Abstract
Chlorine is considered a chemical threat agent to which humans may be exposed as a result of accidental or intentional release. Chlorine is highly reactive, and inhalation of the gas causes cellular damage to the respiratory tract, inflammation, pulmonary edema, and airway hyperreactivity. Drugs that increase intracellular levels of the signaling molecule cyclic AMP (cAMP) may be useful for treatment of acute lung injury through effects on alveolar fluid clearance, inflammation, and airway reactivity. This article describes mechanisms by which cAMP regulates cellular processes affecting lung injury and discusses the basis for investigating drugs that increase cAMP levels as potential treatments for chlorine-induced lung injury. The effects of β2-adrenergic agonists, which stimulate cAMP synthesis, and phosphodiesterase inhibitors, which inhibit cAMP degradation, on acute lung injury are reviewed, and the relative advantages of these approaches are compared.
Keywords: β-adrenergic agonists, phosphodiesterase inhibitors, acute lung injury
CHLORINE-INDUCED LUNG INJURY
Chlorine gas is a highly toxic and widely used industrial chemical. Chlorine is employed in the purification of drinking water and in the production of plastics, solvents, pharmaceuticals, and various other chemicals. Because of its toxicity and the large amounts that are transported and used in the United States, chorine is considered a chemical threat agent that could inflict large numbers of casualties following accidental or intentional release. Chlorine has been used as a chemical weapon in the Iraq war, and accidental releases of chlorine leading to human casualties have occurred within the United States.
Chlorine is a strong oxidant that, when inhaled, causes acute lung injury through direct damage to cells of the respiratory tract. Clinical symptoms of chlorine intoxication include dyspnea, airway obstruction, cough, pulmonary edema, pneumonitis, cyanosis, nausea, vomiting, and loss of consciousness (1–3). Lung injury induced by chlorine inhalation has been investigated using animal models in multiple species, including pigs, rats, rabbits, mice, and dogs (4–9). Common features of lung injury in such models are epithelial cell damage, vascular leakage, pulmonary edema, airway hyperreactivity, production of inflammatory mediators, and influx of neutrophils into lung tissue. Efforts to develop medical countermeasures for chlorine poisoning have focused on preventing or reversing these aspects of lung injury.
CYCLIC AMP AS A SIGNALING MEDIATOR
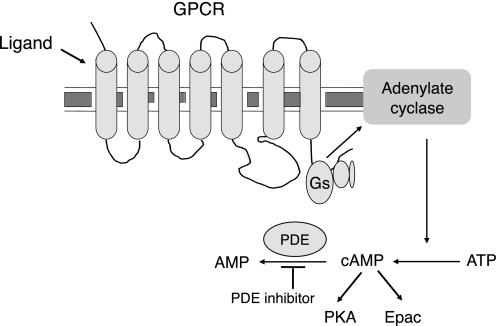
Cyclic AMP (cAMP) is an intracellular signaling molecule that regulates a broad range of cellular processes. cAMP is formed from ATP by the action of the enzyme adenylate cyclase (Figure 1). Adenylate cyclase is a transmembrane protein whose activity is regulated by G protein–coupled receptors (GPCRs), specifically those receptors for which ligand binding is coupled to activation of the Gs type of G protein. Examples of GPCRs coupled to Gs and adenylate cyclase are the β2-adrenergic receptor, EP2 and EP4 prostaglandin receptors, A2A and A2B adenosine receptors, V2 vasopressin receptor, and VPAC receptors for vasoactive intestinal peptide and pituitary adenylate cyclase activating peptide. Signaling initiated by cAMP formation is terminated in part through the degradation of the compound by phosphodiesterases (PDE). Intracellular cAMP concentrations are therefore determined by the balance between cAMP synthesis by adenylate cyclase and its breakdown by PDE. Consequently, cAMP levels can be increased through pharmacologic means by either stimulating production with GPCR ligands such as β2-adrenergic agonists (β agonists) or inhibiting degradation with PDE inhibitors (Figure 1).
Figure 1.
Production and degradation of cyclic AMP (cAMP). The production of cAMP is stimulated by binding of ligand to a Gs-coupled G protein–coupled receptor, leading to activation of adenylate cyclase. Adenylate cyclase catalyzes the formation of cAMP from ATP. Phosphodiesterase (PDE) enzymes catalyze the degradation of cAMP to AMP. PDE inhibitors block degradation of cAMP, leading to increased intracellular concentrations of this mediator. Downstream effects of cAMP are mediated through protein kinase A and Epac pathways.
Intracellular cAMP formed as a result of adenylate cyclase activation triggers cellular responses via two main signal transduction pathways. One pathway involves the activation of protein kinase A (PKA), which occurs though binding of cAMP to the regulatory subunit of the kinase resulting in its dissociation from the catalytic subunit (10, 11). PKA directly phosphorylates a variety of proteins, such as phosphorylase kinase and pyruvate kinase, to directly regulate their activity. In addition, PKA regulates the transcription of an additional set of genes through its ability to activate specific members of the bZIP family of transcription factors, most notably cAMP response element–binding protein (CREB). CREB and related factors bind to an 8–base pair DNA sequence in the promoter region of responsive genes (12). Phosphorylation of CREB by PKA allows binding by the transcriptional coactivator proteins CBP (CREB-binding protein) or p300, leading to the recruitment of RNA polymerase II and initiation of transcription (13). The types of genes regulated by cAMP/CREB signaling are diverse and include hormones, growth factors, enzymes, transcription factors, and structural proteins (14).
A second, PKA-independent, signal transduction pathway triggered by cAMP production is the activation of proteins of the Epac family (exchange proteins directly activated by cAMP). Epac1 and Epac2 are guanine nucleotide exchange factors (GEFs) that activate members of the Ras family of small GTPases, including Rap1 and Rap2. The binding of cAMP to Epac proteins stimulates their GEF function, resulting in release of GDP from, and subsequent binding of GTP to, Rap1 and Rap2 (15, 16). Through the action of these small GTPases, Epac signaling affects multiple cellular processes, such as ion channel function, exocytosis, cell motility, proliferation, and survival (17, 18).
BENEFICIAL EFFECTS OF INCREASED CAMP LEVELS IN LUNG INJURY
Elevation of cAMP levels by pharmacologic means has the potential to result in multiple beneficial effects in the injured lung. Agents that raise cAMP levels are known to stimulate alveolar fluid transport, to inhibit inflammation, and to induce bronchodilation. Increased alveolar fluid transport following acute lung injury is beneficial because of the potential to speed the resolution of pulmonary edema. Alveolar epithelial cells mediate the movement of water from the airspaces to the lung interstitium through the production of an osmotic pressure gradient via the transport of ions. Increased cAMP production stimulates alveolar fluid transport through its effects on the expression and function of ion channels such as the epithelial sodium channel (ENaC) and the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel (19). cAMP regulates ENaC function by increasing subunit gene expression (20–22), enhancing the open probability of the channel (23, 24), and altering intracellular trafficking to increase the number of channels on the cell surface (22, 25). These effects appear to occur through both PKA-dependent (26, 24) and -independent mechanisms (25), including Epac (23). CFTR expression is up-regulated by a transcriptional mechanism mediated by PKA (27) and by a post-transcriptional mechanism that is independent of PKA (28). PKA also directly phosphorylates CFTR to activate the channel (29, 30).
Agents that raise cAMP levels produce widespread dampening of inflammatory processes, including decreased inflammatory mediator production and inhibition of macrophage, lymphocyte, eosinophil, and neutrophil function (31). In neutrophils, which are predominant inflammatory cells in acute lung injury, cAMP-elevating agents reduce neutrophil adhesion (32), chemotaxis (33), and degranulation (34). Treatments that increase cAMP levels also inhibit endothelial permeability (35), and this may contribute to suppression of inflammatory cell influx from the circulation into the injured lung. The anti-inflammatory effects of cAMP appear to occur through diverse mechanisms dependent on both PKA and Epac (36–41).
An additional beneficial effect of elevated cAMP levels in the lung is bronchodilation produced by smooth muscle relaxation. Airway smooth muscle constriction is produced though the action of GPCRs coupled to the Gq family of G proteins. Gq signaling leads to intracellular calcium release, which activates myosin light chain kinase, resulting in myosin phosphorylation and muscle contraction. Raising cAMP levels by stimulation of Gs-coupled GPCRs, or by other means, activates PKA. This enzyme in turn inhibits smooth muscle contraction by inhibition of calcium release, stimulation of myosin light chain phosphatase, and inhibition of Gq signaling (42). cAMP signaling appears to play a minor role in maintenance of basal airway smooth muscle tone under normal conditions, but agents that raise cAMP levels are effective inhibitors of bronchoconstriction and airway hyperreactivity in pathologic states.
β2-ADRENERGIC AGONISTS
The β2-adrenergic receptor is a Gs-coupled GPCR that, upon ligand binding, stimulates the production of cAMP. In the lung, prominent sites of β2-adrenergic receptor expression are alveolar epithelial cells, in which it mediates alveolar fluid transport, and airway smooth muscle cells, in which it mediates bronchodilation. Effects of β-agonists on alveolar fluid transport were first suggested in experiments using cultured rat alveolar epithelial cells (43). Subsequent studies have shown that this phenomenon is likely due to combined effects on ENaC, CFTR, and Na,K-ATPase, which are active in both type I and type II cells (44, 45). Treatment with β-agonists stimulates alveolar fluid transport in isolated lungs (46) and in intact, uninjured lungs (47, 48). In animal models of acute lung injury, β-agonists show beneficial effects on alveolar fluid transport as well as limitation or resolution of pulmonary edema (49, 50). In pigs exposed to chlorine gas, aerosolized terbutaline led to increased arterial oxygen tension and lung compliance (51). In chlorine-exposed mice, intranasal delivery of formoterol resulted in increased alveolar fluid clearance and decreased airway reactivity to methacholine (52). In human subjects, prophylactic treatment with salmeterol inhibited the development of high-altitude pulmonary edema (53). Administration of aerosolized salbutamol reduced post-surgical increases in extravascular lung water and improved oxygenation in patients undergoing lung resection (54). In patients with adult respiratory stress syndrome (ARDS), intravenous salbutamol resulted in a decrease in pulmonary edema as measured by extravascular lung water (55). In contrast, a larger trial involving aerosolized albuterol did not show any difference in mortality or ventilator-free days (56). Thus substantial evidence has been gathered from animal models to support the concept of using β-agonists to treat acute lung injury in general and chlorine injury in particular, yet results from human ARDS trials are mixed. Targeting delivery methods and treatment regimens specifically for chlorine-induced lung injury may potentially result in increased efficacy toward this particular type of acute lung injury.
PHOSPHODIESTERASE INHIBITORS
PDE enzymes have been grouped into eleven families based on their structural and functional properties, including whether they can metabolize cAMP, cyclic GMP, or both. Type 4 PDEs are cAMP-specific enzymes that are important in regulating cAMP turnover in lung and inflammatory cells. Type 4 PDEs represent major isozymes in lung epithelial cells (57, 58), airway smooth muscle cells (59), and multiple inflammatory cell types (60). Therapeutic effects of PDE4 inhibitors such as rolipram, roflumilast, and cilomilast have been tested in lung injury models. For example, rolipram has been shown to inhibit lung injury and/or inflammation induced by LPS (61), hyperoxia (62), and cardiopulmonary bypass (63). Treatment with roflumilast inhibited lung injury induced by cigarette smoke (64) and bleomycin (65). Rolipram has been shown to inhibit airway hyperreactivity induced by various stimuli, including allergen (66), respiratory syncytial virus (67), cigarette smoke (68), and LPS (69). Additional support for the use of PDE inhibitors in acute lung injury caused by chemical agents comes from studies with pentoxifylline, a nonspecific PDE inhibitor, which has shown significant effects in ameliorating injury following exposure to acid (70, 71). In clinical trials for chronic obstructive pulmonary disease (COPD), roflumilast and cilomilast have been shown to improve airflow obstruction, reduce exacerbations, and inhibit inflammation (72–75).
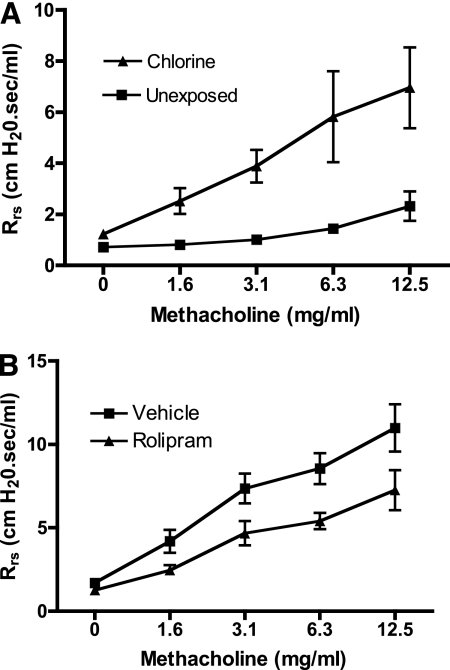
We have tested the effects of the PDE4 inhibitor rolipram on airway hyperreactivity induced by chlorine inhalation. Inbred FVB/N mice were exposed to chlorine, and the following day, respiratory system resistance in anesthetized, mechanically ventilated mice was measured at baseline and after increasing doses of aerosolized methacholine. Chlorine exposure produced a pronounced airway hyperreactivity to inhaled methacholine (Figure 2A). Delivery of rolipram to the lungs via intranasal administration 1 hour and 10 hours after chlorine exposure inhibited chlorine-induced airway hyperreactivity (Figure 2B).
Figure 2.
Effect of rolipram on airway hyperreactivity in chlorine-exposed mice. (A) Mice were exposed to a dose of 250 ppm-hour chlorine (8). The following day, respiratory mechanics were measured in anesthetized, mechanically ventilated mice at baseline and after inhalation of aerosolized methacholine using a FlexiVent system (SCIREQ, Montreal, PQ, Canada). The responses of chlorine-exposed and unexposed mice were significantly different (P < 0.05 by repeated measures ANOVA; n = 4 mice/group). (B) Mice were exposed to 256 ± 3 ppm-hour chlorine (mean ± SE, three exposures). Mice received rolipram (300 μg/kg) or vehicle intranasally 1 hour and then again 10–11 hours after exposure. The day after exposure, airway reactivity was measured as in A. The responses of rolipram- and vehicle-treated mice were significantly different (P < 0.05 by repeated measures ANOVA; n = 8–9 mice/group).
β-AGONISTS VERSUS PDE INHIBITORS FOR THE TREATMENT CHLORINE-INDUCED LUNG INJURY
Relative advantages and disadvantages of β-agonists versus PDE inhibitors for the treatment of chlorine-induced lung injury are summarized in Table 1. An advantage of β-agonists is their long history of use to treat asthma and other lung diseases, and the evidence that they are safe in the vast majority of patients. However, a disadvantage of their use is the fact that continuous stimulation of β2-adrenergic receptors typically leads to significant reductions in response over time. This phenomenon, which is known as desensitization or tolerance, results from multiple mechanisms, including uncoupling of receptor from G protein signaling, increased PDE activity, receptor internalization, receptor degradation, and down-regulation of receptor expression (76). β-agonists typically retain effectiveness for the treatment of asthma in part because the main target of these drugs in this disease is airway smooth muscle, which appears to be more refractory to desensitization than other cell types (76). The efficacy of β-agonists in treating chlorine-induced lung injury is dependent on activity in other cell types such as alveolar epithelial cells. Although the importance of desensitization may be mitigated when considering short-term treatment following chlorine exposure in otherwise healthy individuals, reduced responses to β-agonists in individuals with asthma who regularly receive these drugs may limit the effectiveness of such a strategy for treating chlorine injury in this population. In addition, injury to the lung can result in impaired β-receptor function, which could potentially reduce the efficacy of β-agonist treatment (45). For example, in a model of lung injury induced by hemorrhagic shock in rats, endogenously released nitric oxide was shown to inhibit catecholamine-mediated alveolar fluid clearance (77) and TGF-β1 was shown to impair β-adrenergic receptor–mediated chloride transport and alveolar fluid clearance through desensitization mechanisms dependent on phosphatidylinositol 3-kinase (78). A final disadvantage of β-agonists is that some drugs of this class have been associated with increased, although rare, serious adverse effects in individuals with asthma (79).
TABLE 1.
ADVANTAGES AND DISADVANTAGES OF β-AGONISTS AND PHOSPHODIESTERASE INHIBITORS FOR THE TREATMENT OF CHLORINE-INDUCED ACUTE LUNG INJURY
| Drug Type | Advantages | Disadvantages |
|---|---|---|
| β-agonist | Long history of use in patients with lung disease | Desensitization or tolerance |
| Rare adverse effects | ||
| Phosphodiesterase inhibitor | No desensitization or tolerance | Gastrointestinal side effects |
| More significant antiinflammatory activity |
In contrast to β-agonists, PDE inhibitors do not appear to lose significant activity with continued administration, which represents an advantage for this class of compounds in treating lung injury. In addition, the anti-inflammatory effects of PDE inhibitors are more widespread and of greater magnitude than those induced by β-agonists. This phenomenon may be in part a result of desensitization induced by β-agonists in inflammatory cells. The major disadvantage of PDE inhibitors relative to β-agonists is the occurrence of gastrointestinal side effects caused by the action of the former class of drugs in emesis centers in the brainstem and in intestinal epithelial cells (80, 81). Such side effects represent the major barrier to the use of orally administered PDE4 inhibitors in chronic lung diseases such as asthma and COPD. It is possible that this problem could be obviated by local delivery of PDE inhibitors to the lung via the inhaled route. In addition, PDE inhibitors would be administered for a limited period of time for acute injury induced by chlorine gas inhalation, so any potential side effects may be limited or better tolerated.
CONCLUSIONS
Cyclic AMP regulates multiple cellular processes that regulate normal cell function and responses to injury. Manipulating the production and/or degradation of cAMP to raise the levels of this signaling mediator represents a therapeutic approach for the treatment of chlorine-induced lung injury. Raising cAMP levels stimulates alveolar fluid transport, inhibits inflammation, and relaxes airway smooth muscle. β-agonists and PDE inhibitors have both been documented to raise cAMP levels and produce beneficial effects in injured lungs. The current key challenge is to identify an optimal combination of drug and delivery method to produce the desired therapeutic effects in the chlorine-injured lung while minimizing unwanted effects elsewhere.
Acknowledgments
The author acknowledges the technical assistance of Weiyuan Chang, Jing Chen, and Connie S. Schlueter.
The research is supported by the CounterACT Program, National Institutes Of Health Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number NIH U01 ES015673.
Conflict of Interest Statement: G.W.H. served as a consultant for Sepracor ($1,000–$5,000). He received grant support from Sepracor ($10,001–$50,000) and the National Institutes of Health (more than $100,001).
References
- 1.Adelson L, Kaufman J. Fatal chlorine poisoning: report of two cases with clinicopathologic correlation. Am J Clin Pathol 1971;56:430–442. [DOI] [PubMed] [Google Scholar]
- 2.Joyner RE, Durel EG. Accidental liquid chlorine spill in a rural community. J Occup Med 1962;4:152–154. [PubMed] [Google Scholar]
- 3.Weill H, George R, Schwarz M, Ziskind M. Late evaluation of pulmonary function after acute exposure to chlorine gas. Am Rev Respir Dis 1969;99:374–379. [PubMed] [Google Scholar]
- 4.Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol 1998;18:249–255. [DOI] [PubMed] [Google Scholar]
- 5.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 2008;295:L733–L743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 2003;168:568–574. [DOI] [PubMed] [Google Scholar]
- 7.Menaouar A, Anglade D, Baussand P, Pelloux A, Corboz M, Lantuejoul S, Benchetrit G, Grimbert FA. Chlorine gas induced acute lung injury in isolated rabbit lung. Eur Respir J 1997;10:1100–1107. [DOI] [PubMed] [Google Scholar]
- 8.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 2008;20:783–793. [DOI] [PubMed] [Google Scholar]
- 9.Winternitz MC, Lambert RA, Jackson L, Smith GH. The pathology of chlorine poisoning. New Haven: Yale University School of Medicine; 1920.
- 10.Hofmann F, Beavo JA, Bechtel PJ, Krebs EG. Comparison of adenosine 3′:5′-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem 1975;250:7795–7801. [PubMed] [Google Scholar]
- 11.Tao M, Salas ML, Lipmann F. Mechanism of activation by adenosine 3′:5′-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci USA 1970;67:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montminy MR, Sevarino KA, Wagner JA, Mandel G, Goodman RH. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci USA 1986;83:6682–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 1999;68:821–861. [DOI] [PubMed] [Google Scholar]
- 14.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem 1997;66:807–822. [DOI] [PubMed] [Google Scholar]
- 15.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 1998;396:474–477. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 1998;282:2275–2279. [DOI] [PubMed] [Google Scholar]
- 17.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol 2006;577:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol 2008;377:345–357. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thor Soc 2005;2:206–213. [DOI] [PubMed] [Google Scholar]
- 20.Dagenais A, Denis C, Vives MF, Girouard S, Masse C, Nguyen T, Yamagata T, Grygorczyk C, Kothary R, Berthiaume Y. Modulation of alpha-ENaC and alpha1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L217–L230. [DOI] [PubMed] [Google Scholar]
- 21.Minakata Y, Suzuki S, Grygorczyk C, Dagenais A, Berthiaume Y. Impact of beta-adrenergic agonist on Na+ channel and Na+-K+-ATPase expression in alveolar type II cells. Am J Physiol 1998;275:L414–L422. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CP, Campbell JR, Wright PJ, Husted RF. cAMP-stimulated Na+ transport in H441 distal lung epithelial cells: role of PKA, phosphatidylinositol 3-kinase, and sgk1. Am J Physiol Lung Cell Mol Physiol 2004;287:L843–L851. [DOI] [PubMed] [Google Scholar]
- 23.Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L710–L722. [DOI] [PubMed] [Google Scholar]
- 24.Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem 1997;272:14037–14040. [DOI] [PubMed] [Google Scholar]
- 25.Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's beta- and gamma-subunit. J Biol Chem 2006;281:9859–9868. [DOI] [PubMed] [Google Scholar]
- 26.Mustafa SB, Castro R, Falck AJ, Petershack JA, Henson BM, Mendoza YM, Choudary A, Seidner SR. Protein kinase A and mitogen-activated protein kinase pathways mediate cAMP induction of alpha-epithelial Na+ channels (alpha-ENaC). J Cell Physiol 2008;215:101–110. [DOI] [PubMed] [Google Scholar]
- 27.McDonald RA, Matthews RP, Idzerda RL, McKnight GS. Basal expression of the cystic fibrosis transmembrane conductance regulator gene is dependent on protein kinase A activity. Proc Natl Acad Sci USA 1995;92:7560–7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taouil K, Hinnrasky J, Hologne C, Corlieu P, Klossek JM, Puchelle E. Stimulation of beta 2-adrenergic receptor increases cystic fibrosis transmembrane conductance regulator expression in human airway epithelial cells through a cAMP/protein kinase A-independent pathway. J Biol Chem 2003;278:17320–17327. [DOI] [PubMed] [Google Scholar]
- 29.Berger HA, Anderson MP, Gregory RJ, Thompson S, Howard PW, Maurer RA, Mulligan R, Smith AE, Welsh MJ. Identification and regulation of the cystic fibrosis transmembrane conductance regulator-generated chloride channel. J Clin Invest 1991;88:1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabcharani JA, Chang XB, Riordan JR, Hanrahan JW. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature 1991;352:628–631. [DOI] [PubMed] [Google Scholar]
- 31.Souness JE, Aldous D, Sargent C. Immunosuppressive and anti-inflammatory effects of cyclic AMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacol 2000;47:127–162. [DOI] [PubMed] [Google Scholar]
- 32.Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cAMP modulators. J Immunol 1995;154:308–317. [PubMed] [Google Scholar]
- 33.Harvath L, Robbins JD, Russell AA, Seamon KB. cAMP and human neutrophil chemotaxis: elevation of cAMP differentially affects chemotactic responsiveness. J Immunol 1991;146:224–232. [PubMed] [Google Scholar]
- 34.Barnette MS, Bartus JO, Burman M, Christensen SB, Cieslinski LB, Esser KM, Prabhakar US, Rush JA, Torphy TJ. Association of the anti-inflammatory activity of phosphodiesterase 4 (PDE4) inhibitors with either inhibition of PDE4 catalytic activity or competition for [3H]rolipram binding. Biochem Pharmacol 1996;51:949–956. [DOI] [PubMed] [Google Scholar]
- 35.Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest 1993;91:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 2008;215:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bopp T, Dehzad N, Reuter S, Klein M, Ullrich N, Stassen M, Schild H, Buhl R, Schmitt E, Taube C. Inhibition of cAMP degradation improves regulatory T cell-mediated suppression. J Immunol 2009;182:4017–4024. [DOI] [PubMed] [Google Scholar]
- 38.Flamand N, Surette ME, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol 2002;62:250–256. [DOI] [PubMed] [Google Scholar]
- 39.Manning CD, McLaughlin MM, Livi GP, Cieslinski LB, Torphy TJ, Barnette MS. Prolonged beta adrenoceptor stimulation up-regulates cAMP phosphodiesterase activity in human monocytes by increasing mRNA and protein for phosphodiesterases 4A and 4B. J Pharmacol Exp Ther 1996;276:810–818. [PubMed] [Google Scholar]
- 40.O'Dowd YM, El-Benna J, Perianin A, Newsholme P. Inhibition of formyl-methionyl-leucyl-phenylalanine-stimulated respiratory burst in human neutrophils by adrenaline: inhibition of Phospholipase A2 activity but not p47phox phosphorylation and translocation. Biochem Pharmacol 2004;67:183–190. [DOI] [PubMed] [Google Scholar]
- 41.Orlic T, Loomis WH, Shreve A, Namiki S, Junger WG. Hypertonicity increases cAMP in PMN and blocks oxidative burst by PKA-dependent and -independent mechanisms. Am J Physiol Cell Physiol 2002;282:C1261–C1269. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande DA, Penn RB. Targeting G protein-coupled receptor signaling in asthma. Cell Signal 2006;18:2105–2120. [DOI] [PubMed] [Google Scholar]
- 43.Goodman BE, Brown SE, Crandall ED. Regulation of transport across pulmonary alveolar epithelial cell monolayers. J Appl Physiol 1984;57:703–710. [DOI] [PubMed] [Google Scholar]
- 44.Johnson M, Allen L, Dobbs L. Characteristics of Cl- uptake in rat alveolar type I cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L816–L827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mutlu GM, Factor P. Alveolar epithelial beta2-adrenergic receptors. Am J Respir Cell Mol Biol 2008;38:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med 1997;155:506–512. [DOI] [PubMed] [Google Scholar]
- 47.Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest 1987;79:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol 1994;76:2636–2642. [DOI] [PubMed] [Google Scholar]
- 49.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem 2006;281:19892–19898. [DOI] [PubMed] [Google Scholar]
- 50.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med 2004;32:1470–1476. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Zhang L, Walther SM. Administration of aerosolized terbutaline and budesonide reduces chlorine gas-induced acute lung injury. J Trauma 2004;56:850–862. [DOI] [PubMed] [Google Scholar]
- 52.Wei S, Song W, Doran S, Estell K, Schwiebert LM, Matalon S. Mitigation of chlorine induced lung injury by Brovana. Am J Respir Crit Care Med 2009;179:A5656. [Google Scholar]
- 53.Sartori C, Allemann Y, Duplain H, Lepori M, Egli M, Lipp E, Hutter D, Turini P, Hugli O, Cook S, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med 2002;346:1631–1636. [DOI] [PubMed] [Google Scholar]
- 54.Licker M, Tschopp JM, Robert J, Frey JG, Diaper J, Ellenberger C. Aerosolized salbutamol accelerates the resolution of pulmonary edema after lung resection. Chest 2008;133:845–852. [DOI] [PubMed] [Google Scholar]
- 55.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 2006;173:281–287. [DOI] [PubMed] [Google Scholar]
- 56.Matthay MA, Brower R, Thompson BT, Schoenfeld D, Eisner MD, Carson S, Moss M, Douglas I, Hite D, MacIntyre N, et al. Randomized, placebo-controlled trial of an aerosolized beta-2 adrenergic agonist (albuterol) for the treatment of acute lung injury. Am J Respir Crit Care Med 2009;179:A2166. [Google Scholar]
- 57.Dent G, White SR, Tenor H, Bodtke K, Schudt C, Leff AR, Magnussen H, Rabe KF. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitors. Pulm Pharmacol Ther 1998;11:47–56. [DOI] [PubMed] [Google Scholar]
- 58.Fuhrmann M, Jahn HU, Seybold J, Neurohr C, Barnes PJ, Hippenstiel S, Kraemer HJ, Suttorp N. Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol 1999;20:292–302. [DOI] [PubMed] [Google Scholar]
- 59.Torphy TJ, Undem BJ, Cieslinski LB, Luttmann MA, Reeves ML, Hay DW. Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscle. J Pharmacol Exp Ther 1993;265:1213–1223. [PubMed] [Google Scholar]
- 60.Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998;157:351–370. [DOI] [PubMed] [Google Scholar]
- 61.Miotla JM, Teixeira MM, Hellewell PG. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am J Respir Cell Mol Biol 1998;18:411–420. [DOI] [PubMed] [Google Scholar]
- 62.de Visser YP, Walther FJ, Laghmani EH, van Wijngaarden S, Nieuwland K, Wagenaar GT. Phosphodiesterase-4 inhibition attenuates pulmonary inflammation in neonatal lung injury. Eur Respir J 2008;31:633–644. [DOI] [PubMed] [Google Scholar]
- 63.Hamamoto M, Suga M, Nakatani T, Takahashi Y, Sato Y, Inamori S, Yagihara T, Kitamura S. Phosphodiesterase type 4 inhibitor prevents acute lung injury induced by cardiopulmonary bypass in a rat model. Eur J Cardiothorac Surg 2004;25:833–838. [DOI] [PubMed] [Google Scholar]
- 64.Martorana PA, Beume R, Lucattelli M, Wollin L, Lungarella G. Roflumilast fully prevents emphysema in mice chronically exposed to cigarette smoke. Am J Respir Crit Care Med 2005;172:848–853. [DOI] [PubMed] [Google Scholar]
- 65.Cortijo J, Iranzo A, Milara X, Mata M, Cerda-Nicolas M, Ruiz-Sauri A, Tenor H, Hatzelmann A, Morcillo EJ. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol 2009;156:534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Toward TJ, Broadley KJ. Early and late bronchoconstrictions, airway hyper-reactivity, leucocyte influx and lung histamine and nitric oxide after inhaled antigen: effects of dexamethasone and rolipram. Clin Exp Allergy 2004;34:91–102. [DOI] [PubMed] [Google Scholar]
- 67.Ikemura T, Schwarze J, Makela M, Kanehiro A, Joetham A, Ohmori K, Gelfand EW. Type 4 phosphodiesterase inhibitors attenuate respiratory syncytial virus-induced airway hyper-responsiveness and lung eosinophilia. J Pharmacol Exp Ther 2000;294:701–706. [PubMed] [Google Scholar]
- 68.Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, Sopori ML. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol 2009;183:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toward TJ, Broadley KJ. Chronic lipopolysaccharide exposure on airway function, cell infiltration, and nitric oxide generation in conscious guinea pigs: effect of rolipram and dexamethasone. J Pharmacol Exp Ther 2001;298:298–306. [PubMed] [Google Scholar]
- 70.Kudoh I, Ohtake M, Nishizawa H, Kurahashi K, Hattori S, Okumura F, Pittet JF, Wiener-Kronish J. The effect of pentoxifylline on acid-induced alveolar epithelial injury. Anesthesiology 1995;82:531–541. [DOI] [PubMed] [Google Scholar]
- 71.Pawlik MT, Schreyer AG, Ittner KP, Selig C, Gruber M, Feuerbach S, Taeger K. Early treatment with pentoxifylline reduces lung injury induced by acid aspiration in rats. Chest 2005;127:613–621. [DOI] [PubMed] [Google Scholar]
- 72.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:154–161. [DOI] [PubMed] [Google Scholar]
- 73.Gamble E, Grootendorst DC, Brightling CE, Troy S, Qiu Y, Zhu J, Parker D, Matin D, Majumdar S, Vignola AM, et al. Antiinflammatory effects of the phosphodiesterase-4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;168:976–982. [DOI] [PubMed] [Google Scholar]
- 74.Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563–571. [DOI] [PubMed] [Google Scholar]
- 75.Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest 2006;129:56–66. [DOI] [PubMed] [Google Scholar]
- 76.Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol 2006;117:18–24. [DOI] [PubMed] [Google Scholar]
- 77.Pittet JF, Lu LN, Morris DG, Modelska K, Welch WJ, Carey HV, Roux J, Matthay MA. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol 2001;166:6301–6310. [DOI] [PubMed] [Google Scholar]
- 78.Roux J, Carles M, Koh H, Goolaerts A, Ganter MT, Chesebro BB, Howard M, Houseman BT, Finkbeiner W, Shokat KM, et al. Transforming growth factor beta1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J Biol Chem 2010;285:4278–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levenson M. Long-acting beta-agonists and adverse asthma events meta-analysis: statistical briefing package for joint meeting of the Pulmonary-Allergy Drugs Advisory Committee, Drug Safety and Risk Management Advisory Committee, and Pediatric Advisory Committee on December 10–11, 2008.
- 80.O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol 2002;185:137–144. [DOI] [PubMed] [Google Scholar]
- 81.Spina D. PDE4 inhibitors: current status. Br J Pharmacol 2008;155:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]