Abstract
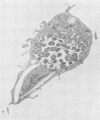
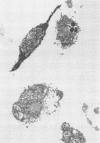
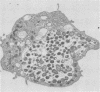
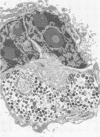
Chlamydia trachomatis is an obligate intracellular parasite of eucaryotic cells. Little is known about the role of the host in supporting chlamydial replication beyond the facts that host cells provide ATP and that de novo host protein synthesis is not required for bacterial growth. To further explore potential contributions of host nuclear function to chlamydial development, we questioned whether murine C. trachomatis could grow in mouse L cells that had been enucleated with cytochalasin B. Following enucleation, cells were infected with chlamydiae and analyzed morphologically and biochemically. Late in infection, substantial numbers of chlamydiae of all developmental stages were seen within large cytoplasmic inclusions that were indistinguishable from those seen in infected intact cells. Normal numbers of infectious progeny particles were produced from enucleated cultures. We conclude that active host cell nuclear function is not required to support the growth of chlamydiae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y. The chlamydia: molecular biology of procaryotic obligate parasites of eucaryocytes. Microbiol Rev. 1978 Jun;42(2):274–306. doi: 10.1128/mr.42.2.274-306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCKER T. T., EASTWOOD J. M. Subcellular cultivation of a virus: growth of ornithosis virus in nonnucleate cytoplasm. Virology. 1963 Jan;19:23–31. doi: 10.1016/0042-6822(63)90020-5. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Al-Hossainy E., Silverman J. A. Adenine nucleotide and lysine transport in Chlamydia psittaci. J Bacteriol. 1982 May;150(2):662–670. doi: 10.1128/jb.150.2.662-670.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal-Drouard L., Weil J. H., Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988 Jun 10;16(11):4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardinia L. M., Segal E., Ganem D. Developmental regulation of the cysteine-rich outer-membrane proteins of murine Chlamydia trachomatis. J Gen Microbiol. 1988 Apr;134(4):997–1004. doi: 10.1099/00221287-134-4-997. [DOI] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Leipzig G. V., Sameshima J. H., Stanbridge E. J. Selective transfer of individual human chromosomes to recipient cells. Mol Cell Biol. 1985 Jan;5(1):140–146. doi: 10.1128/mcb.5.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Higashi N. Purification and chemical composition of reticulate bodies of the meningopneumonitis organisms. J Bacteriol. 1967 Jun;93(6):2003–2008. doi: 10.1128/jb.93.6.2003-2008.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. DNA primase of human mitochondria is associated with structural RNA that is essential for enzymatic activity. Cell. 1986 Jun 20;45(6):817–825. doi: 10.1016/0092-8674(86)90556-8. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978 Mar;19(3):1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]