Google Scholar]
- Leserman J, Barroso J, Pence BW, Salahuddin N, Harmon JL. Trauma, stressful life events and depression predict HIV-related fatigue. AIDS Care. 2008;20:1258–1265. doi: 10.1080/09540120801919410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Dropplemann LF. Manual for the Profile of Mood States. Educational and Industrial Testing Services; San Diego: 1971. [Google Scholar]
- Miaskowski C, Paul SM, Cooper BA, Lee K, Dodd M, West C, Aouizerat BE, Swift PS, Wara W. Trajectories of fatigue in men with prostate cancer before, during and after radiation therapy. Journal of Pain and Symptom Management. 2008;35:632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millikin CP, Rourke SB, Halman MH, Power C. Fatigue in HIV/AIDS is associated with depression and subjective neurocognitive complaints but not neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 2003;25:201–215. doi: 10.1076/jcen.25.2.201.13644. [DOI] [PubMed] [Google Scholar]
- Morris AM, So Y, Lee KA, Lash AA, Becker CE. The P300 event-related potential. The effects of sleep deprivation. Journal of Occupational Medicine. 1992;34:1143–1152. [PubMed] [Google Scholar]
- Nikolaus S, Bode C, Taal E, van de Laar MA. Four different patterns of fatigue in rheumatoid arthritis patients: results of a Q-sort study. Rheumatology (Oxford) 2010 doi: 10.1093/rheumatology/keq210. [DOI] [PubMed] [Google Scholar]
- O’Dell MW, Hubert HB, Lubeck DP, O’Driscoll P. Pre-AIDS physical disability: data from the AIDS Time-Oriented Health Outcome Study. Archives of Physical Medicine and Rehabilitation. 1998;79:1200–1205. doi: 10.1016/s0003-9993(98)90262-3. [DOI] [PubMed] [Google Scholar]
- Paddison J, Fricchione G, Gandhi RT, Freudenreich O. Fatigue in psychiatric HIV patients: a pilot study of psychological correlates. Psychosomatics. 2009;50:455–460. doi: 10.1176/appi.psy.50.5.455. [DOI] [PubMed] [Google Scholar]
- Pence BW, Barroso J, Harmon JL, Leserman J, Salahuddin N, Hammill BG. Chronicity and remission of fatigue in patients with established HIV infection. AIDS Patient Care and STDS. 2009;23:239–244. doi: 10.1089/apc.2008.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Barroso J, Leserman J, Harmon JL, Salahuddin N. Measuring fatigue in people living with HIV/AIDS: psychometric characteristics of the HIV-related fatigue scale. AIDS Care. 2008;20:829–837. doi: 10.1080/09540120701694063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KD, Sowell RL, Rojas M, Tavakoli A, Fulk LJ, Hand GA. Physiological and psychological correlates of fatigue in HIV disease. Biological Research for Nursing. 2004;6:59–74. doi: 10.1177/1099800404264846. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Sobel K, Coyle N, Kemeny N, Norton L. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES–D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reisner SL, Mimiaga MJ, Safren SA, Mayer KH. Stressful or traumatic life events, post-traumatic stress disorder (PTSD) symptoms and HIV sexual risk taking among men who have sex with men. AIDS Care. 2009;21:1481–1489. doi: 10.1080/09540120902893258. [DOI] [PubMed] [Google Scholar]
- Salahuddin N, Barroso J, Leserman J, Harmon JL, Pence BW. Daytime sleepiness, nighttime sleep quality, stressful life events and HIV-related fatigue. Journal of the Association of Nurses In Aids Care. 2009;20:6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki GS, Sellers DE, Robinson WM. Self-reported physical and psychological symptom burden in adults with cystic fibrosis. Journal of Pain and Symptom Management. 2008;35:372–380. doi: 10.1016/j.jpainsymman.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwid SR, Covington M, Segal BM, Goodman AD. Fatigue in multiple sclerosis: current understanding and future directions. Journal of Rehabilitation Research and Development. 2002;39:211–224. [PubMed] [Google Scholar]
- Sewell MC, Goggin KJ, Rabkin JG, Ferrando SJ, McElhiney MC, Evans S. Anxiety syndromes and symptoms among men with AIDS: a longitudinal controlled study. Psychosomatics. 2000;41:294–300. doi: 10.1176/appi.psy.41.4.294. [DOI] [PubMed] [Google Scholar]
- Silverberg MJ, Jacobson LP, French AL, Witt MD, Gange SJ. Age and racial/ethnic differences in the prevalence of reported symptoms in human immunodeficiency virus-infected persons on antiretroviral therapy. Journal of Pain and Symptom Management. 2009;38:197–207. doi: 10.1016/j.jpainsymman.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Ware JE, Sherbourne CD, Wells KB. Psychological distress/well-being and cognitive functioning measures. In: Stewart AL, Wareeds JE, editors. Measuring Function and Well-Being: The Medical Outcomes Study Approach. Duke University Press; Durham, North Carolina: 1992. pp. 102–142. [Google Scholar]
- Sullivan PS, Dworkin MS. Prevalence and correlates of fatigue among persons with HIV infection. Journal of Pain and Symptom Management. 2003;25:329–333. doi: 10.1016/s0885-3924(02)00676-0. [DOI] [PubMed] [Google Scholar]
- Theuninck AC, Lake N, Gibson S. HIV-Related Posttraumatic Stress Disorder: Investigating the Traumatic Events. AIDS Patient Care and STDS. 2010;24:485–491. doi: 10.1089/apc.2009.0231. [DOI] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. International Clinical Psychopharmacology. 1999;14:139–151. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- Voss J, Portillo CJ, Holzemer WL, Dodd MJ. Symptom cluster of fatigue and depression in HIV/AIDS. Journal of Prevention & Intervention in the Community. 2007;33:19–34. doi: 10.1300/J005v33n01_03. [DOI] [PubMed] [Google Scholar]
- Voss JG. Predictors and Correlates of Fatigue in HIV/AIDS. Journal of Pain and Symptom Management. 2005;29:173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Vosvick M, Martin LA, Smith NG, Jenkins SR. Gender differences in HIV-related coping and depression. AIDS Behavior. 2010;14:390–400. doi: 10.1007/s10461-008-9490-1. [DOI] [PubMed] [Google Scholar]
- Walker K, McGown A, Jantos M, Anson J. Fatigue, depression and quality of life in HIV-positive men. Journal of Psychosocial Nursing and Mental Health Services. 1997;35:32–40. doi: 10.3928/0279-3695-19970901-17. [DOI] [PubMed] [Google Scholar]
- Whetten K, Reif S, Whetten R, Murphy-McMillan LK. Trauma, mental health, distrust and stigma among HIV-positive persons: implications for effective care. Psychosomatic Medicine. 2008;70:531–538. doi: 10.1097/PSY.0b013e31817749dc. [DOI] [PubMed] [Google Scholar]
- Woods SP, Carey CL, Moran LM, Dawson MS, Letendre SL, Grant I. Frequency and predictors of self-reported prospective memory complaints in individuals infected with HIV. Archives of Clinical Neuropsychology. 2007;22:187–195. doi: 10.1016/j.acn.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AW, Rubin HR, Mathews WC, Brysk LM, Bozzette SA, Hardy WD, Atkinson JH, Grant I, Spector SA, McCutchan JA. Functional status and well-being in a placebo-controlled trial of zidovudine in early symptomatic HIV infection. Journal of Acquired Immune Deficiency Syndromes. 1993;6:452–458. [PubMed] [Google Scholar]


