Abstract
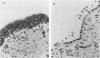
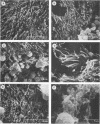
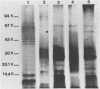
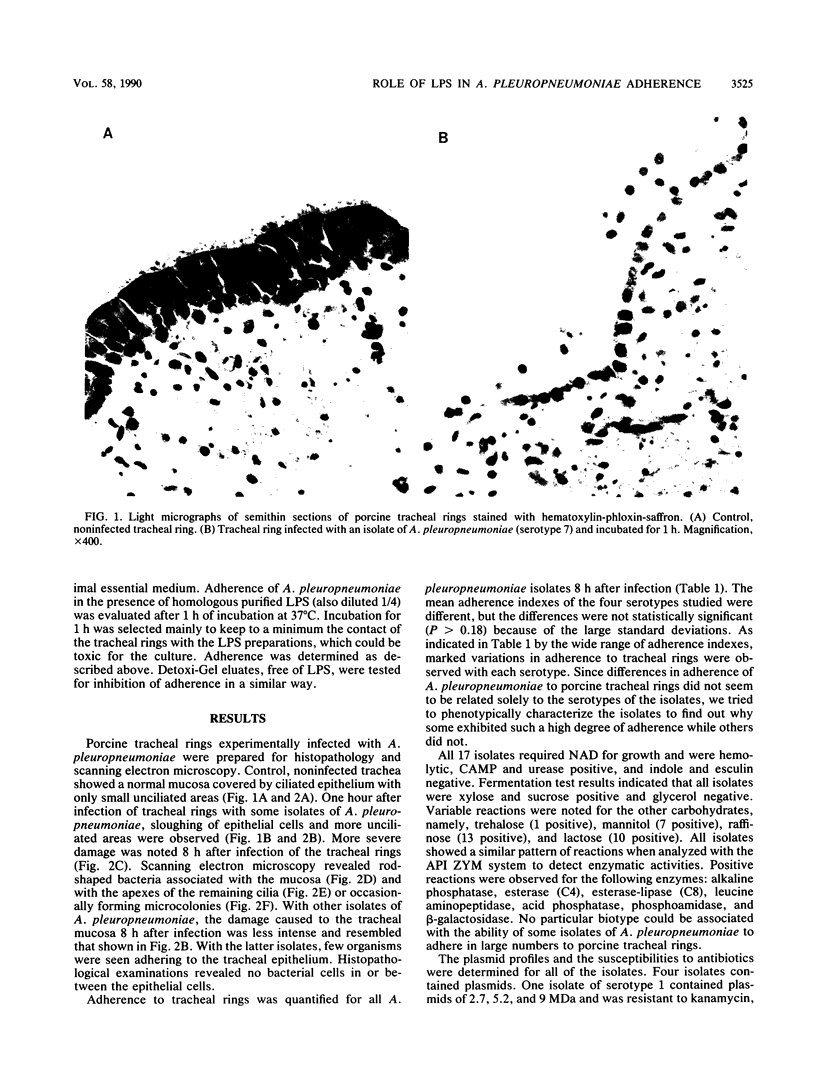
The ability of 17 Actinobacillus pleuropneumoniae isolates, representing serotypes 1, 2, 5, and 7, to adhere to tracheal rings maintained in culture was examined. Porcine tracheal rings were infected, and 8 h after inoculation, adherent bacterial cells were evaluated. A. pleuropneumoniae adhered to tracheal rings, and marked variations were observed between and even within serotypes, suggesting that adherence of this microorganism is not primarily related to the serotype of the isolate. No relationship was found between adherence to porcine tracheal rings and plasmid profiles, virulence in mice, hemagglutination, capsular material thickness, or whole-cell protein profiles. On the other hand, we observed that all isolates of serotypes 1 and 5 had a semirough-type lipopolysaccharide (LPS), whereas isolates of serotypes 2 and 7 had a smooth-type LPS (75%) or a semirough-type LPS (25%). Results showed that 83% of isolates with a smooth-type LPS adhered in large numbers to tracheal rings, whereas 80% of isolates with a semirough-type LPS adhered poorly (P less than 0.007). Our data indicated that the degree of adherence of A. pleuropneumoniae to porcine tracheal rings appeared to be related, at least in part, to LPS profiles. Furthermore, LPS seemed to be the adhesin of A. pleuropneumoniae, since purified LPS blocked adherence of this microorganism to porcine tracheal rings.
Full text
PDF

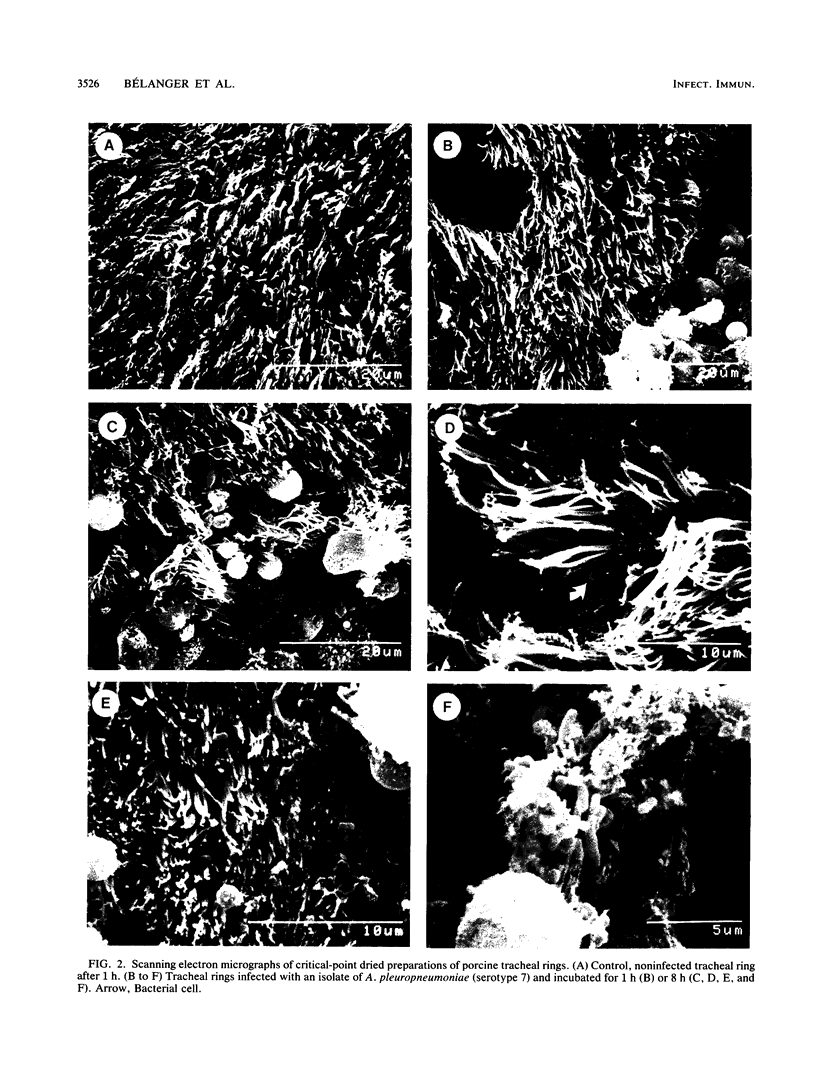
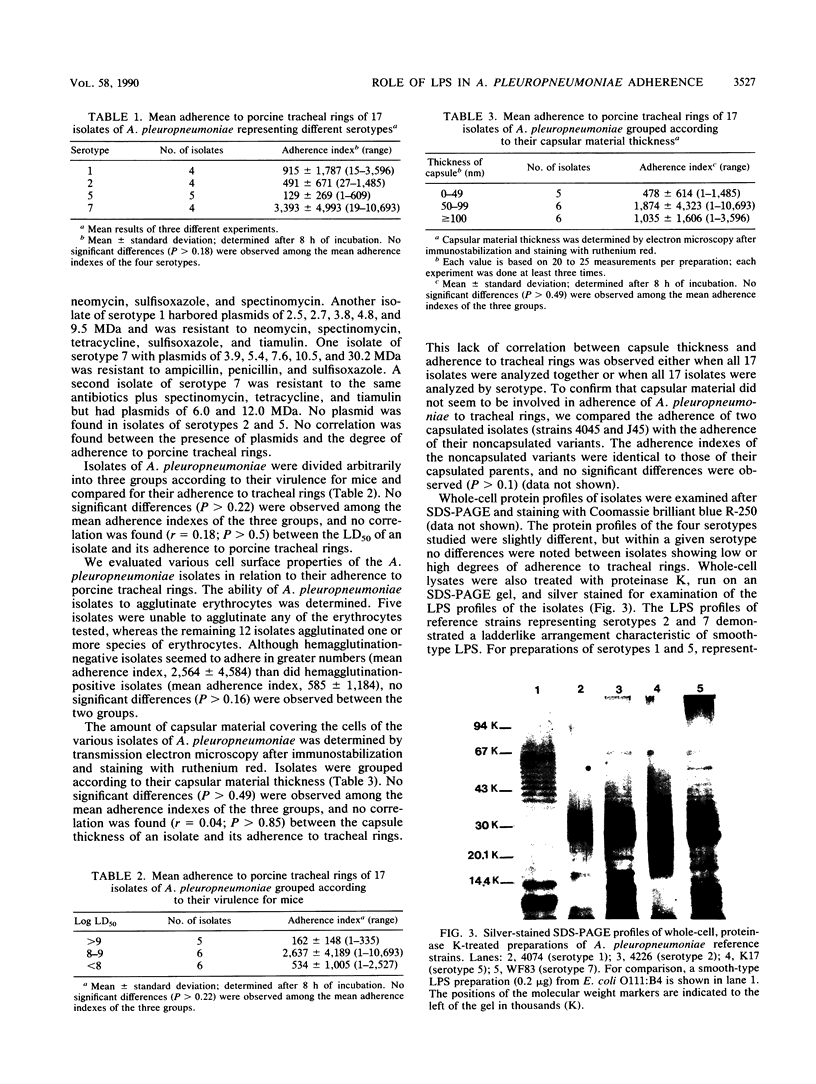



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp L. H., Brooks E. E. An in vivo model for the study of Bordetella avium adherence to tracheal mucosa in turkeys. Am J Vet Res. 1986 Dec;47(12):2614–2617. [PubMed] [Google Scholar]
- Arp L. H., Leyh R. D., Griffith R. W. Adherence of Bordetella avium to tracheal mucosa of turkeys: correlation with hemagglutination. Am J Vet Res. 1988 May;49(5):693–696. [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broes A., Fairbrother J. M., Larivière S., Jacques M., Johnson W. M. Virulence properties of enterotoxigenic Escherichia coli O8: KX105 strains isolated from diarrheic piglets. Infect Immun. 1988 Jan;56(1):241–246. doi: 10.1128/iai.56.1.241-246.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd W., Kadis S. Structures and sugar compositions of lipopolysaccharides isolated from seven Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1989 Dec;57(12):3901–3906. doi: 10.1128/iai.57.12.3901-3906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugal F., Girard C., Jacques M. Adherence of Bordetella bronchiseptica 276 to porcine trachea maintained in organ culture. Appl Environ Microbiol. 1990 Jun;56(6):1523–1529. doi: 10.1128/aem.56.6.1523-1529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I., Olander H. J. Isolation and biological characterization of two lipopolysaccharides and a capsular-enriched polysaccharide preparation from Haemophilus pleuropneumoniae. Am J Vet Res. 1986 Jul;47(7):1433–1441. [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Foiry B., Higgins R., Mittal K. R. Electron microscopic examination of capsular material from various serotypes of Actinobacillus pleuropneumoniae. J Bacteriol. 1988 Jul;170(7):3314–3318. doi: 10.1128/jb.170.7.3314-3318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Parent N., Foiry B. Adherence of Bordetella bronchiseptica and Pasteurella multocida to porcine nasal and tracheal epithelial cells. Can J Vet Res. 1988 Apr;52(2):283–285. [PMC free article] [PubMed] [Google Scholar]
- Jacques M., Roy G., Mittal K. R. Hemagglutinating properties of Actinobacillus pleuropneumoniae. Can J Microbiol. 1988 Sep;34(9):1046–1049. doi: 10.1139/m88-184. [DOI] [PubMed] [Google Scholar]
- Jensen A. E., Bertram T. A. Morphological and biochemical comparison of virulent and avirulent isolates of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1986 Feb;51(2):419–424. doi: 10.1128/iai.51.2.419-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LiPuma J. J., Gilsdorf J. R. Role of capsule in adherence of Haemophilus influenzae type b to human buccal epithelial cells. Infect Immun. 1987 Sep;55(9):2308–2310. doi: 10.1128/iai.55.9.2308-2310.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Martin P. G., Lachance P., Niven D. F. Production of RNA-dependent haemolysin by Haemophilus pleuropneumoniae. Can J Microbiol. 1985 May;31(5):456–462. doi: 10.1139/m85-085. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S. Identification and serotyping of Haemophilus pleuropneumoniae by coagglutination test. J Clin Microbiol. 1983 Dec;18(6):1351–1354. doi: 10.1128/jcm.18.6.1351-1354.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Characterization of the hemolysin produced by haemophilus pleuropneumoniae. Am J Vet Res. 1983 Feb;44(2):344–347. [PubMed] [Google Scholar]
- Nielsen R. Serological characterization of Actinobacillus pleuropneumoniae strains and proposal of a new serotype: serotype 12. Acta Vet Scand. 1986;27(3):453–455. doi: 10.1186/BF03548158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly T., Rosendal S., Niven D. F. Porcine haemophili and actinobacilli: characterization by means of API test strips and possible taxonomic implications. Can J Microbiol. 1984 Oct;30(10):1229–1238. doi: 10.1139/m84-195. [DOI] [PubMed] [Google Scholar]
- Pijoan C. Effect of Pasteurella multocida and Haemophilus pleuropneumoniae toxins on swine alveolar macrophages. Vet Immunol Immunopathol. 1986 Sep;13(1-2):141–149. doi: 10.1016/0165-2427(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F. Antibody response of swine to outer membrane components of Haemophilus pleuropneumoniae during infection. Infect Immun. 1986 Dec;54(3):751–760. doi: 10.1128/iai.54.3.751-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Jacobs R. F., Haas J. E., Smith A. L. Adherence of Haemophilus influenzae to monkey respiratory tissue in organ culture. J Gen Microbiol. 1984 Jun;130(6):1437–1447. doi: 10.1099/00221287-130-6-1437. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]