Abstract
Aims
Many cities have banned indoor smoking in public places. Thus, an updated recommendation for a breath carbon monoxide (CO) cut-off is needed that optimally determines smoking status. We evaluated and compared the performance of breath CO and semiquantitative cotinine immunoassay test strips (urine and saliva NicAlert®) alone and in combination.
Design
Cross-sectional study.
Setting
Urban drug addiction research and treatment facility.
Participants
Ninety non-treatment-seeking smokers and 82 non-smokers.
Measurements
Participants completed smoking histories and provided breath CO, urine and saliva specimens. Urine and saliva specimens were assayed for cotinine by NicAlert® and liquid chromatography-tandem mass spectrometry (LCMSMS).
Findings
An optimal breath CO cut-off was established using self-report and LCMSMS analysis of cotinine, an objective indicator, as reference measures. Performance of smoking indicators and combinations were compared to the reference measures. Breath CO ≥5 parts per million (p.p.m.) optimally discriminated smokers from non-smokers. Saliva NicAlert® performance was less effective than the other indicators.
Conclusions
In surveys of smokers and non-smokers in areas with strong smoke-free laws, the breath carbon monoxide cut-off that discriminates most effectively appears to be ≥5 p.p.m. rather than the ≥10 p.p.m. cut-off often used. These findings may not generalize to clinical trials, regions with different carbon monoxide pollution levels or areas with less stringent smoke-free laws.
Keywords: Assays, biomarker, carbon monoxide, cigarette smokers, cotinine, nicotine, non-smokers, saliva, urine
INTRODUCTION
In many clinical and research settings, tobacco exposure is assessed by self-report. In settings with increased social pressure to abstain from smoking, biochemical verification is useful to identify smokers [1,2]. Breath carbon monoxide (CO) and semiquantitative measurement of cotinine, the major metabolite of nicotine, by immunoassay-based test strips (urine and saliva NicA-lert®) are commonly used, rapid and relatively inexpensive biochemical indicators of smoking [3].
As markers of smoking, breath CO and cotinine have different merits and limitations. Breath CO is a good indicator of recent smoking, but its short half-life (2–3 hours) limits its sensitivity, and environmental CO sources (e.g. passive smoke) limit its specificity [4]. In addition, there is no consensus on the breath CO cut-off that optimally discriminates smokers from non-smokers. Cut-offs ranging from ≥3–10 parts per million (p.p.m.) have been recommended [2,5]. Many studies employ the Society for Research on Nicotine and Tobacco’s (SRNT) recommended cut-off of ≥8–10 p.p.m. [2]. Recent indoor smoking bans have probably decreased passive smoke exposure among non-smokers than when previous cut-offs were recommended. For example, breath CO levels of European non-smokers were associated negatively with strength of tobacco control policies, suggesting that Europe’s antismoking policies reduced passive smoke exposure in non-smokers [6]. Thus, new data are needed to determine if lower breath CO cut-offs optimally distinguish smokers and non-smokers.
In contrast to breath CO, cotinine has a long half-life (10–30 hours) [7] and a greater window of detection for recent tobacco exposure. Cotinine is assayed semiquantitatively by immunoassay test strips (urine and saliva NicAlert®) and quantitatively by liquid chromatography-tandem mass spectrometry (LCMSMS) or gas chromatography-mass spectrometry (GCMS). NicAlert® is relatively inexpensive, easy to use and provides onsite results, but cotinine antibodies might cross-react with other nicotine metabolites and endogenous and exogenous analytes present in the specimen [8]. Thus, the immunoassay might indicate greater cotinine levels than are actually present, and might not be a truly semiquantitative indicator of cotinine concentration. Quantitative LCMSMS or GCMS analysis is an objective reference measure for verification of tobacco exposure, but there is disagreement about which cut-off optimally discriminates smoking from passive smoke exposure in different biological matrices. The method is also expensive, time-consuming, unavailable immediately upon collection and requires special instrumentation and highly trained staff.
The merits and limitations of smoking indicators are well known, but to date no study has directly compared the performance of self-report, breath CO, urine NicA-lert®, saliva NicAlert® and their combinations. Currently, clinicians and researchers rely on these markers without data on their relative performance. In this study, smokers and non-smokers completed self-reported smoking histories and provided breath CO, urine and saliva specimens. Urine and saliva specimens were assayed for cotinine by NicAlert® and LCMSMS. An optimal breath CO cut-off was established with self-report and LCMSMS cotinine analysis, an objective indicator, as the reference measures. Self-report and LCMSMS results determined performance of the indicators and combinations. We hypothesized that the optimal breath CO cut-off would be lower than SRNT’s previous recommendation of ≥8–10 p.p.m. [2], and that breath CO and NicAlert® tests together would perform optimally because of their differential sensitivities to recent tobacco exposure.
METHODS
Participants
Participants were non-treatment-seeking smokers (n = 90) and non-smokers (n = 82). To capture a wide range of tobacco exposure, we recruited smokers reporting >10 cigarettes per day (heavy smokers, n = 46), smokers reporting one to 10 cigarettes per day (light smokers, n = 44), non-smokers reporting regular passive smoke exposure (exposed non-smokers, n = 36) and non-smokers reporting no passive smoke exposure (not exposed non-smokers, n = 46). Exposed non-smokers reported passive smoke exposure at least three times per month; most reported that significant people in their lives (e.g. partners, parents, roommates) were smokers. Not exposed non-smokers reported no regular passive smoke exposure.
For all participants, exclusion criteria included chronic pulmonary disease and cannabis use greater than five times in the past 2 weeks or within the past 24 hours to avoid CO confounding. Additional exclusion criteria for smokers included: current interest in decreasing use or quitting, treatment for tobacco dependence within the past 3 months and current use of tobacco products other than cigarettes. Additional exclusion criteria for non-smokers included use of any tobacco or nicotine product in the past 3 months.
The National Institute on Drug Addiction (NIDA) Institutional Review Board approved the study. Participants gave written informed consent according to guidelines for the protection of research volunteers of the US Department of Health and Human Services and were compensated for their time.
Procedure
Participants followed a standardized protocol. After giving consent, participants provided a breath CO sample, completed smoking history forms, gave two saliva specimens, and finally gave a urine specimen. Breath CO readings were obtained by a Vitalograph monitor (Vitalograph, Lenexa, KS, USA). Time since last cigarette was the interval from last reported cigarette to the breath CO sample. Saliva specimens were collected for NicAlert® testing via expectoration (Nymox Corp., Hasbrouck Heights, NJ, USA) and for LCMSMS analysis via a Quantisal™ collection device (Immunalysis, Pomona, CA, USA). Data were collected at different times of the day to mimic a research or clinical setting.
Cotinine assays
Urine and saliva specimens were assayed by NicAlert® test strips and by LCMSMS. We followed the manufacturer’s instructions for NicAlert® testing. The tests results included seven zones representing a range of cotinine concentrations from 0 (0–10 ng/ml) to 6 (>1000 ng/ml). The manufacturer’s cut-offs of ≥3 (≥100 ng/ml) for urine and ≥1 (≥10 ng/ml) for saliva indicated tobacco smoking. All NicAlert® strips were read by a staff member who was blind to participants’ smoking status. A second blinded staff member read 75% of specimens. Inter-rater reliability was r = 0.97, P < 0.0001.
Oral fluid cotinine concentrations were quantified by LCMSMS according to a previously validated method [9], and urine cotinine was quantified by LCMSMS using a modified version of the oral fluid method that was also fully validated. Oral fluid (500 μl) collected with the Quantisal™ device or 1 ml urine was fortified with cotinine-d3, diluted with 2 ml 0.1 M sodium phosphate buffer, pH 6.0, and extracted with CleanScreen solid phase extraction columns (part no. ZSDAU020; United Chemical Technologies, Bristol, PA, USA) before LCMSMS analysis [9]. The LCMSMS instrument consisted of a Sil-20AC autosampler, LC-20AD pumps and a CTO-20A column oven (Shimadzu, Columbia, MD, USA) coupled to a MDS Sciex API 3200 Qtrap® triple quadrupole/linear ion trap mass spectrometer with a TurboIonSpray source (Applied Biosystems, Foster City, CA, USA). A Synergi Polar RP column (100 × 2.0 mm, 2.5 μm) with a similar phase guard column (4.0 × 2.0 mm) (Phenomenex, Torrance, CA, USA) was used with a 250 μl/minute flow rate. Gradient elution was performed using mobile phase A: 0.1% formic acid in water (v/v) and phase B: 0.1% formic acid in acetonitrile (v/v). The following MSMS transitions were monitored: 177.2 to 80.1 and 98.1 for cotinine and 180.2 to 80.2 and 101.2 for cotinine-d3 (transitions for quantification are italicized). Cotinine extraction efficiencies were 77–93% and 92–110% from oral fluid and urine, respectively; n = 5. Limits of detection and limits of quantification (LOQ) were 0.1 and 0.2 ng/ml, and upper limits of linearity (ULOL) were 2000 and 500 ng/ml for cotinine in oral fluid and urine, respectively. Calibration curves were constructed with 8 and 10 points for urine and oral fluid, respectively. Cotinine inter-assay biases were 88–116% and 82–110% for oral fluid and urine, respectively; n = 20. Inter-assay imprecisions expressed as percentage coefficient of variation were less than 10% for cotinine in oral fluid and urine; n = 20. Matrix effect (ion suppression) was less than 25% for both oral fluid and urine.
Urine contains significant concentrations of cotinine–glucuronide and free cotinine. However, our objective was to compare NicAlert® with LCMSMS results. Because the antibody in the NicAlert® device does not cross-react with cotinine–glucuronide (as described by the manufacturer, NyMox), we chose not to hydrolyze specimens prior to LCMSMS analysis.
Statistical analyses
Self-reported tobacco use and LCMSMS cotinine analysis were the reference measures for performance of the other indicators of smoking status. There is no consensus on the LCMSMS cotinine cut-off that optimally discriminates passive smoke exposure from smoking. We present data from three LCMSMS urine cotinine cut-offs to classify participants as smokers and non-smokers: ≥0.2 ng/ml (the LOQ of the method), ≥25 ng/ml and ≥100 ng/ml. The ≥ 0.2 ng/ml (LOQ) cut-off is the most sensitive and conservative; it classifies all participants with detectable cotinine levels as smokers. The ≥25 ng/ml urine cotinine cut-off was recommended as a level that optimally discriminates passive smoke exposure from tobacco smoking [10,11]. In 2002, SRNT recommended ≥50 ng/ml as an optimal urine cotinine cut-off [2]. In our study, only one subject’s smoking status changed from smoker to non-smoker (urine cotinine = 28.5 ng/ml; saliva cotinine = 2.5 ng/ml) when we examined the ≥50 compared to the ≥25 ng/ml cut-off. Thus, the results of the ≥25 ng/ml cut-off are nearly identical to those of ≥50 ng/ml cut-off (data not shown). The ≥100 ng/ml cut-off is the manufacturer’s recommended level for the urine NicAlert® assay. Two LCMSMS saliva cotinine cutoffs classified participants as smokers and non-smokers: ≥0.2 and ≥10 ng/ml. The ≥0.2 ng/ml cut-off is equal to the LOQ of the LCMSMS assay for this matrix, and the ≥10 ng/ml cut-off is recommended by the manufacturer for the saliva NicAlert® assay. Non-smokers with heavy passive smoke exposure rarely have saliva cotinine concentrations ≥10 ng/ml [12,13].
To determine the optimal breath CO cut-off, we performed receiver operating characteristic (ROC) curve analyses [14]. Reference measures were self-report and the previously described LCMSMS cut-offs. The breath CO cut-off that optimally discriminated smokers from non-smokers was the value corresponding to the maximum of the Youden index: J = max [SEi + SPi − 1], where SEi and SPi are the sensitivity and specificity, respectively, at all possible cut-off values [15].
Performance of each indicator of smoking status was evaluated using several measures of predictive accuracy: sensitivity (smokers identified as smokers), specificity (non-smokers identified as non-smokers), efficiency (all correct identifications), positive and negative likelihood ratio and positive and negative predictive value [16]. For indicator combinations (e.g. breath CO and urine NicA-lert®), participants were considered smokers if at least one indicator identified them as smokers; participants were considered non-smokers if both indicators identified them as non-smokers.
Group differences between smokers and non-smokers were examined with t-tests. Differences between heavy smokers, light smokers, exposed non-smokers and not exposed non-smokers were examined with one-way analysis of variance (Table 1 only). Differences in categorical variables (e.g. sex) were examined with Pearson’s χ2 tests. Several specimens had cotinine concentrations below the LOQ (0.2 ng/ml) of the LCMSMS assay. We assigned a value of half the LOQ (0.1 ng/ml) for statistical analyses [17]. All data were analyzed with SPSS version 17.0 (Chicago, IL, USA). Results were considered statistically significant at P < 0.05.
Table 1.
Demographic, smoking, and carbon monoxide (CO) exposure characteristics of participants based on self-reported smoking status.
| Heavy smokers | Light smokers | Exposed non-smokers | Not exposed non-smokers | Group differences | Smokers versus non-smokers | |
|---|---|---|---|---|---|---|
| (n = 46) | (n = 44) | (n = 36) | (n = 46) | |||
| Age (mean ± SD) | 38.0 ± 9.5 | 39.3 ± 10.5 | 36.4 ± 12.8 | 30.9 ± 11.5 | P = 0.002 | P = 0.002 |
| Years education (mean ± SD) | 12.4 ± 1.7 | 12.9 ± 2.0 | 12.8 ± 2.3 | 13.7 ± 2.0 | P = 0.010 | P = 0.028 |
| Gender (% female) | 34.8 | 29.5 | 38.9 | 43.5 | NS | NS |
| Race (%) | P = 0.035 | NS | ||||
| African American | 56.5 | 68.3 | 91.7 | 71.7 | ||
| Asian | 0 | 4.5 | 0 | 2.2 | ||
| Caucasian | 41.3 | 22.7 | 8.3 | 23.9 | ||
| Other | 2.2 | 4.5 | 0 | 2.2 | ||
| Ethnicity (% Hispanic) | 2.2 | 6.8 | 0 | 4.3 | NS | NS |
| Minutes since last cigarette (mean ± SD) | 109.3 ± 132.7 | 142.6 ± 216.1 | NA | NA | NS | NA |
| Number of cigarettes today (mean ± SD) | 4.6 ± 3.3 | 2.6 ± 1.6 | NA | NA | P = 0.001 | NA |
| Cigarettes per day (mean ± SD) | 18.4 ± 6.5 | 8.7 ± 1.7 | NA | NA | P < 0.001 | NA |
| Years smoking (mean ± SD) | 19.3 ± 10.0 | 20.1 ± 12.8 | NA | NA | NS | NA |
| Exposed to passive smoke at home (%) | 54.3 | 43.2 | 91.7 | 0 | P < 0.001 | NS |
| Frequency exposed at home (%) | P < 0.001 | P = 0.003 | ||||
| Daily | 45.7 | 34.1 | 41.7 | 0 | ||
| Weekly, less than daily | 8.7 | 9.1 | 38.9 | 0 | ||
| Monthly, less than weekly | 0 | 0 | 11.1 | 0 | ||
| Never or rarely | 45.6 | 56.8 | 8.3 | 100.0 | ||
| When exposed, hours per day (mean ± SD) | 5.2 ± 4.1 | 4.8 ± 3.3 | 2.2 ± 3.1 | NA | P = 0.004 | P = 0.001 |
| If exposed, hours since last exposure at home (mean ± SD) | 10.7 ± 13.4 | 18.5 ± 30.1 | 35.0 ± 39.5 | NA | P = 0.012 | P = 0.004 |
| Exposed to passive smoke at work (%) | 19.6 | 9.1 | 13.9 | 0 | P = 0.019 | NS |
| Frequency exposed at work (%) | NS | NS | ||||
| Daily | 15.2 | 9.1 | 8.3 | 0 | ||
| Weekly, less than daily | 4.3 | 0 | 5.6 | 0 | ||
| Monthly, less than weekly | 0 | 0 | 0 | 0 | ||
| Never or rarely | 80.4 | 90.9 | 86.1 | 100.0 | ||
| When exposed, hours per day (mean ± SD) | 3.4 ± 3.0 | 3.1 ± 3.5 | 1.4 ± 1.8 | NA | NS | NS |
| If exposed, hours since last exposure at work (mean ± SD) | 31.6 ± 28.2 | 24.1 ± 24.8 | 43.4 ± 46.0 | NA | NS | NS |
| Drive car daily (%) | 23.9 | 29.5 | 50.0 | 41.3 | NS | P = 0.03 |
| If yes, minutes in car per day (mean ± SD) | 245.9 ± 248.6 | 190.8 ± 143.0 | 145.8 ± 189.5 | 112.9 ± 102.2 | NS | NS |
| Ride public transit daily (%) | 63.0 | 61.4 | 47.2 | 40.0 | P = 0.033 | P = 0.006 |
| If yes, minutes waiting for and riding transit per day (mean ± SD) | 144.0 ± 90.8 | 152.4 ± 96.6 | 157.8 ± 82.4 | 178.5 ± 104.9 | NS | NS |
| Home in city (%) | 76.1 | 79.5 | 75.0 | 58.7 | NS | NS |
| Occupational exposure to CO (%) | 26.1 | 22.7 | 11.1 | 8.7 | NS | P = 0.011 |
| LCMSMS urine cotinine (ng/ml) | 1482.0 ± 737.4 | 1361.8 ± 767.6 | 5.7 ± 12.6 | 11.8 ± 53.2 | P < 0.001 | P < 0.001 |
| LCMSMS saliva cotinine (ng/ml) | 339.0 ± 190.4 | 269.8 ± 139.4 | 0.8 ± 2.1 | 0.4 ± 1.1 | P < 0.001 | P < 0.001 |
Occupational exposures include construction; cooking; bus, taxi, limousine driver; industrial plant; and car repair. Group differences based on F-values, except for comparisons of smoking behaviors between light and heavy smokers. These comparisons and comparisons between smokers and non-smokers are based on t-tests. Differences between categorical variables are based on Pearson χ2 tests. SD: standard deviation; LCMSMS: liquid chromatography-tandem mass spectrometry; NA: not applicable; NS: not significant.
RESULTS
Participants and biochemical indicator variability
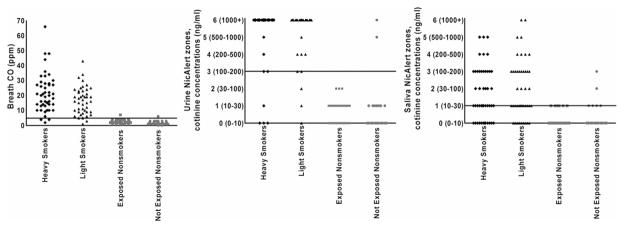
Table 1 shows participant characteristics based on self-reported tobacco and CO exposure. Figure 1 shows scatter-plots indicating variability in breath CO, urine NicAlert® and saliva NicAlert® levels based on participants’ self-reported tobacco exposure. Smokers had breath CO of 19.2 ± 11.4 p.p.m. [mean ± standard deviation (SD)] (range = 2–66 p.p.m. for heavy and 1–43 p.p.m. for light smokers). Non-smokers had breath CO of 2.1 ± 1.2 p.p.m. (range = 0–6 p.p.m. for not exposed and 1–7 p.p.m. for exposed non-smokers); 81.1% of smokers (38 of 46 heavy and 35 of 44 light smokers) had urine NicAlert® readings in zone 6 (>1000 ng/ml cotinine), consistent with the LCMSMS data (not shown) and previous reports of average urine cotinine concentrations among smokers [13,18]. Saliva NicAlert® readings were variable among all groups, but especially smokers; 23.3% of smokers (12 of 46 heavy and nine of 44 light smokers) had saliva NicAlert® readings in zone 0 (0–10 ng/ml cotinine) and were therefore identified as non-smokers. When saliva NicAlert® identified self-reported smokers as smokers, most readings were in zones 1, 2 and 3 (10–200 ng/ml).
Figure 1.
Variability of breath carbon monoxide (CO), urine NicAlert® and saliva NicAlert® based on self-reported smoking status. Each dot represents an individual participant. Horizontal lines represent cut-offs discriminating smokers from non-smokers for each indicator (breath CO ≥5 parts per million (p.p.m.), urine NicAlert® ≥zone 3/100 ng/ml cotinine, saliva NicAlert® ≥zone 1/>10 ng/ml cotinine)
Optimal breath CO cut-off
Table 2 lists the sensitivity and specificity of breath CO at cut-offs ranging from ≥2–11 p.p.m. with self-report and LCMSMS as reference measures. Breath CO at a cut-off of ≥5 p.p.m. optimally discriminated smokers from non-smokers with all reference measures.
Table 2.
Breath carbon monoxide (CO) performance with self-report and liquid chromatography-tandem mass spectrometry (LCMSMS) urine and saliva cotinine as reference measures.
| Reference measure | Cut-off (ng/ml) | Breath CO cut-off (p.p.m.) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Self-report | NA | ≥2 | 98.9 | 32.9 |
| ≥3 | 97.8 | 72.0 | ||
| ≥4 | 96.7 | 89.0 | ||
| ≥5 | 94.4 | 97.6 | ||
| ≥6 | 92.2 | 97.6 | ||
| ≥7 | 88.9 | 98.8 | ||
| ≥8 | 86.7 | 100.0 | ||
| ≥9 | 85.6 | 100.0 | ||
| ≥10 | 81.1 | 100.0 | ||
| ≥11 | 73.3 | 100.0 | ||
| LCMSMS urine cotinine | ≥0.2 | ≥2 | 85.6 | 26.9 |
| ≥3 | 71.9 | 76.9 | ||
| ≥4 | 64.4 | 90.3 | ||
| ≥5 | 58.9 | 96.2 | ||
| ≥6 | 57.5 | 96.2 | ||
| ≥7 | 54.8 | 96.2 | ||
| ≥8 | 53.4 | 100.0 | ||
| ≥9 | 52.7 | 100.0 | ||
| ≥10 | 50.0 | 100.0 | ||
| ≥11 | 45.2 | 100.0 | ||
| LCMSMS urine cotinine | ≥25 | ≥2 | 100.0 | 35.4 |
| ≥3 | 96.8 | 73.4 | ||
| ≥4 | 95.7 | 91.1 | ||
| ≥5 | 92.5 | 98.7 | ||
| ≥6 | 90.3 | 98.7 | ||
| ≥7 | 86.0 | 98.7 | ||
| ≥8 | 83.9 | 100.0 | ||
| ≥9 | 82.8 | 100.0 | ||
| ≥10 | 78.5 | 100.0 | ||
| ≥11 | 71.0 | 100.0 | ||
| LCMSMS urine cotinine | ≥100 | ≥2 | 100.0 | 34.1 |
| ≥3 | 97.8 | 72.0 | ||
| ≥4 | 96.7 | 89.0 | ||
| ≥5 | 94.4 | 97.6 | ||
| ≥6 | 92.2 | 97.6 | ||
| ≥7 | 88.9 | 98.8 | ||
| ≥8 | 86.7 | 100.0 | ||
| ≥9 | 85.6 | 100.0 | ||
| ≥10 | 81.1 | 100.0 | ||
| ≥11 | 73.3 | 100.0 | ||
| LCMSMS saliva cotinine | ≥0.2 | ≥2 | 99.1 | 41.5 |
| ≥3 | 91.6 | 80.0 | ||
| ≥4 | 84.1 | 90.8 | ||
| ≥5 | 79.4 | 96.9 | ||
| ≥6 | 77.6 | 96.9 | ||
| ≥7 | 73.8 | 96.9 | ||
| ≥8 | 72.0 | 98.5 | ||
| ≥9 | 71.0 | 98.5 | ||
| ≥10 | 67.3 | 98.5 | ||
| ≥11 | 60.7 | 98.5 | ||
| LCMSMS saliva cotinine | ≥10 | ≥2 | 100.0 | 33.7 |
| ≥3 | 98.9 | 72.3 | ||
| ≥4 | 97.8 | 89.2 | ||
| ≥5 | 94.4 | 96.4 | ||
| ≥6 | 92.1 | 96.4 | ||
| ≥7 | 88.8 | 97.6 | ||
| ≥8 | 86.5 | 98.8 | ||
| ≥9 | 85.4 | 98.8 | ||
| ≥10 | 80.9 | 98.8 | ||
| ≥11 | 73.0 | 98.8 |
Sensitivity and specificity at cut-offs ≥2–11 parts per million (p.p.m.). NA: not applicable.
Performance of breath CO, urine NicAlert®, saliva NicAlert® and combinations
Table 3 includes the performance of breath CO, urine NicAlert®, saliva NicAlert® and combinations, with self-report as the reference measure. When compared to self-report, breath CO and urine NicAlert® had high sensitivity, specificity and efficiency alone and in combination. High positive likelihood ratios and high positive and negative predictive values and low negative likelihood ratios reflect high sensitivity and specificity. Saliva NicA-lert® did not perform as well as breath CO and urine NicAlert®.
Table 3.
Performance of biochemical indicators and combinations with self-report as the reference measure.
| Biochemical indicator | Biochemical indicator cut-off |
True positives |
True negatives |
False positives |
False negatives |
Sensitivity
|
Specificity
|
Efficiency
|
Positive likelihood ratio |
Negative likelihood ratio |
Positive predictive value (%) |
Negative predictive value (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | ||||||||||
| Breath CO | ≥5 | 85 | 80 | 2 | 5 | 94.4 | 97.6 | 95.9 | 38.72 | 0.06 | 97.7 | 94.1 |
| Urine NicAlert | ≥3 | 83 | 80 | 2 | 7 | 92.2 | 97.6 | 94.8 | 37.80 | 0.08 | 97.7 | 92.0 |
| Saliva NicAlert | ≥1 | 69 | 70 | 12 | 21 | 76.7 | 85.4 | 80.8 | 5.23 | 0.27 | 85.2 | 76.9 |
| Breath CO/urine NicAlert | ≥5/≥3 | 88 | 78 | 4 | 2 | 97.8 | 95.1 | 96.5 | 20.04 | 0.02 | 95.7 | 97.5 |
| Breath CO/saliva NicAlert | ≥5/≥1 | 88 | 68 | 14 | 2 | 97.8 | 82.9 | 90.7 | 5.73 | 0.03 | 86.3 | 91.1 |
| Urine NicAlert/saliva NicAlert | ≥3/≥1 | 89 | 69 | 13 | 1 | 98.9 | 84.2 | 91.9 | 6.24 | 0.01 | 87.3 | 98.6 |
True positive: self-reported smoker and indicator smoker. True negative: self-reported non-smoker and indicator non-smoker. False positive: self-reported non-smoker and indicator smoker. False negative: self-reported smoker and indicator non-smoker. CO: carbon monoxide. NicAlert is a Registered Trademark.
Table 4 shows the performance of self-report, breath CO, urine NicAlert®, saliva NicAlert® and combinations, with LCMSMS urine and saliva cotinine results as the reference. Indicators performed poorly at the LOQ cut-off compared to higher LCMSMS cut-offs. This is consistent with the LOQ as a high sensitivity cut-off, classifying any measurable cotinine as a smoking indicator.
Table 4.
Performance of indicators and combinations with liquid chromatography-tandem mass spectrometry (LCMSMS) urine and saliva cotinine as reference measures.
| LCMSMS specimen |
LCMSMS cotinine cut-off (ng/ml) |
Biochemical indicator | Biochemical indicator cut-off |
True positives |
True negatives |
False positives |
False negatives |
Sensitivity (%) |
Specificity (%) |
Efficiency (%) |
Positive likelihood ratio |
Negative likelihood ratio |
Positive predictive value (%) |
Negative predictive value (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine | ≥0.2 | Self-report | NA | 90 | 26 | 0 | 56 | 61.6 | 100.0 | 67.4 | NA | 0.38 | 100.0 | 31.7 |
| Urine | ≥25 | Self-report | NA | 89 | 78 | 1 | 4 | 95.7 | 98.7 | 97.1 | 75.60 | 0.04 | 98.9 | 95.1 |
| Urine | ≥100 | Self-report | NA | 89 | 81 | 1 | 1 | 98.9 | 98.8 | 98.8 | 81.09 | 0.01 | 98.9 | 98.8 |
| Saliva | ≥0.2 | Self-report | NA | 88 | 63 | 2 | 19 | 82.2 | 96.9 | 87.8 | 26.70 | 0.18 | 97.8 | 76.8 |
| Saliva | ≥10 | Self-report | NA | 88 | 81 | 2 | 1 | 98.9 | 97.6 | 98.3 | 41.03 | 0.01 | 98.7 | 98.8 |
| Urine | ≥0.2 | Breath CO | ≥5 | 86 | 25 | 1 | 60 | 58.9 | 96.2 | 64.5 | 15.32 | 0.43 | 98.9 | 29.4 |
| Urine | ≥25 | Breath CO | ≥5 | 86 | 78 | 1 | 7 | 92.5 | 98.7 | 95.3 | 73.05 | 0.08 | 98.9 | 91.8 |
| Urine | ≥100 | Breath CO | ≥5 | 85 | 80 | 2 | 5 | 94.4 | 97.6 | 94.8 | 38.72 | 0.06 | 97.7 | 94.1 |
| Saliva | ≥0.2 | Breath CO | ≥5 | 85 | 63 | 2 | 22 | 79.4 | 96.9 | 86.1 | 25.82 | 0.21 | 97.7 | 74.1 |
| Saliva | ≥10 | Breath CO | ≥5 | 84 | 80 | 3 | 5 | 94.4 | 96.4 | 95.3 | 26.11 | 0.06 | 96.6 | 94.1 |
| Urine | ≥0.2 | Urine NicAlert | ≥3 | 84 | 25 | 1 | 62 | 57.5 | 96.2 | 63.3 | 14.96 | 0.44 | 98.8 | 28.7 |
| Urine | ≥25 | Urine NicAlert | ≥3 | 84 | 78 | 1 | 9 | 90.3 | 98.7 | 94.2 | 71.35 | 0.10 | 98.8 | 89.7 |
| Urine | ≥100 | Urine NicAlert | ≥3 | 83 | 80 | 2 | 7 | 92.2 | 97.6 | 94.8 | 37.81 | 0.08 | 97.7 | 92.0 |
| Saliva | ≥0.2 | Saliva NicAlert | ≥1 | 71 | 55 | 10 | 36 | 66.4 | 84.6 | 73.3 | 4.31 | 0.40 | 87.7 | 60.4 |
| Saliva | ≥10 | Saliva NicAlert | ≥1 | 68 | 70 | 13 | 21 | 76.4 | 84.3 | 80.2 | 4.88 | 0.28 | 84.0 | 77.0 |
| Urine | ≥0.2 | Self-report/breath CO | NA/≥5 | 91 | 25 | 1 | 55 | 62.3 | 96.2 | 67.4 | 16.21 | 0.39 | 98.9 | 31.3 |
| Urine | ≥25 | Self-report/breath CO | NA/≥5 | 90 | 77 | 2 | 3 | 96.8 | 97.5 | 97.1 | 38.23 | 0.03 | 97.8 | 96.3 |
| Urine | ≥100 | Self-report/breath CO | NA/≥5 | 89 | 79 | 3 | 1 | 98.9 | 96.3 | 97.7 | 27.03 | 0.01 | 96.7 | 98.8 |
| Saliva | ≥0.2 | Self-report/breath CO | NA/≥5 | 89 | 62 | 3 | 18 | 83.2 | 95.4 | 87.8 | 18.02 | 0.18 | 96.7 | 77.5 |
| Saliva | ≥10 | Self-report/breath CO | NA/≥5 | 84 | 79 | 4 | 5 | 94.4 | 95.2 | 94.8 | 19.58 | 0.06 | 95.5 | 94.1 |
| Urine | ≥0.2 | Self-report/urine NicAlert | NA/≥3 | 91 | 25 | 1 | 55 | 62.3 | 96.2 | 67.4 | 16.21 | 0.39 | 98.9 | 31.3 |
| Urine | ≥25 | Self-report/urine NicAlert | NA/≥3 | 90 | 77 | 2 | 3 | 96.8 | 97.5 | 97.1 | 38.23 | 0.03 | 97.8 | 96.3 |
| Urine | ≥100 | Self-report/urine NicAlert | NA/≥3 | 89 | 79 | 3 | 1 | 98.9 | 96.3 | 97.7 | 27.03 | 0.01 | 96.7 | 98.8 |
| Saliva | ≥0.2 | Self-report/saliva NicAlert | NA/≥1 | 92 | 55 | 10 | 15 | 86.0 | 84.6 | 85.5 | 5.59 | 0.17 | 90.2 | 78.6 |
| Saliva | ≥10 | Self-report/saliva NicAlert | NA/≥1 | 89 | 70 | 13 | 0 | 100.0 | 84.3 | 92.4 | 6.39 | 0.00 | 87.3 | 100.0 |
| Urine | ≥0.2 | Breath CO/urine NicAlert | ≥5/≥3 | 90 | 24 | 2 | 56 | 61.6 | 92.3 | 66.3 | 8.01 | 0.42 | 97.8 | 30.0 |
| Urine | ≥25 | Breath CO/urine NicAlert | ≥5/≥3 | 90 | 77 | 2 | 3 | 96.8 | 97.5 | 97.1 | 38.23 | 0.03 | 97.8 | 96.3 |
| Urine | ≥100 | Breath CO/urine NicAlert | ≥5/≥3 | 88 | 79 | 2 | 3 | 96.7 | 97.5 | 97.1 | 39.16 | 0.03 | 97.8 | 96.3 |
| Saliva | ≥0.2 | Breath CO/saliva NicAlert | ≥5/≥1 | 91 | 54 | 11 | 16 | 85.1 | 83.1 | 84.3 | 5.03 | 0.18 | 89.2 | 77.1 |
| Saliva | ≥10 | Breath CO/saliva NicAlert | ≥5/≥1 | 87 | 68 | 15 | 2 | 97.8 | 81.9 | 90.1 | 5.41 | 0.03 | 85.3 | 97.1 |
| Urine | ≥0.2 | Urine NicAlert/saliva NicAlert | ≥3/≥1 | 100 | 24 | 2 | 46 | 68.5 | 92.3 | 72.1 | 8.90 | 0.34 | 98.0 | 34.1 |
| Urine | ≥25 | Urine NicAlert/saliva NicAlert | ≥3/≥1 | 90 | 67 | 12 | 3 | 96.8 | 84.8 | 91.3 | 6.37 | 0.04 | 88.2 | 95.7 |
| Urine | ≥100 | Urine NicAlert/saliva NicAlert | ≥3/≥1 | 88 | 68 | 14 | 2 | 97.8 | 82.9 | 90.7 | 5.73 | 0.03 | 86.3 | 97.1 |
| Saliva | ≥0.2 | Urine NicAlert/saliva NicAlert | ≥3/≥1 | 92 | 55 | 10 | 15 | 86.0 | 84.6 | 85.5 | 5.59 | 0.17 | 90.2 | 78.6 |
| Saliva | ≥10 | Urine NicAlert/saliva NicAlert | ≥3/≥1 | 87 | 70 | 14 | 1 | 98.8 | 83.3 | 91.3 | 5.93 | 0.01 | 86.1 | 98.6 |
True positive: LCMSMS smoker and indicator smoker. True negative: LCMSMS non-smoker and indicator non-smoker. False positive: LCMSMS non-smoker and indicator smoker. False negative: LCMSMS smoker and indicator non-smoker. CO: carbon monoxide; NA: not applicable. NicAlert is a Registered Trademark.
Self-report, breath CO and urine NicAlert® performed well in predicting smoking status determined by LCMSMS (Table 4). They performed similarly in all possible combinations. Saliva NicAlert® alone was not as reliable a test. Combinations of saliva NicAlert® with the other indicators improved performance relative to saliva NicAlert® alone.
DISCUSSION
This study established an optimal breath CO cut-off for individuals experiencing a wide range of tobacco exposure in an urban setting that bans indoor smoking in many public spaces, such as bars and restaurants. We determined the optimal cut-off by comparing breath CO levels to six reference measures: self-report, three LCMSMS urine cotinine cut-offs and two LCMSMS saliva cotinine cut-offs. The breath CO cut-offs recommended by SRNT in 2002 (≥8–10 p.p.m.) had considerably lower sensitivity and slightly better specificity compared to the ≥5 p.p.m. cut-off (Table 2). We also documented performance of several other smoking indicators alone and in combination. Saliva NicAlert® performance was less effective than the other indicators (Tables 3 and 4). A combination of breath CO (cut-off ≥ 5 p.p.m.) and urine NicAlert® testing optimally determined smoking status (Tables 3 and 4).
Because most previous breath CO data were collected before expansion of indoor smoking bans, new data are needed to define the appropriate cut-off to optimally distinguish smokers from non-smokers. In support of our hypothesis, we found an optimal breath CO cut-off (≥5 p.p.m.), which was in the lower range of previously recommended cut-offs. Cropsey et al. [5] recommended a breath CO cut-off of ≥3 p.p.m. (sensitivity = 98.1%, specificity = 95.8%; self-report reference measure) in female prisoners. Kauffman et al. [17] recommended ≥4 p.p.m. (sensitivity = 88.3%, specificity = 94.9%; self-report reference measure) in male prisoners. Our breath CO data seem more consistent with that of prison populations than previous data based on the general population. Perhaps this is due to similar patterns of tobacco exposure in our sample and prison populations. In locations where indoor smoking bans have been enacted, tobacco smoking and exposure are confined to specific times and locations, which is also true of prisons [5,17]. Reduced tobacco exposure in these populations as a result of smoking restrictions might explain lower breath CO levels compared to earlier data from the general population [5,6,17].
Our study is the first to evaluate breath CO, urine NicAlert®, and saliva NicAlert® and their various combinations in the same group of participants. Acosta et al. [19] reported that breath CO (>8 p.p.m. cut-off) had 83.1% sensitivity and 100% specificity, and urine NicA-lert® had 98.5% sensitivity and 58.5% specificity against GCMS urine cotinine (≥100 ng/ml) in treatment seekers. This suggested that breath CO and urine NicAlert® in combination might be more effective in verifying smoking status than either method alone [19]. Schepis et al. [20] similarly obtained daily breath CO, urine NicAlert® and urine GCMS cotinine levels for treatment seekers. Breath CO was the best indicator of abstinence during early interventions, whereas urine NicAlert® was particularly useful to identify later lapses in smoking [20]. Consistent with these findings and our hypothesis, we found that breath CO in combination with urine NicAlert® performed well in predicting smoking status determined by quantitative analysis of cotinine (urine cut-offs ≥ 25 and ≥100 ng/ml); efficiency was greater when these indicators were combined versus either indicator alone (Table 4).
Our study compared the performance of several smoking indicators against several reference measures, whereas most studies have assessed only the performance of one indicator with quantitative analysis of cotinine at a single cut-off. Current disagreement over optimal quantitative cut-offs makes previous data difficult to interpret. For example, differences between our findings and Montalto & Wells [21] might be related to differences in saliva cotinine cut-offs for quantitative reference measures. Montalto & Wells [21] found that saliva NicAlert® had 99% sensitivity and 96% specificity when LCMS saliva cotinine (cut-off ≥ 50 ng/ml) was the reference measure. Similarly, Cooke et al. [22] found that with GCMS saliva cotinine (cut-off ≥ 10 ng/ml) as the reference measure, saliva NicAlert® had 93% sensitivity, 95% specificity, 95% positive predictive value and 93% negative predictive value. With self-report as the reference, saliva NicA-lert® had 100% sensitivity, specificity, positive predictive value and negative predictive value. We found that performance of saliva NicAlert® was improved with self-report as the reference measure but, in general, the performance of saliva NicAlert® was less accurate than reported previously [21,22].
A possible explanation for the relatively poorer performance with saliva NicAlert® is that endogenous metabolites in saliva cross-reacted in the saliva NicAlert® assay. In this case, a higher saliva NicAlert® cut-off might discriminate smokers from non-smokers more clearly. An additional analysis of saliva NicAlert® performance using cut-offs of ≥2 and ≥3 improved specificity, but sensitivity decreased to about 40% at these cut-offs. Specificity improved because there were self-reported not exposed non-smokers with saliva NicAlert® readings in zones 2 and 3; their urine NicAlert® readings were in zone 0, and their breath CO levels were 1 and 2 p.p.m., respectively (Fig. 1). Breath CO, urine NicAlert® and saliva NicAlert® rarely identified the same participants as false positives and false negatives. Three participants had two and only one participant had all biochemical indicators inconsistent with their LCMSMS urine and saliva cotinine classifications as smokers/non-smokers. This is consistent with our findings that combinations of indicators performed well in predicting smoking status, and suggests that similar performance was not related to the indicators misidentifying the same participants.
One limitation of this study is the potential lack of generality to other populations. This study was conducted in one city, and future studies will need to determine if a breath CO cut-off ≥ 5 p.p.m. is optimal for additional locations. In addition, a larger sample size might have allowed us to examine a wider range of tobacco exposure, especially among non-smokers with environmental exposure and individuals with CO exposure unrelated to tobacco. Our optimal cut-off of ≥ 5 p.p.m. is based on current smokers and non-smokers. It may not apply to populations with different patterns of tobacco exposure, such as adolescents or treatment seekers. For example, differences in breath CO levels between abstinent and non-abstinent smokers in treatment are unlikely to be equivalent to smokers and non-smokers in this study. Because smoking policies are evolving, continued research is needed to update optimal cut-off values for clinical trials. The same argument also applies to cotinine cut-offs [23].
Another limitation of our study was the smoking prevalence of 52%, compared with 20% in the general population [24]. A future study should determine if the indicators perform as well when smoking prevalence is constrained to 20%. Future studies should also examine alternatives to the saliva NicAlert® assay, such as the Salimetrics assay (State College, PA, USA). This assay is more labor-intensive than NicAlert®, but less labor-intensive than LCMSMS. We believe that the poorer performance of saliva NicAlert® is probably related to the immunoassay, which was developed originally for urine, and not to use of saliva as a matrix. Several saliva cotinine assays have been validated as reliable indicators of tobacco exposure, and collection of saliva offers several advantages, such as ability to observe collection non-invasively.
In summary, we found that self-report, breath CO, urine NicAlert® and combinations of these techniques are good indicators of smoking. In our study, self-report was a good indicator of smoking status, but it can be influenced by social factors [1]. Because there was little social pressure to misreport smoking status and we collected multiple specimens for biochemical quantification, we might have created self-report data that cannot be replicated easily. In situations where self-reported smoking might be questioned, combined use of breath CO and urine NicAlert® would provide a reliable indication of smoking status.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse. We thank Rebecca Lange for assistance in conducting the study. We also thank Jaclyn Javerbaum and Rima Chakraborty for assistance with the NicAlert® assays.
Footnotes
Declarations of interest
None.
References
- 1.Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull. 1992;111:23–41. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Society for Research on Nicotine and Tobacco (SRNT) Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 3.Marrone GF, Paulpillai M, Evans RJ, Singleton EG, Heishman SJ. Breath carbon monoxide and semiquantitative saliva cotinine as biomarkers for smoking. Hum Psychopharmacol. 2010;25:80–3. doi: 10.1002/hup.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarvis MJ, Russell MA. Measurement and estimation of smoke dosage to non-smokers from environmental tobacco smoke. Eur J Respir Dis Suppl. 1984;133:68–75. [PubMed] [Google Scholar]
- 5.Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML. Expired carbon monoxide levels in self-reported smokers and non-smokers in prison. Nicotine Tob Res. 2006;8:653–9. doi: 10.1080/14622200600789684. [DOI] [PubMed] [Google Scholar]
- 6.Tual S, Piau JP, Jarvis MJ, Dautzenberg B, Annesi-Maesano I. Impact of tobacco control policies on exhaled carbon monoxide in non-smokers. J Epidemiol Commun Health. 2010;64:554–6. doi: 10.1136/jech.2008.086256. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107:349–55. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Best D, Green EM, Smith JH, Perry DC. Dipstick tests for secondhand smoke exposure. Nicotine Tob Res. 2010;12:551–6. doi: 10.1093/ntr/ntq043. [DOI] [PubMed] [Google Scholar]
- 9.Shakleya DM, Huestis MA. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human oral fluid. Anal Bioanal Chem. 2009;395:2349–57. doi: 10.1007/s00216-009-3157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavazzano P, Perico A, Boddi V, Lorini C, Cavaciocchi D, Lanciotti E. Most sensitive urinary cotinine cut-off level for environmental tobacco smoke exposure assessment: a pilot study. Ann Ig. 2007;19:225–33. [PubMed] [Google Scholar]
- 11.Man CN, Fatherlrahman AI, Harn GL, Lajis R, Samin ASM, Omar M, et al. Correlation between urinary nicotine, cotinine and self-reported smoking status among educated adults. Environ Toxicol Pharmacol. 2009;28:92–6. doi: 10.1016/j.etap.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med. 1990;19:190–7. doi: 10.1016/0091-7435(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 13.Wall MA, Johnson J, Jacob P, Benowitz NL. Cotinine in the serum, saliva, and urine of non-smokers, passive smokers, and active smokers. Am J Public Health. 1988;78:699–701. doi: 10.2105/ajph.78.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–77. [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan HI, Sadock BJ. Epidemiology, Biostatistics, and Social Psychiatry. 6. Baltimore: Williams and Wilkins; 1991. [Google Scholar]
- 17.Kauffman RM, Ferketich AK, Murray DM, Bellair PE, Wewers ME. Measuring tobacco use in a prison population. Nicotine Tob Res. 2010;12:582–8. doi: 10.1093/ntr/ntq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 19.Acosta M, Buchhalter A, Breland A, Hamilton D, Eissenberg T. Urine cotinine as an index of smoking status in smokers during 96-hour abstinence: comparison between gas chromatography/mass spectrometry and immunoassay test strips. Nicotine Tob Res. 2004;6:615–20. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- 20.Schepis TS, Duhig AM, Liss T, McFetridge A, Wu R, Cavallo DA, et al. Contingency management for smoking cessation: enhancing feasibility through use of immunoassay test strips measuring cotinine. Nicotine Tob Res. 2008;10:1495–501. doi: 10.1080/14622200802323209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: a rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16:1858–62. doi: 10.1158/1055-9965.EPI-07-0189. [DOI] [PubMed] [Google Scholar]
- 22.Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N, et al. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10:607–12. doi: 10.1080/14622200801978680. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz N, Bernert J, Caraballo R, Holiday D, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and non-smokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–48. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Cigarette smoking among adults and trends in smoking cessation—United States, 2008. Morb Mortal Wkly Rep. 2009;58:1227–32. [PubMed] [Google Scholar]