Abstract
Many pathological effects of gram-negative bacteria are produced by their cell wall-derived lipopolysaccharides (LPSs). Differing pathogenicity of gram-negative LPSs, however, may depend on their capacities to induce cytokines. Thus, we studied the lethal toxicity of four nonenterobacterial LPSs and compared it with their capacity to induce mononuclear cell (MNC)-derived interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor (TNF). Unstimulated MNC did not release these cytokines. LPS from the phototrophic strain Rhodobacter capsulatus 37b4 elaborated little toxicity in galactosamine-treated mice (10 micrograms of LPS per mouse was the 100% lethal dose [LD100]) and induced IL-1 and IL-6 release only at high concentrations (10 to 50 micrograms of LPS per ml). R. capsulatus LPS failed to induce TNF activity even at the highest concentration tested (100 micrograms of LPS per ml). In contrast, LPS derived from Pseudomonas diminuta NCTC 8545 or the nodulating species Bradyrhizobium lupini DSM 30140 and Rhizobium meliloti 10406 expressed lethal toxicity (LD100, 1,000, 100, and 10 ng per mouse, respectively) and induced IL-1 or IL-6 (10 to 100, 10, and 1 ng of LPS per ml, respectively) at concentrations 1,000- to 10,000-fold lower than effective levels of R. capsulatus LPS. LPSs from P. diminuta, B. lupini, and R. meliloti also stimulated TNF production and release. MNC accumulated cell-associated IL-1 activities under circumstances in which released activity was readily detected. The cells contained only scant IL-6 activity, indicating release of this mediator rather than intracellular accumulation. Antisera to the respective cytokines inactivated biological activities of the samples selectively. The R. capsulatus LPS inhibited cytokine induction by LPS from P. diminuta, B. lupini, and R. meliloti in coincubation experiments. These results show that the in vivo lethality of the LPSs tested correlates with the induction of monocyte-derived cytokines in vitro. The results of this study suggest that the different lethality of various LPSs from gram-negative bacteria may be due to the differential ability of these LPSs to induce cytokine production.
Full text
PDF
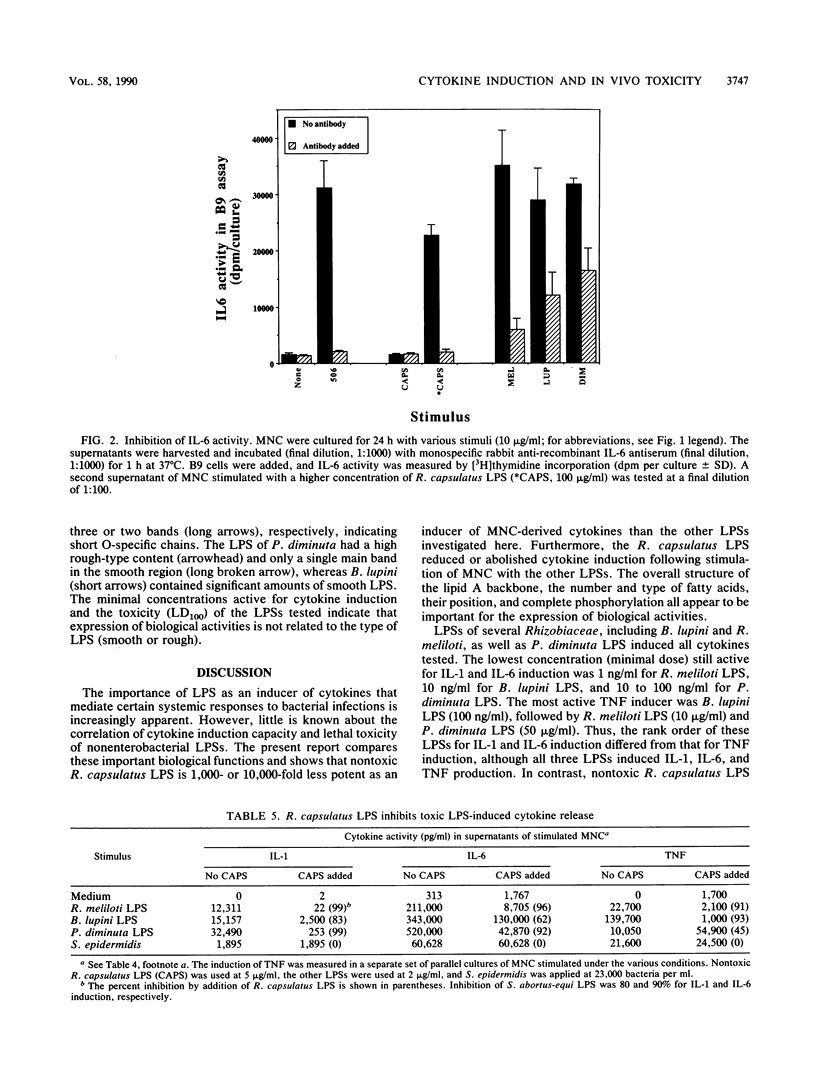
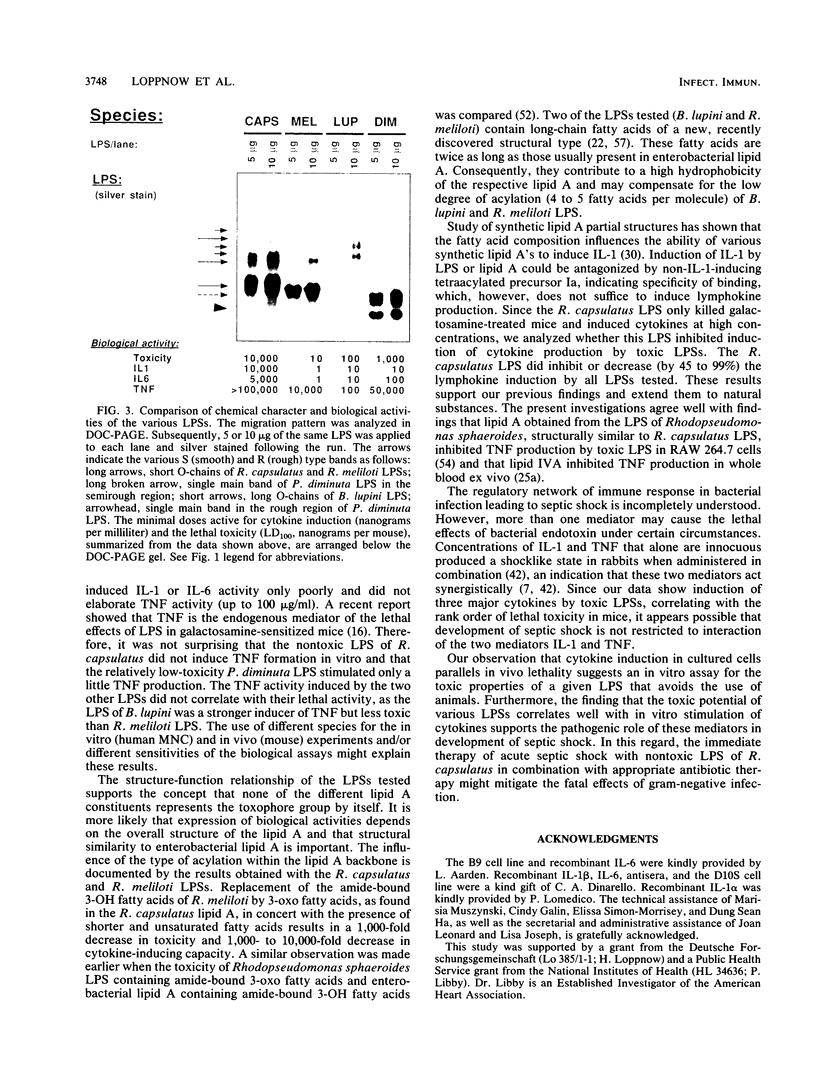


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Bauer J., Ganter U., Geiger T., Jacobshagen U., Hirano T., Matsuda T., Kishimoto T., Andus T., Acs G., Gerok W. Regulation of interleukin-6 expression in cultured human blood monocytes and monocyte-derived macrophages. Blood. 1988 Oct;72(4):1134–1140. [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Burt D. W., Kelley V. E. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988 Nov 1;141(9):3050–3054. [PubMed] [Google Scholar]
- Cady A. B., Kotani S., Shiba T., Kusumoto S., Krueger J. M. Somnogenic activities of synthetic lipid A. Infect Immun. 1989 Feb;57(2):396–403. doi: 10.1128/iai.57.2.396-403.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Dinarello C. A. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985 Mar 8;227(4691):1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Chia J. K., Pollack M., Guelde G., Koles N. L., Miller M., Evans M. E. Lipopolysaccharide (LPS)-reactive monoclonal antibodies fail to inhibit LPS-induced tumor necrosis factor secretion by mouse-derived macrophages. J Infect Dis. 1989 May;159(5):872–880. doi: 10.1093/infdis/159.5.872. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Keppler D., Galanos C. Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun. 1986 Mar;51(3):891–895. doi: 10.1128/iai.51.3.891-895.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Rietschel E. T., Westphal O., Brade H., Brade L., Freudenberg M., Schade U., Imoto M., Yoshimura H. Synthetic and natural Escherichia coli free lipid A express identical endotoxic activities. Eur J Biochem. 1985 Apr 1;148(1):1–5. doi: 10.1111/j.1432-1033.1985.tb08798.x. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hiernaux J. R., Stashak P. W., Cantrell J. L., Rudbach J. A., Baker P. J. Immunomodulatory activity of monophosphoryl lipid A in C3H/HeJ and C3H/HeSnJ mice. Infect Immun. 1989 May;57(5):1483–1490. doi: 10.1128/iai.57.5.1483-1490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Carlson R. W. 27-Hydroxyoctacosanoic acid is a major structural fatty acyl component of the lipopolysaccharide of Rhizobium trifolii ANU 843. J Biol Chem. 1989 Jun 5;264(16):9300–9303. [PubMed] [Google Scholar]
- Kanegasaki S., Tanamoto K., Yasuda T., Homma J. Y., Matsuura M., Nakatsuka M., Kumazawa Y., Yamamoto A., Shiba T., Kusumoto S. Structure-activity relationship of lipid A: comparison of biological activities of natural and synthetic lipid A's with different fatty acid compositions. J Biochem. 1986 Apr;99(4):1203–1210. doi: 10.1093/oxfordjournals.jbchem.a135583. [DOI] [PubMed] [Google Scholar]
- Kasai N., Arata S., Mashimo J., Akiyama Y., Tanaka C., Egawa K., Tanaka S. Pseudomonas diminuta LPS with a new endotoxic lipid A structure. Biochem Biophys Res Commun. 1987 Feb 13;142(3):972–978. doi: 10.1016/0006-291x(87)91509-9. [DOI] [PubMed] [Google Scholar]
- Kovach N. L., Yee E., Munford R. S., Raetz C. R., Harlan J. M. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990 Jul 1;172(1):77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss J. H., Seydel U., Weckesser J., Mayer H. Structural analysis of the nontoxic lipid A of Rhodobacter capsulatus 37b4. Eur J Biochem. 1989 Apr 1;180(3):519–526. doi: 10.1111/j.1432-1033.1989.tb14677.x. [DOI] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lepe-Zuniga J. L., Zigler J. S., Jr, Zimmerman M. L., Gery I. Differences between intra- and extracellular interleukin-1. Mol Immunol. 1985 Dec;22(12):1387–1392. doi: 10.1016/0161-5890(85)90061-6. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- Loppnow H., Brade L., Brade H., Rietschel E. T., Kusumoto S., Shiba T., Flad H. D. Induction of human interleukin 1 by bacterial and synthetic lipid A. Eur J Immunol. 1986 Oct;16(10):1263–1267. doi: 10.1002/eji.1830161013. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Flad H. D., Dürrbaum I., Musehold J., Fetting R., Ulmer A. J., Herzbeck H., Brandt E. Detection of interleukin 1 with human dermal fibroblasts. Immunobiology. 1989 Jun;179(2-3):283–291. doi: 10.1016/S0171-2985(89)80023-3. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Adult human vascular endothelial cells express the IL6 gene differentially in response to LPS or IL1. Cell Immunol. 1989 Sep;122(2):493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Comparative analysis of cytokine induction in human vascular endothelial and smooth muscle cells. Lymphokine Res. 1989 Fall;8(3):293–299. [PubMed] [Google Scholar]
- Loppnow H., Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990 Mar;85(3):731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G., Chan M. K., Dinarello C. A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed Proc. 1987 Jan;46(1):97–104. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Orencole S. F., Dinarello C. A. Characterization of a subclone (D10S) of the D10.G4.1 helper T-cell line which proliferates to attomolar concentrations of interleukin-1 in the absence of mitogens. Cytokine. 1989 Nov;1(1):14–22. doi: 10.1016/1043-4666(89)91044-2. [DOI] [PubMed] [Google Scholar]
- Rackow E. C., Astiz M. E., Kim Y. B., Weil M. H. Monophosphoryl lipid A blocks the hemodynamic effects of lethal endotoxemia. J Lab Clin Med. 1989 Jan;113(1):112–117. [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Strittmatter W., Weckesser J., Salimath P. V., Galanos C. Nontoxic lipopolysaccharide from Rhodopseudomonas sphaeroides ATCC 17023. J Bacteriol. 1983 Jul;155(1):153–158. doi: 10.1128/jb.155.1.153-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor D. R., Burchett S. K., Jacobs R. F. Enhanced production of monokines by canine alveolar macrophages in response to endotoxin-induced shock. Proc Soc Exp Biol Med. 1988 Apr;187(4):408–415. doi: 10.3181/00379727-187-42681. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Kaufman E. N., Tate M. D., Neta R. Recombinant interleukin-1 alpha and recombinant tumor necrosis factor alpha synergize in vivo to induce early endotoxin tolerance and associated hematopoietic changes. Infect Immun. 1988 Oct;56(10):2650–2657. doi: 10.1128/iai.56.10.2650-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]