Abstract
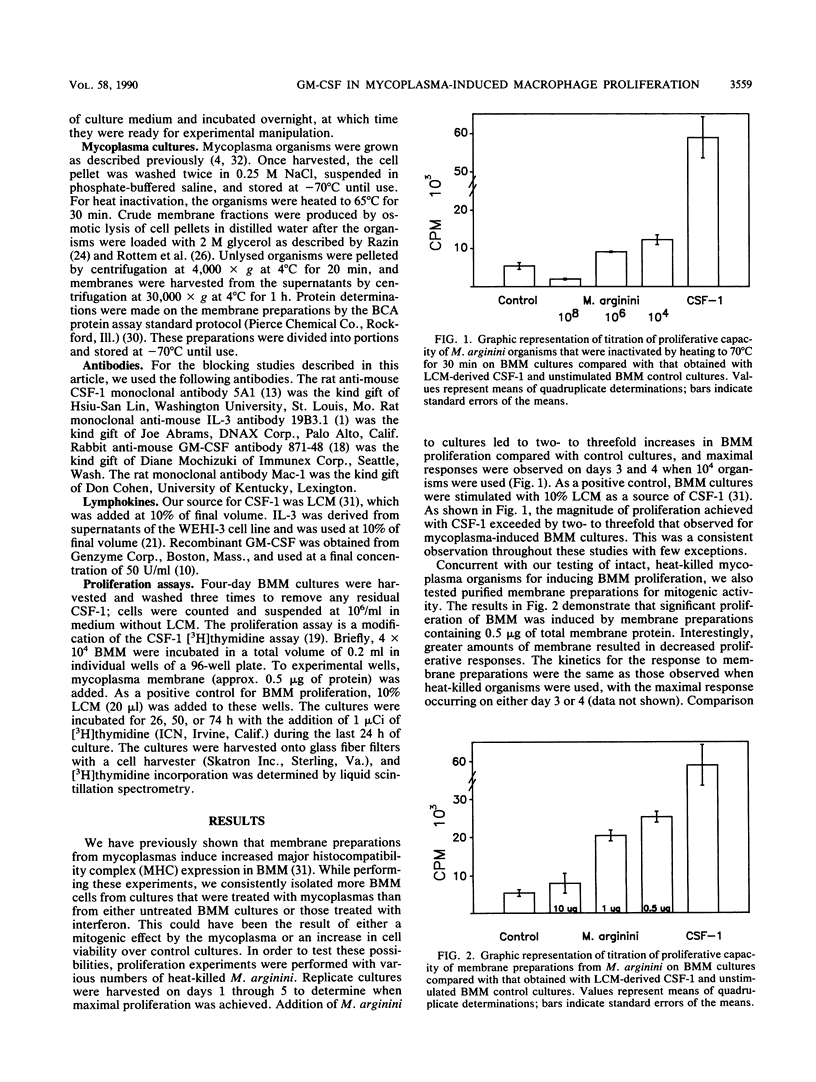
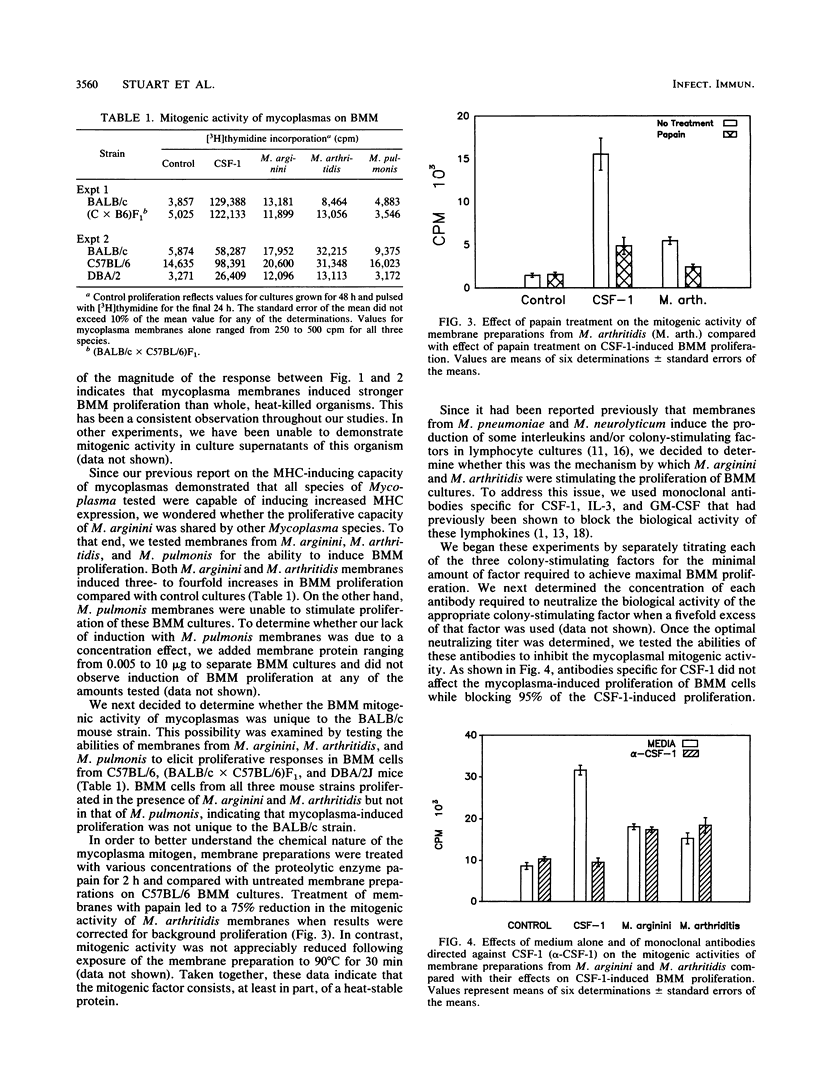
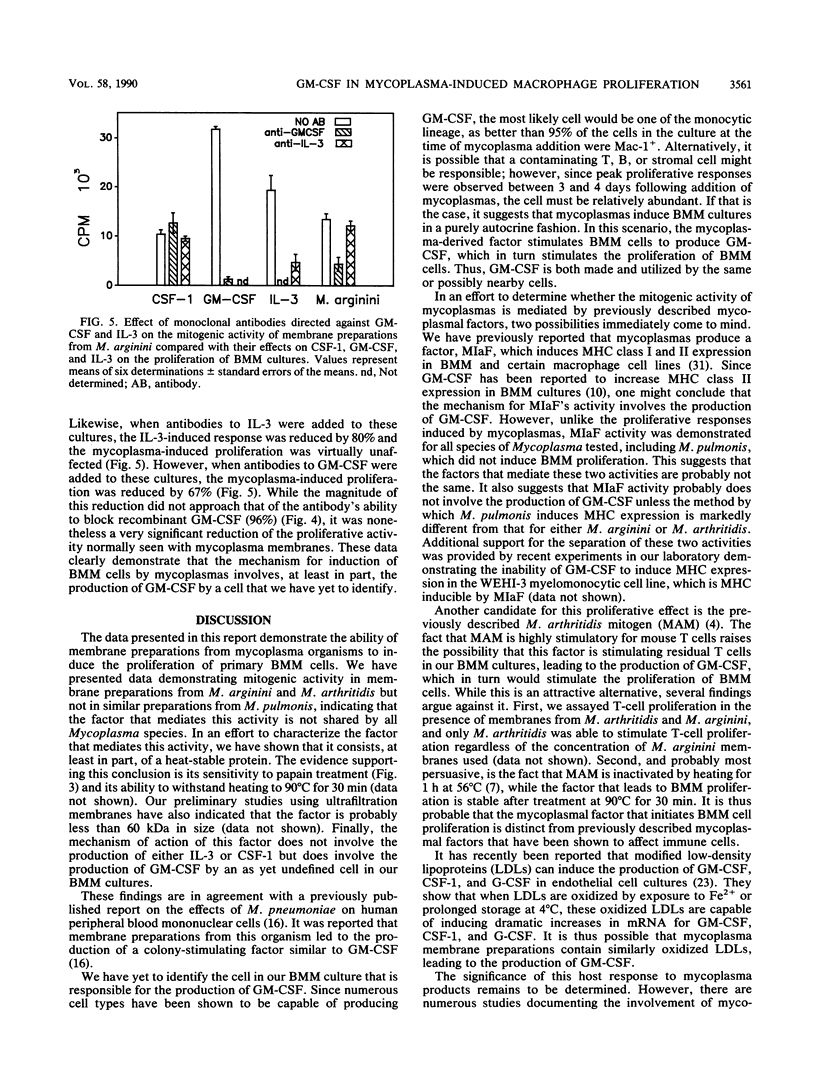
We have studied the ability of three different Mycoplasma species to induce proliferation of bone marrow-derived macrophages (BMM). We observed a significant mitogenic effect when BMM cells from BALB/c, DBA/2J, SJL, and C57BL/6 mice were incubated with membranes derived from Mycoplasma arginini or M. arthritidis but not when they were incubated with an equivalent amount of M. pulmonis membrane. We also determined that pretreatment of mycoplasma membrane preparations with papain eliminated the ability of these preparations to induce BMM proliferation. To determine whether these membrane fractions acted indirectly by stimulating the production of soluble factors known to stimulate proliferation of BMM cells, we performed blocking studies with antibodies directed against colony-stimulating factor 1 (CSF-1), interleukin-3 (IL-3), and granulocyte-macrophage colony-stimulating factor. Our results indicate that antibodies directed against either CSF-1 or IL-3 failed to block mycoplasma-initiated proliferation of BMM cells. However, when anti-GM-CSF was added to proliferative cultures at the time of initiation, we saw a dose-dependent reduction of mycoplasma-initiated proliferation. We conclude that the ability of mycoplasma membranes to initiate the proliferation of BMM is not shared by all species of mycoplasma and that it involves the production of GM-CSF by an as yet undetermined cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Pearce M. K. Development of rat anti-mouse interleukin 3 monoclonal antibodies which neutralize bioactivity in vitro. J Immunol. 1988 Jan 1;140(1):131–137. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis (MAM). VIII. Selective activation of T cells expressing distinct V beta T cell receptors from various strains of mice by the "superantigen" MAM. J Immunol. 1990 Jan 15;144(2):425–431. [PubMed] [Google Scholar]
- Demczuk S., Baumberger C., Mach B., Dayer J. M. Differential effects of in vitro mycoplasma infection on interleukin-1 alpha and beta mRNA expression in U937 and A431 cells. J Biol Chem. 1988 Sep 15;263(26):13039–13045. [PubMed] [Google Scholar]
- Fischer H. G., Frosch S., Reske K., Reske-Kunz A. B. Granulocyte-macrophage colony-stimulating factor activates macrophages derived from bone marrow cultures to synthesis of MHC class II molecules and to augmented antigen presentation function. J Immunol. 1988 Dec 1;141(11):3882–3888. [PubMed] [Google Scholar]
- Gershon H., Naot Y. Induction of interleukin-2 and colony-stimulating factors in lymphoid cell cultures activated by mitogenic mycoplasmas. Isr J Med Sci. 1984 Sep;20(9):882–885. [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Loewenstein J., Gallily R. Studies on the mechanism of macrophage-mediated tumor cell lysis induced by Mycoplasma orale. Isr J Med Sci. 1984 Sep;20(9):895–897. [PubMed] [Google Scholar]
- Lokeshwar B. L., Lin H. S. Development and characterization of monoclonal antibodies to murine macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):483–488. [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Secretion of colony-stimulating factors by T cell clones. Role in adoptive protection against Listeria monocytogenes. J Immunol. 1989 Oct 1;143(7):2336–2341. [PubMed] [Google Scholar]
- Makhoul N., Merchav S., Tatarsky I., Naot Y. Mycoplasma-induced in vitro production of interleukin-2 and colony-stimulating activity. Isr J Med Sci. 1987 May;23(5):480–484. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Park L. S., Urdal D. L. Development and characterization of antiserum to murine granulocyte-macrophage colony-stimulating factor. J Immunol. 1986 May 15;136(10):3706–3709. [PubMed] [Google Scholar]
- Moore R. N., Rouse B. T. Enhanced responsiveness of committed macrophage precursors to macrophage-type colony-stimulating factor (CSF-1) induced in vitro by interferons alpha + beta 1. J Immunol. 1983 Nov;131(5):2374–2378. [PubMed] [Google Scholar]
- Naot Y., Merchav S., Ben-David E., Ginsburg H. Mitogenic activity of Mycoplasma pulmonis. I. Stimulation of rat B and T lymphocytes. Immunology. 1979 Mar;36(3):399–406. [PMC free article] [PubMed] [Google Scholar]
- Prestidge R. L., Watson J. D., Urdal D. L., Mochizuki D., Conlon P., Gillis S. Biochemical comparison of murine colony-stimulating factors secreted by a T cell lymphoma and a myelomonocytic leukemia. J Immunol. 1984 Jul;133(1):293–298. [PubMed] [Google Scholar]
- Proust J. J., Buchholz M. A., Nordin A. A. A "lymphokine-like" soluble product that induces proliferation and maturation of B cells appears in the serum-free supernatant of a T cell hybridoma as a consequence of mycoplasmal contamination. J Immunol. 1985 Jan;134(1):390–396. [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Nathan C. F., Pihl D. L., Rodricks P., Shanebeck K., Conlon P. J., Grabstein K. H. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. doi: 10.1084/jem.166.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Stein O., Razin S. Reassembly of Mycoplasma membranes disaggregated by detergents. Arch Biochem Biophys. 1968 Apr;125(1):46–56. doi: 10.1016/0003-9861(68)90637-1. [DOI] [PubMed] [Google Scholar]
- Ruuth E., Lundgren E. Enhancement of immunoglobulin secretion by the lymphokine-like activity of a Mycoplasma arginini strain. Scand J Immunol. 1986 May;23(5):575–580. doi: 10.1111/j.1365-3083.1986.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Sheldon P. Specific cell-mediated responses to bacterial antigens and clinical correlations in reactive arthritis, Reiter's syndrome and ankylosing spondylitis. Immunol Rev. 1985 Aug;86:5–25. doi: 10.1111/j.1600-065x.1985.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Cassell G. H., Woodward J. G. Induction of class II MHC antigen expression in macrophages by Mycoplasma species. J Immunol. 1989 May 15;142(10):3392–3399. [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Washburn L. R. The Derrick Edward award lecture. Immunologic aspects of Mycoplasma arthritidis-induced arthritis. Isr J Med Sci. 1987 May;23(5):326–333. [PubMed] [Google Scholar]
- Wayner E. A., Brooks C. G. Induction of NKCF-like activity in mixed lymphocyte-tumor cell culture: direct involvement of mycoplasma infection of tumor cells. J Immunol. 1984 Apr;132(4):2135–2142. [PubMed] [Google Scholar]
- Weisbart R. H., Gasson J. C., Golde D. W. Colony-stimulating factors and host defense. Ann Intern Med. 1989 Feb 15;110(4):297–303. doi: 10.7326/0003-4819-110-4-297. [DOI] [PubMed] [Google Scholar]


