Abstract
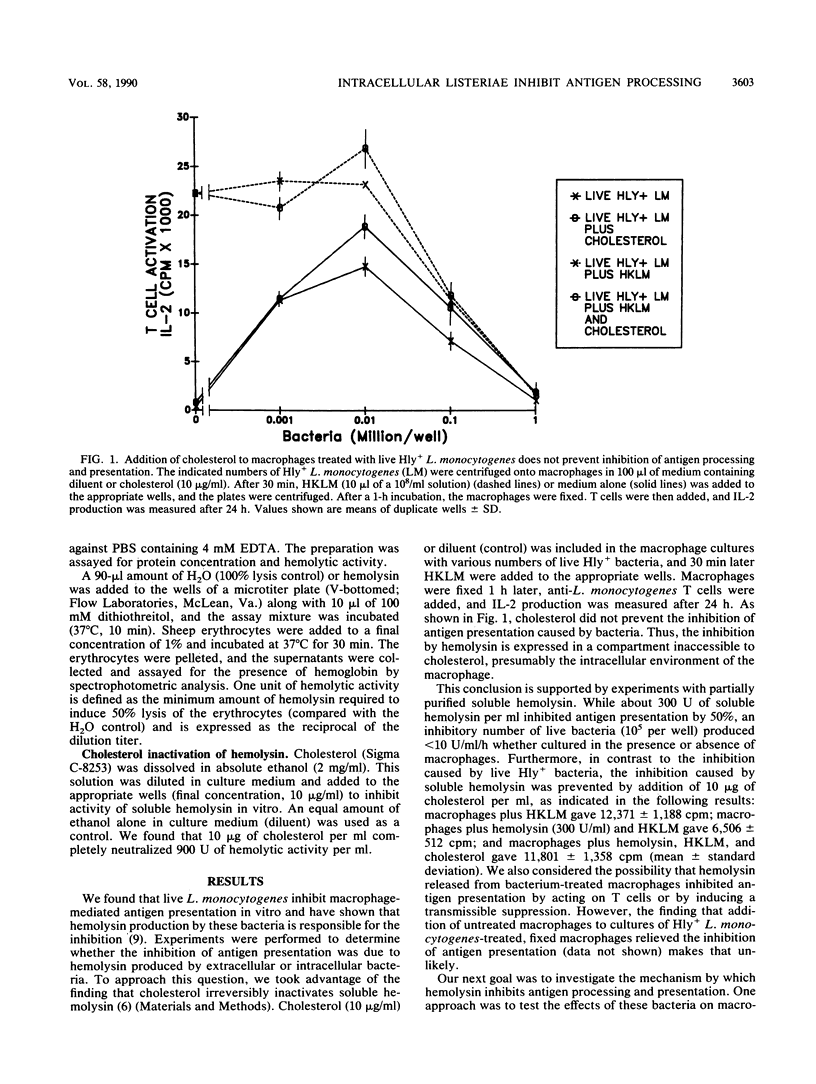
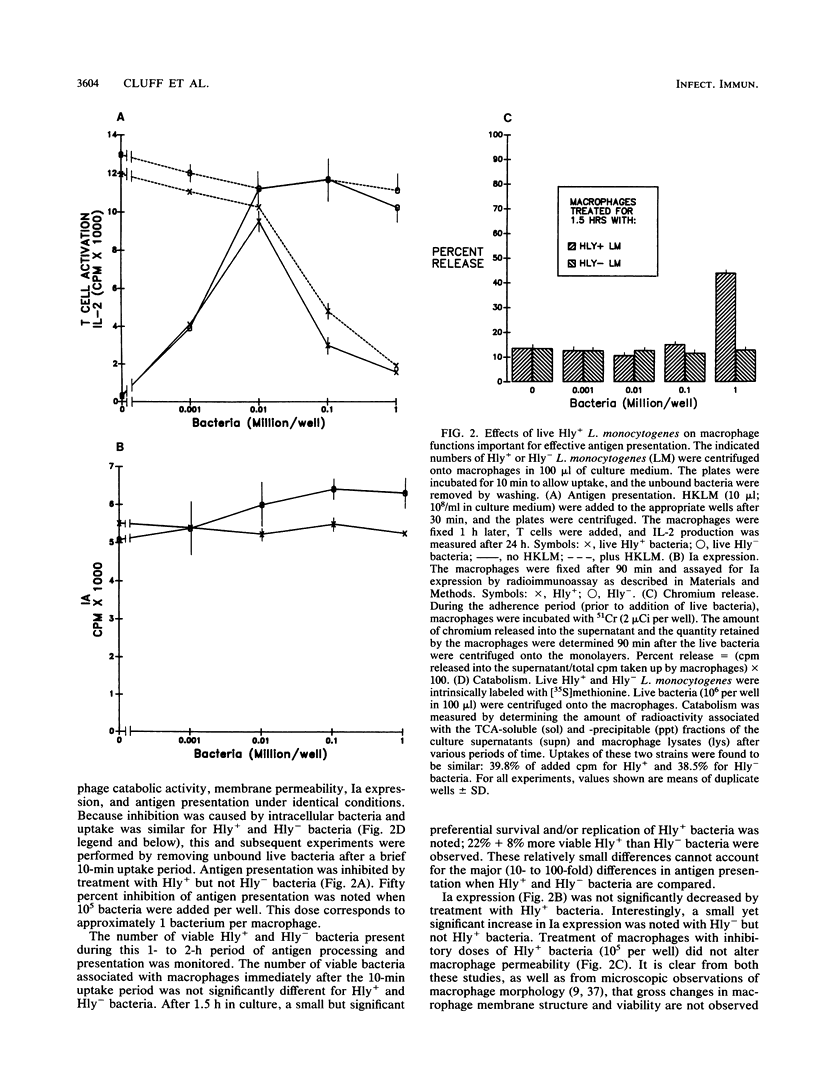
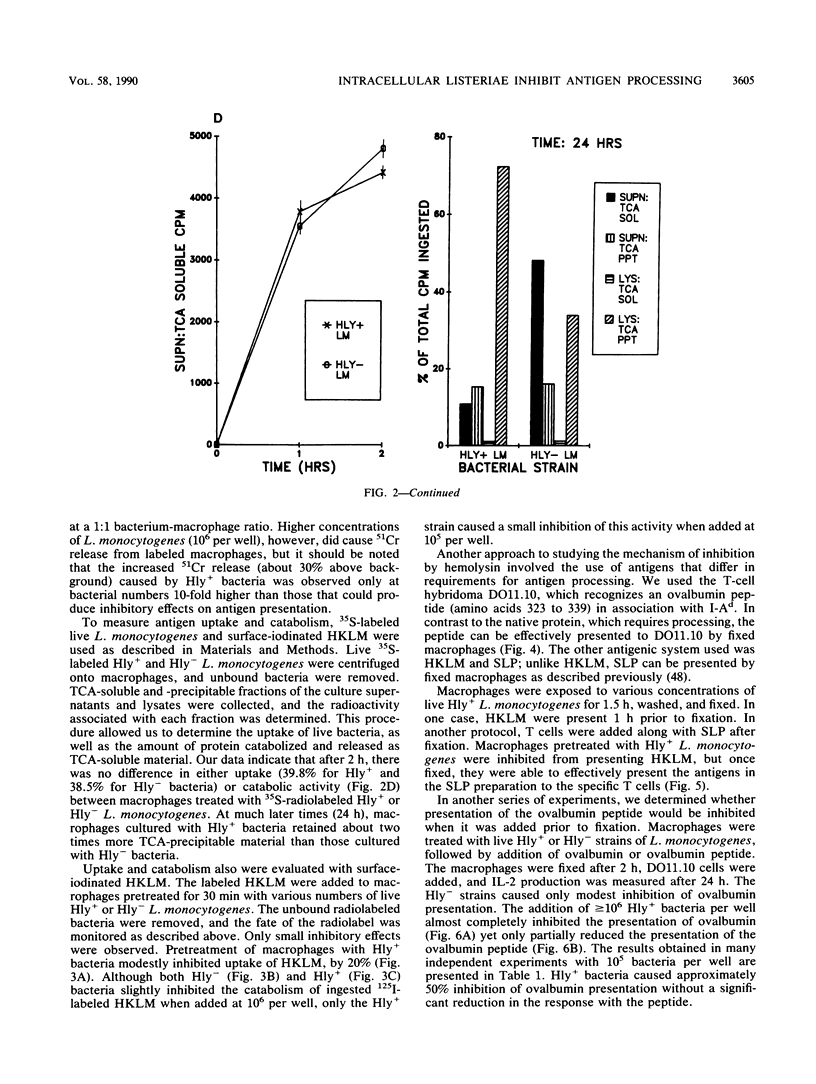
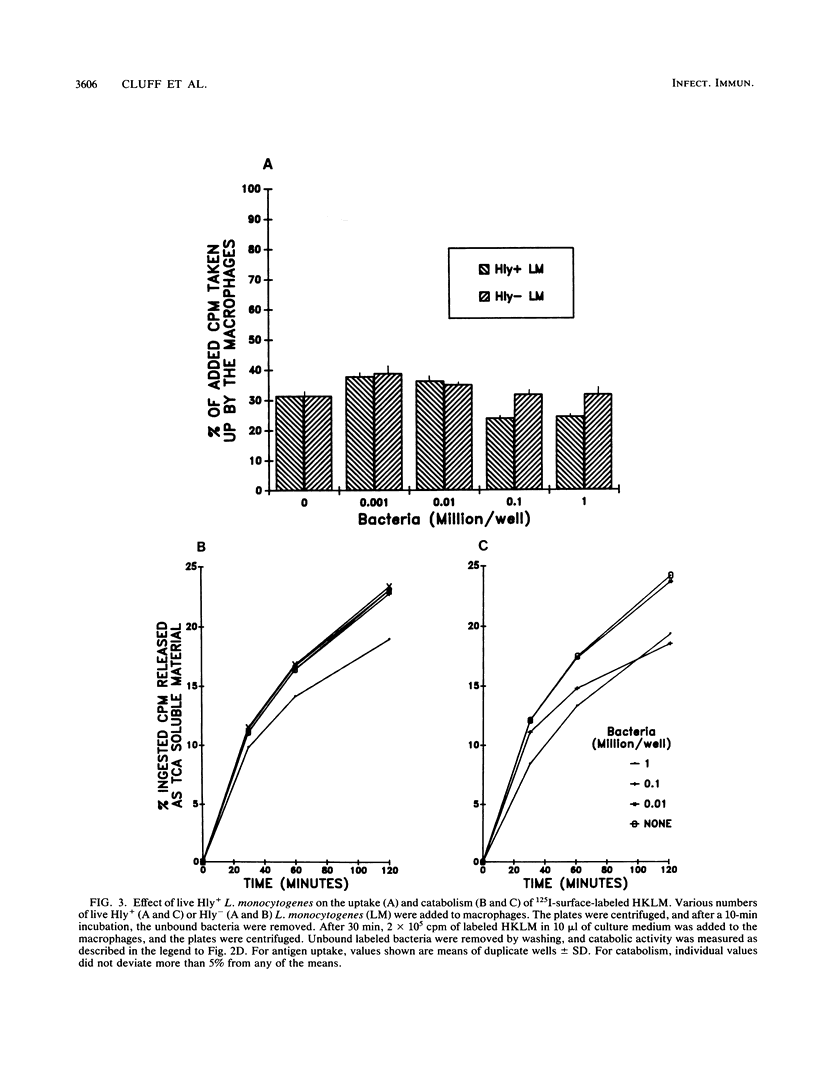
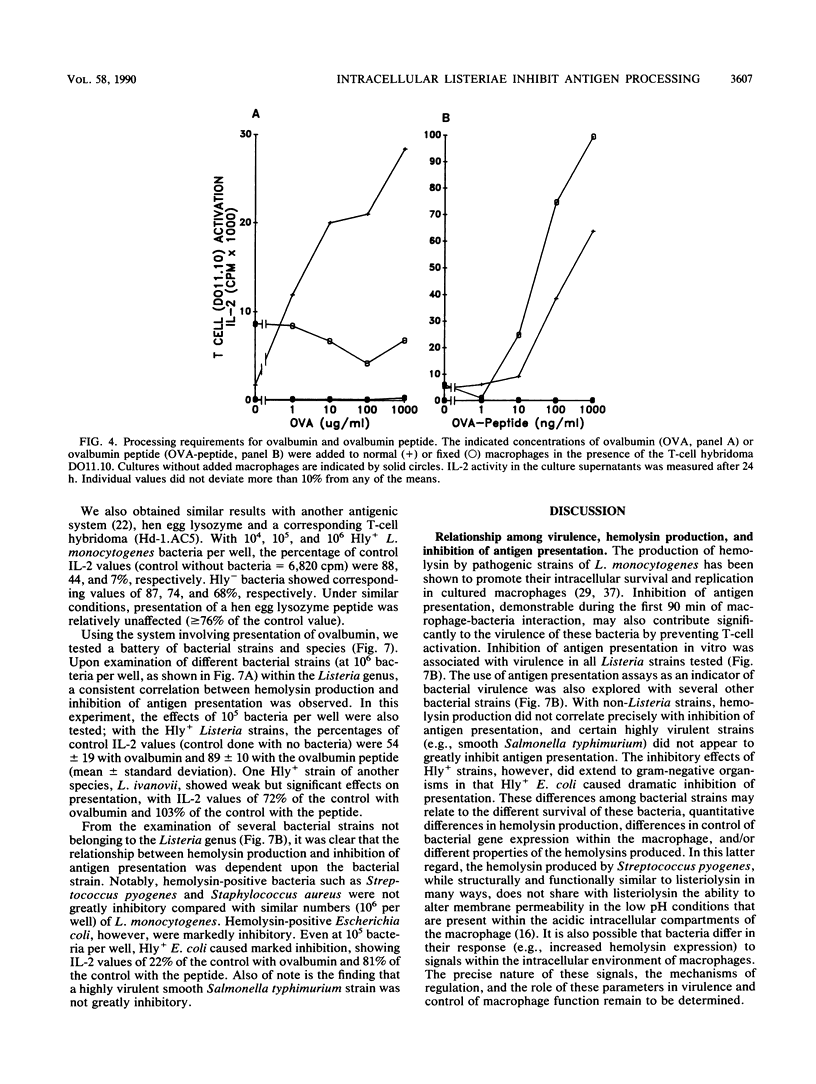
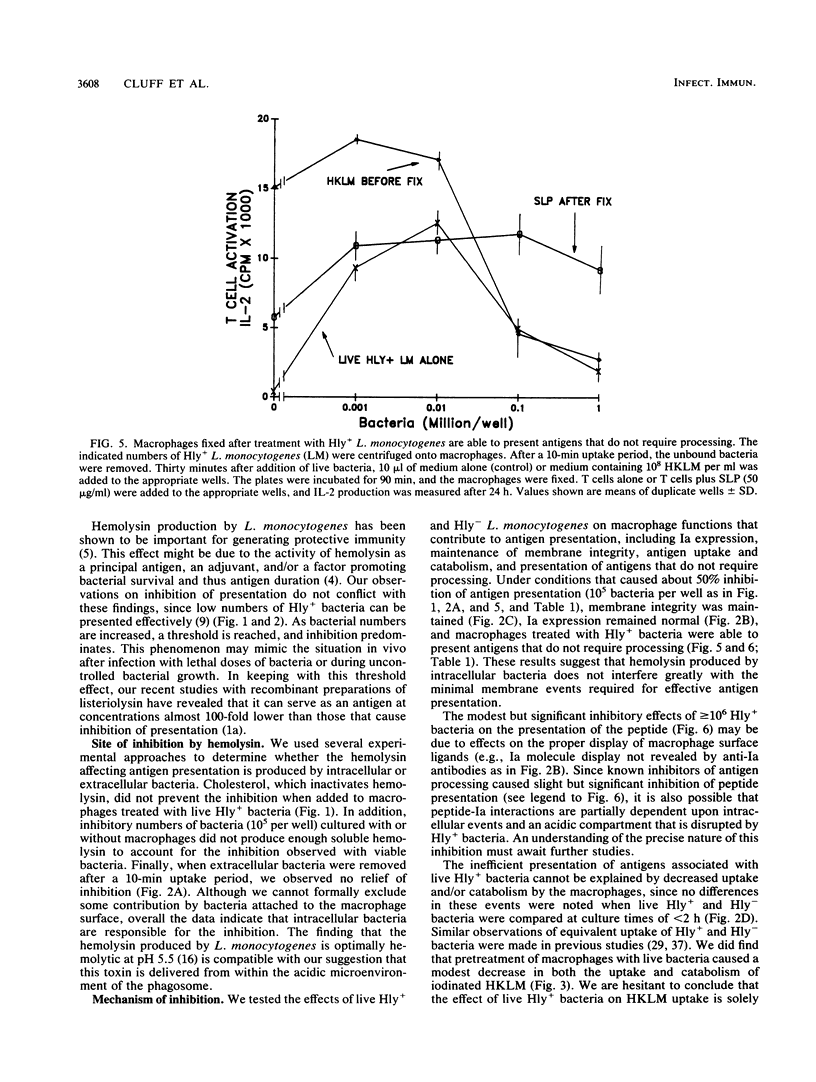
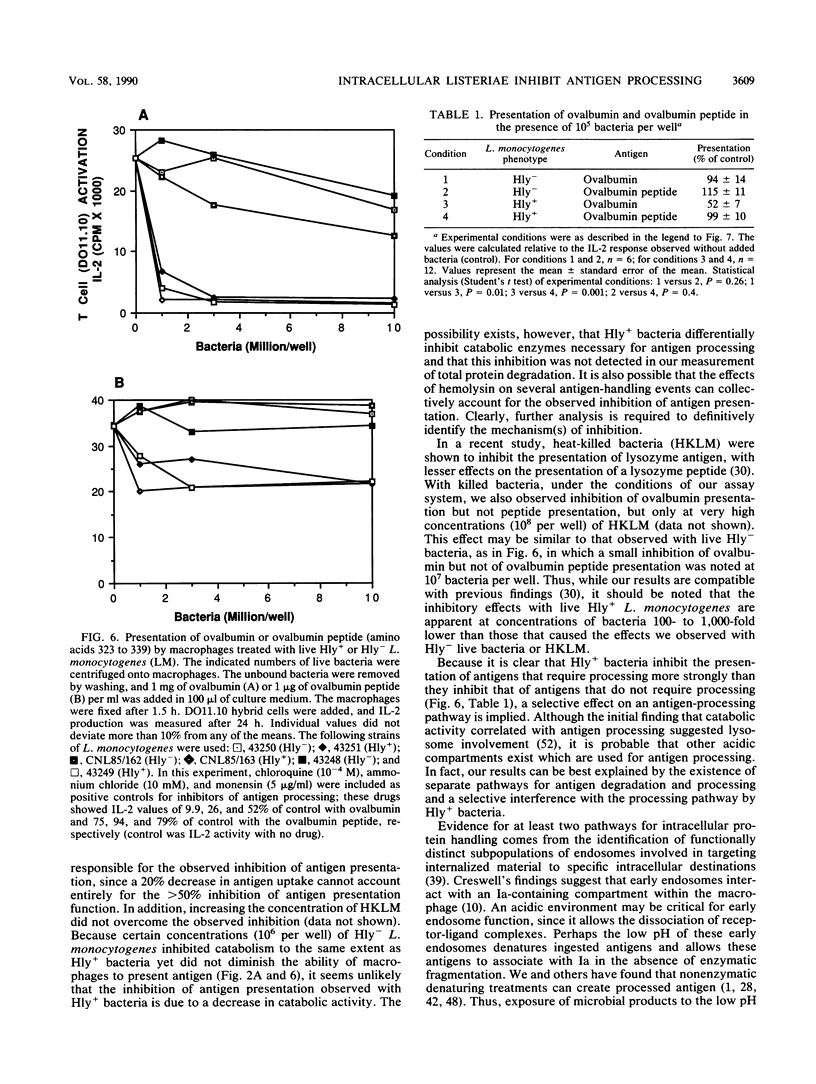
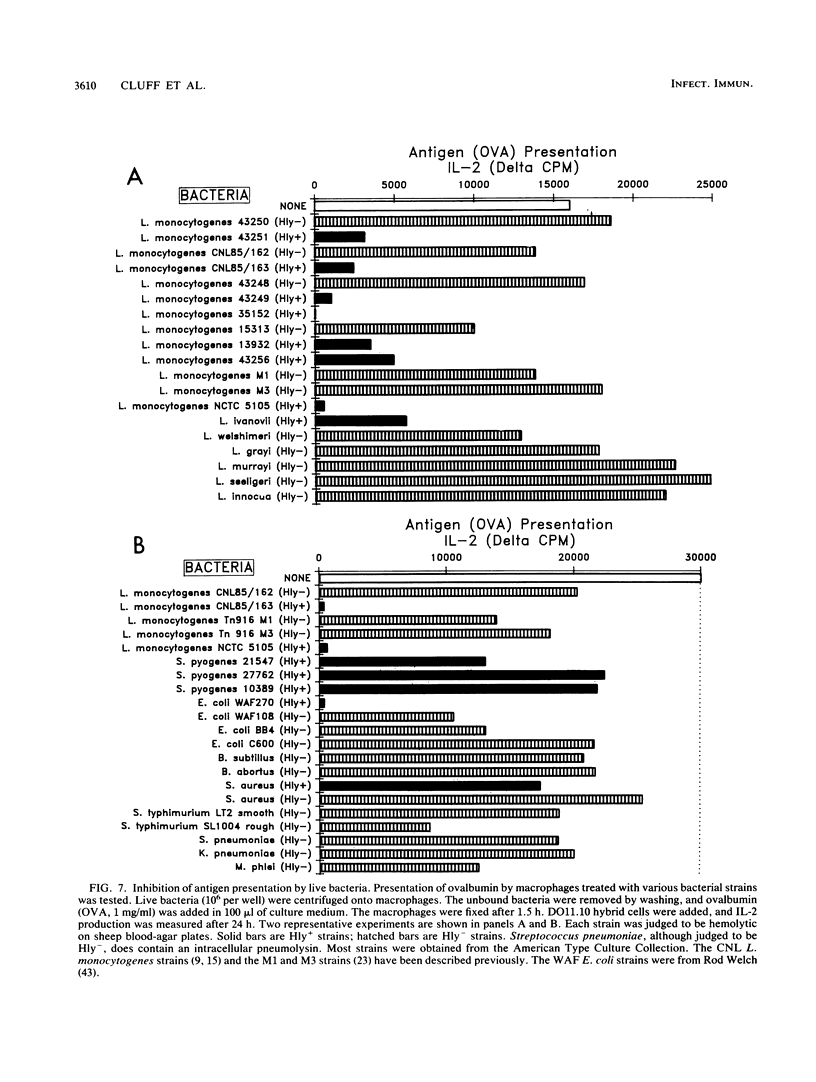
We found that virulent hemolysin-producing (Hly+) Listeria monocytogenes strains inhibit antigen processing and presentation when added to macrophages in vitro. A virulent Hly- bacteria caused little or no inhibition. Live Hly+ bacteria inhibited presentation of both heat-killed L. monocytogenes and ovalbumin. Several observations indicate that hemolysin produced by intracellular bacteria was responsible for the inhibition. First, inhibition was observed even when extracellular bacteria were removed after a brief 10-min bacterial uptake period. Second, inhibition was not prevented by the addition of cholesterol, a substance which inactivates soluble hemolysin. Third, only very high concentrations of soluble hemolysin were inhibitory. Under conditions which inhibit antigen presentation (10(5) per well), macrophages retained normal levels of Ia, maintained normal morphology, and were not permeable when assayed by chromium release. The uptake and catabolism of 35S-labeled live bacteria by macrophages were similar for both Hyl+ and Hly- bacteria. Only a small decrease in uptake and catabolism of surface-iodinated heat-killed L. monocytogenes by macrophages pretreated with inhibitory numbers of live Hly+ bacteria was observed. Additionally, macrophages pretreated with live Hly+ bacteria and fixed 1.5 h later were able to effectively present an ovalbumin peptide (amino acids 323 to 339) to the T-cell hybridoma DO11.10. Hemolysin-producing bacteria inhibited the presentation of antigens that need processing better than they did of antigens that do not require a processing event. Thus, we have demonstrated inhibition of an intracellular antigen processing pathway by hemolysin-producing L. monocytogenes, which may contribute to the virulence of this pathogen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Beattie I. A., Swaminathan B., Ziegler H. K. Cloning and characterization of T-cell-reactive protein antigens from Listeria monocytogenes. Infect Immun. 1990 Sep;58(9):2792–2803. doi: 10.1128/iai.58.9.2792-2803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beller D. I., Ho K. Regulation of macrophage populations. V. Evaluation of the control of macrophage Ia expression in vitro. J Immunol. 1982 Sep;129(3):971–976. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. Regulation of macrophage populations. II. Synthesis and expression of Ia antigens by peritoneal exudate macrophages is a transient event. J Immunol. 1981 Jan;126(1):263–269. [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Geoffroy C., Alouf J. E. T cell recognition of listeriolysin O is induced during infection with Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3813–3821. [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff C. W., Ziegler H. K. An early response to lipopolysaccharide is the elicitation of macrophages specialized for antigen degradation with negative regulatory effects on the induction of specific immune responses. Infect Immun. 1987 Jun;55(6):1346–1354. doi: 10.1128/iai.55.6.1346-1354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluff C. W., Ziegler H. K. Inhibition of macrophage-mediated antigen presentation by hemolysin-producing Listeria monocytogenes. J Immunol. 1987 Dec 1;139(11):3808–3812. [PubMed] [Google Scholar]
- Cresswell P. Intracellular class II HLA antigens are accessible to transferrin-neuraminidase conjugates internalized by receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8188–8192. doi: 10.1073/pnas.82.23.8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Eisenlohr L. C., Gerhard W., Hackett C. J. Acid-induced conformational modification of the hemagglutinin molecule alters interaction of influenza virus with antigen-presenting cells. J Immunol. 1988 Sep 15;141(6):1870–1876. [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R. D., Welshimer H. J. Separation of pathogenic from apathogenic Listeria monocytogenes by three in vitro reactions. J Clin Microbiol. 1977 Jun;5(6):559–563. doi: 10.1128/jcm.5.6.559-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS E. M., NJOKU-OBI A. N., ADAMS E. W. PURIFICATION OF THE SOLUBLE HEMOLYSINS OF LISTERIA MONOCYTOGENES. J Bacteriol. 1964 Aug;88:418–424. doi: 10.1128/jb.88.2.418-424.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Protein synthesis in antigen processing. J Immunol. 1988 Oct 15;141(8):2545–2550. [PubMed] [Google Scholar]
- Jensen P. E. Regulation of antigen presentation by acidic pH. J Exp Med. 1990 May 1;171(5):1779–1784. doi: 10.1084/jem.171.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kovac Z., Schwartz R. H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985 May;134(5):3233–3240. [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Cobian F., Unanue E. R. Intracellular interference with antigen presentation. J Immunol. 1988 Sep 1;141(5):1445–1450. [PubMed] [Google Scholar]
- Magee D. M., Wing E. J. Cloned L3T4+ T lymphocytes protect mice against Listeria monocytogenes by secreting IFN-gamma. J Immunol. 1988 Nov 1;141(9):3203–3207. [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay D. A., Cluff C. W., Wentworth P. A., Ziegler H. K. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986 Jun 15;136(12):4348–4353. [PubMed] [Google Scholar]
- Pine L., Weaver R. E., Carlone G. M., Pienta P. A., Rocourt J., Goebel W., Kathariou S., Bibb W. F., Malcolm G. B. Listeria monocytogenes ATCC 35152 and NCTC 7973 contain a nonhemolytic, nonvirulent variant. J Clin Microbiol. 1987 Nov;25(11):2247–2251. doi: 10.1128/jcm.25.11.2247-2251.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Shimonkevitz R., Colon S., Kappler J. W., Marrack P., Grey H. M. Antigen recognition by H-2-restricted T cells. II. A tryptic ovalbumin peptide that substitutes for processed antigen. J Immunol. 1984 Oct;133(4):2067–2074. [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth P. A., Ziegler H. K. Induction of macrophage Ia expression by lipopolysaccharide and Listeria monocytogenes in congenitally athymic nude mice. J Immunol. 1987 May 15;138(10):3167–3173. [PubMed] [Google Scholar]
- Wilson C. B., Westall J. Activation of neonatal and adult human macrophages by alpha, beta, and gamma interferons. Infect Immun. 1985 Aug;49(2):351–356. doi: 10.1128/iai.49.2.351-356.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Orlin C. A. Analysis of Listeria monocytogenes antigens with monoclonal antibodies. Clin Invest Med. 1984;7(4):239–242. [PubMed] [Google Scholar]
- Ziegler H. K., Orlin C. A., Cluff C. W. Differential requirements for the processing and presentation of soluble and particulate bacterial antigens by macrophages. Eur J Immunol. 1987 Sep;17(9):1287–1296. doi: 10.1002/eji.1830170911. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Staffileno L. K., Wentworth P. Modulation of macrophage Ia-expression by lipopolysaccharide. I. Induction of Ia expression in vivo. J Immunol. 1984 Oct;133(4):1825–1835. [PubMed] [Google Scholar]
- Ziegler H. K. The processing and presentation of Listeria monocytogenes antigens by macrophages. Clin Invest Med. 1984;7(4):269–272. [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. The specific binding of Listeria monocytogenes-immune T lymphocytes to macrophages. I. Quantitation and role of H-2 gene products. J Exp Med. 1979 Nov 1;150(5):1143–1160. doi: 10.1084/jem.150.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]


