Abstract
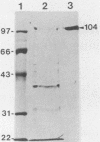
Five cesarean-derived, colostrum-deprived pigs were given three adjuvant-supplemented subcutaneous and one intravenous injection of the purified 104-kDa hemolysin from serotype 1 Actinobacillus pleuropneumoniae CM-5. Six control animals received phosphate-buffered saline only. Five of six control pigs died within 24 h after challenge. The sixth control pig was moribund and euthanized after 48 h. All six pigs had pleuropneumonia, and A. pleuropneumoniae was isolated from all six lungs. None of the vaccinated pigs died as a result of challenge. After being euthanized, two pigs in this group had no lung lesions but three had chronic pleuropneumonia involving 10, 20, and 40% of the lung tissue. A. pleuropneumoniae was isolated from lung lesions of these three animals but not from the two pigs without lesions. The prechallenge hemolysin-neutralizing antibody titers in the vaccinated pigs were 1:10,900, 1:10,600, 1:4,800, 1:3,900, and 1:3,000, in order of increasing lung involvement. None of the control pigs had neutralizing antibodies. Enzyme-linked immunosorbent assay (ELISA) antibodies to capsule, lipopolysaccharide, and hemolysin were not detected in serum samples collected from the control pigs. In the vaccinated group, prechallenge sera did not contain ELISA antibodies to capsule or lipopolysaccharide. ELISA antibodies to the hemolysin were detected only in the prechallenge and postchallenge serum samples. These results indicate that pigs immunized with the 104-kDa hemolysin of serotype 1 A. pleuropneumoniae are protected against challenge with virulent bacteria. The association between neutralizing antibodies and protection indicates indirectly that the hemolysin is an important virulence factor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram T. A. Quantitative morphology of peracute pulmonary lesions in swine induced by Haemophilus pleuropneumoniae. Vet Pathol. 1985 Nov;22(6):598–609. doi: 10.1177/030098588502200615. [DOI] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Rosendal S. Capsular polysaccharide antigens for detection of serotype-specific antibodies to Actinobacillus pleuropneumoniae. Can J Vet Res. 1990 Jun;54(3):320–325. [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Bossé J. T., Wilkie B. N., Johnson R. Prevalence of seroreactors to the 104-kilodalton hemolysin of Actinobacillus pleuropneumoniae in swine herds. J Clin Microbiol. 1990 Apr;28(4):789–791. doi: 10.1128/jcm.28.4.789-791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S. Identification of the heat-labile hemolysin of Actinobacillus pleuropneumoniae serotype 1. Can J Vet Res. 1989 Apr;53(2):251–254. [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Johnson R., Hubler S. Immunoserological comparison of 104-kilodalton proteins associated with hemolysis and cytolysis in Actinobacillus pleuropneumoniae, Actinobacillus suis, Pasteurella haemolytica, and Escherichia coli. Infect Immun. 1989 Oct;57(10):3210–3213. doi: 10.1128/iai.57.10.3210-3213.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka-Cray P. J., Huether M. J., Stine D. L., Anderson G. A. Efficacy of a cell extract from Actinobacillus (Haemophilus) pleuropneumoniae serotype 1 against disease in swine. Infect Immun. 1990 Feb;58(2):358–365. doi: 10.1128/iai.58.2.358-365.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W., Cullor J. S., Osburn B. I., Olander H. J. Mechanisms involved in protection provided by immunization against core lipopolysaccharides of Escherichia coli J5 from lethal Haemophilus pleuropneumoniae infections in swine. Infect Immun. 1986 Aug;53(2):298–304. doi: 10.1128/iai.53.2.298-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick B. W. Virulence attributes of the liposaccharides of the HAP group organisms. Can J Vet Res. 1990 Apr;54 (Suppl):S28–S32. [PubMed] [Google Scholar]
- Frey J., Nicolet J. Regulation of hemolysin expression in Actinobacillus pleuropneumoniae serotype 1 by Ca2+. Infect Immun. 1988 Oct;56(10):2570–2575. doi: 10.1128/iai.56.10.2570-2575.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J. Capsules and virulence in the HAP group of bacteria. Can J Vet Res. 1990 Apr;54 (Suppl):S22–S27. [PubMed] [Google Scholar]
- Inzana T. J., Mathison B. Serotype specificity and immunogenicity of the capsular polymer of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1987 Jul;55(7):1580–1587. doi: 10.1128/iai.55.7.1580-1587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A. E., Bertram T. A. Morphological and biochemical comparison of virulent and avirulent isolates of Haemophilus pleuropneumoniae serotype 5. Infect Immun. 1986 Feb;51(2):419–424. doi: 10.1128/iai.51.2.419-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett A. D., Harrison L. R., Farrell R. L. Acute inflammatory effects of intratracheally instilled Escherichia coli endotoxin and sonicated suspension of Haemophilus pleuropneumoniae in swine. Can J Vet Res. 1986 Oct;50(4):526–531. [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y. Molecular characterization of cytotoxins produced by Haemophilus, Actinobacillus, Pasteurella. Can J Vet Res. 1990 Apr;54 (Suppl):S33–S35. [PubMed] [Google Scholar]
- Lombin L. H., Rosendal S., DeMoor J. Biochemical and serological identification of strains of Haemophilus pleuropneumoniae. Vet Microbiol. 1985 Jun;10(4):393–397. doi: 10.1016/0378-1135(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Maudsley J. R., Kadis S., Mayberry W. R. Isolation, purification, and partial characterization of a lipopolysaccharide from Haemophilus pleuropneumoniae. Infect Immun. 1986 Feb;51(2):501–506. doi: 10.1128/iai.51.2.501-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Carpenter D. S., Mitchell W. R., Wilson M. R. Vaccination against pleuropneumonia of pigs caused by Haemophilus pleuropneumoniae. Can Vet J. 1981 Feb;22(2):34–35. [PMC free article] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Rosendal S., MacInnes J. I. Characterization of an attenuated strain of Actinobacillus pleuropneumoniae, serotype 1. Am J Vet Res. 1990 May;51(5):711–717. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R., Osborne A. D. A model aerosol exposure system for induction of porcine Haemophilus pleuropneumonia. Can J Comp Med. 1983 Jan;47(1):48–53. [PMC free article] [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]