Abstract
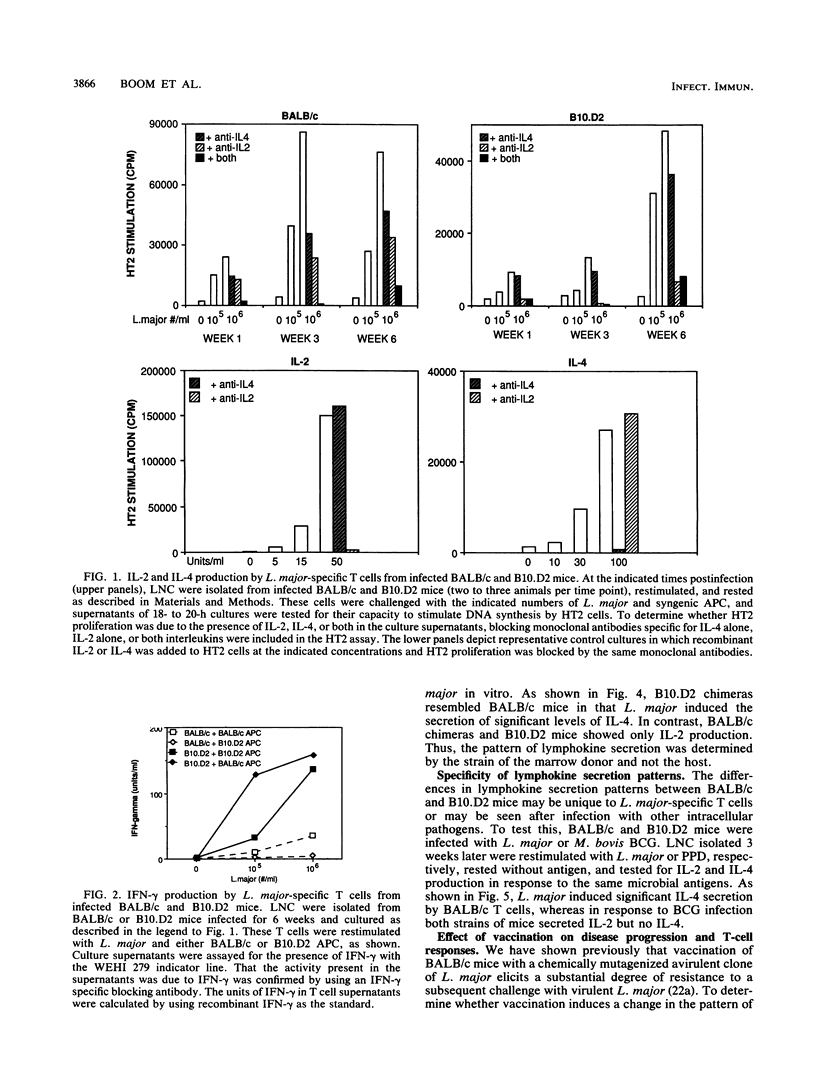
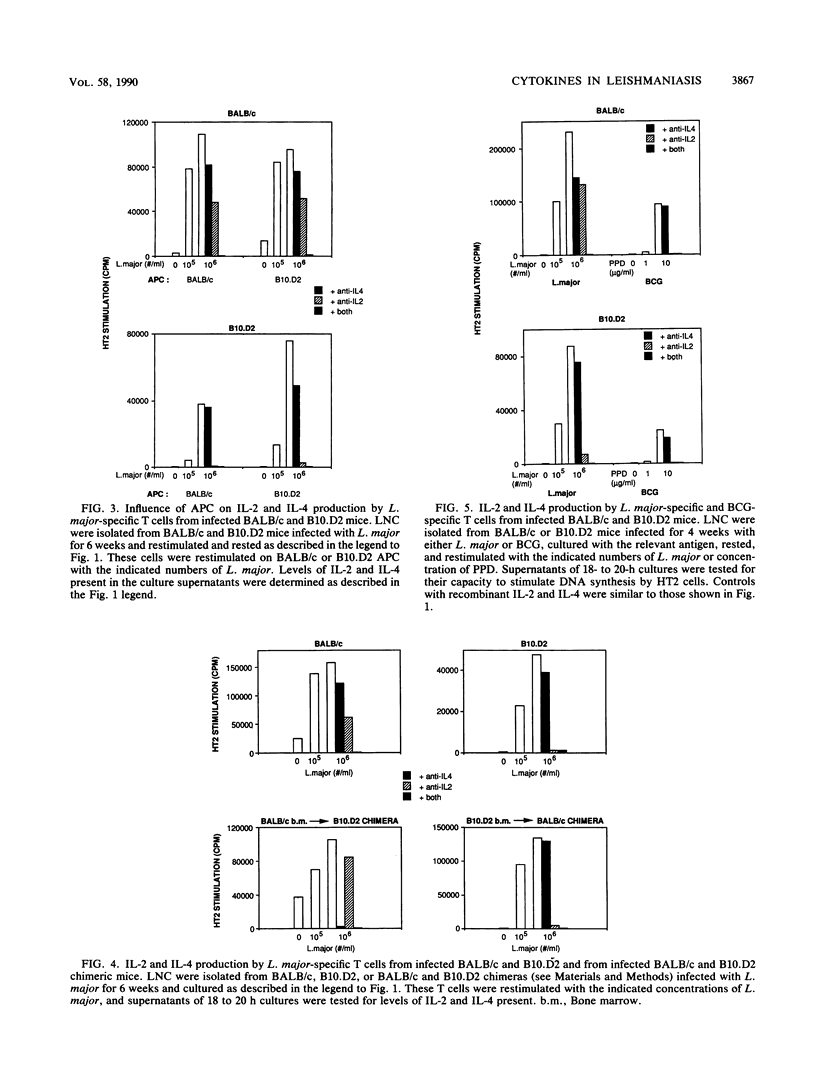
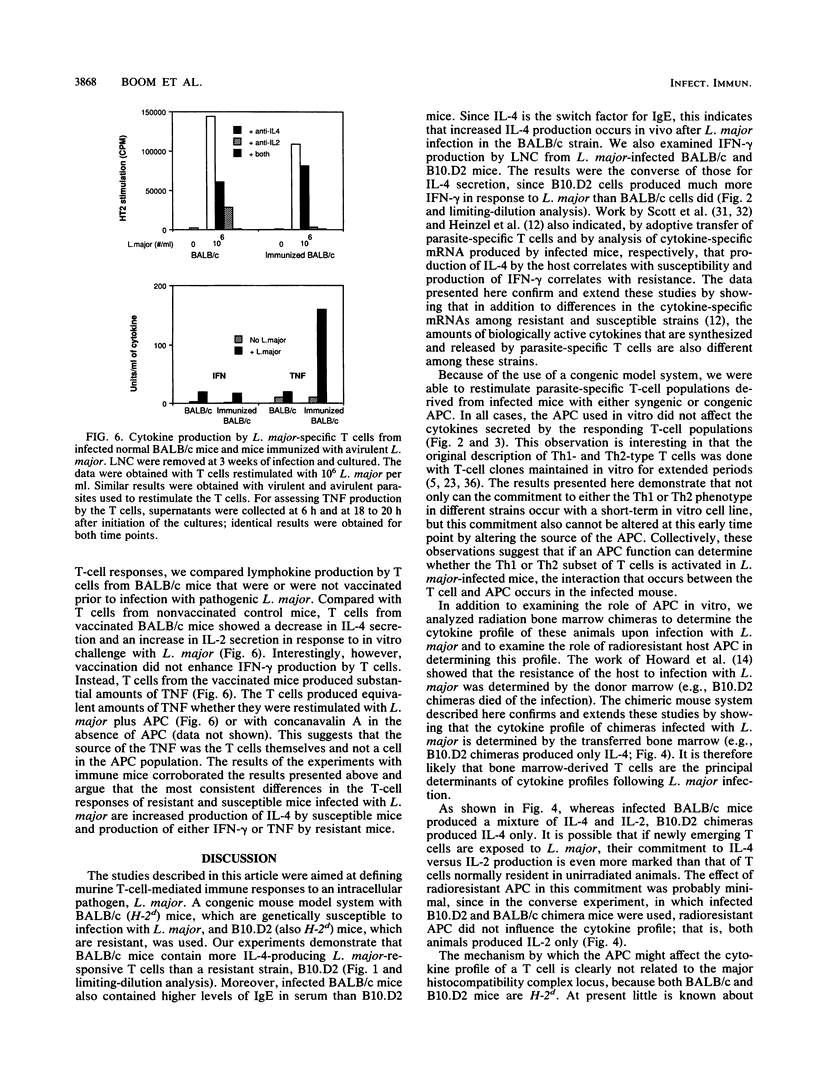
Susceptibility or resistance to infection with Leishmania major correlates with the ability of mice to produce characteristic panels of lymphokines in response to the parasite. To investigate the role of antigen-presenting cells in this phenomenon, we developed a model system which used congenic (H-2d) susceptible and resistant mice. L. major-specific T cells were isolated from infected BALB/c and B10.D2 mice, and the cells were restimulated in vitro on syngenic or congenic antigen-presenting cells. BALB/c L. major-reactive T cells restimulated with either antigen-presenting cell produced high levels of interleukin-4 and low levels of gamma interferon. In contrast, T cells from B10.D2 mice produced gamma interferon. Radiation-induced chimeras reconstituted with BALB/c bone marrow also produced more interleukin-4 in response to L. major than did chimeras reconstituted with B10.D2 bone marrow. To test whether this pattern of cytokine secretion was unique to infection with L. major, we infected the mice with a second intracellular pathogen, Mycobacterium bovis BCG. Mycobacterium-specific T cells from both BALB/c and B10.D2 mice produced interleukin-2 and no interleukin-4. Finally, when BALB/c mice were vaccinated with avirulent L. major, the induced resistance correlated with reduced production of interleukin-4 but no increase in gamma interferon production. Instead, T cells from the vaccinated mice produced high levels of tumor necrosis factor. This suggests that tumor necrosis factor, in addition to gamma interferon, may be involved in resistance to L. major and that interleukin-4 may inhibit the leishmanicidal activity of tumor necrosis factor and/or gamma interferon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. L., Gallin J. I. IL-4 inhibits superoxide production by human mononuclear phagocytes. J Immunol. 1990 Jan 15;144(2):625–630. [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Gallagher G., Baillie A. J., Alexander J. The induction of protective immunity to Leishmania major in the BALB/c mouse by interleukin 4 treatment. Eur J Immunol. 1989 Apr;19(4):779–782. doi: 10.1002/eji.1830190432. [DOI] [PubMed] [Google Scholar]
- Cillari E., Dieli M., Maltese E., Milano S., Salerno A., Liew F. Y. Enhancement of macrophage IL-1 production by Leishmania major infection in vitro and its inhibition by IFN-gamma. J Immunol. 1989 Sep 15;143(6):2001–2005. [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Essner R., Rhoades K., McBride W. H., Morton D. L., Economou J. S. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989 Jun 1;142(11):3857–3861. [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Nicklin S., Hale C., Liew F. Y. Prophylactic immunization against experimental leishmaniasis: I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982 Nov;129(5):2206–2212. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Lichtman A. H., Chin J., Schmidt J. A., Abbas A. K. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Li Y., Lelchuk R., Chan W. L., Ziltener H. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL)3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989 Jul;19(7):1227–1232. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Parkinson C., Millott S., Severn A., Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–573. [PMC free article] [PubMed] [Google Scholar]
- Maggi E., Del Prete G., Macchia D., Parronchi P., Tiri A., Chrétien I., Ricci M., Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur J Immunol. 1988 Jul;18(7):1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- McGurn M., Boon T., Louis J. A., Titus R. G. Leishmania major: nature of immunity induced by immunization with a mutagenized avirulent clone of the parasite in mice. Exp Parasitol. 1990 Jul;71(1):81–89. doi: 10.1016/0014-4894(90)90010-a. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mueller D. L., Jenkins M. K., Schwartz R. H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Müller I., Louis J. A. Immunity to experimental infection with Leishmania major: generation of protective L3T4+ T cell clones recognizing antigen(s) associated with live parasites. Eur J Immunol. 1989 May;19(5):865–871. doi: 10.1002/eji.1830190513. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Pearce E., Cheever A. W., Coffman R. L., Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989 Dec;112:161–182. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Korty P. E. Ly-6: a multigene family in search of a function. Immunol Today. 1989 Jun;10(6):195–200. doi: 10.1016/0167-5699(89)90324-1. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Zlotnik A., Woodward J. G. Induction of class I and class II MHC antigen expression on murine bone marrow-derived macrophages by IL-4 (B cell stimulatory factor 1). J Immunol. 1988 Mar 1;140(5):1542–1547. [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Dutton R. W., Tonkonogy S. L., English M. The role of IL4 and IL5: characterization of a distinct helper T cell subset that makes IL4 and IL5 (Th2) and requires priming before induction of lymphokine secretion. Immunol Rev. 1988 Feb;102:77–105. doi: 10.1111/j.1600-065x.1988.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Lauener R. P., Geha R. S. IL-4 inhibits the synthesis of IFN-gamma and induces the synthesis of IgE in human mixed lymphocyte cultures. J Immunol. 1990 Jan 15;144(2):570–573. [PubMed] [Google Scholar]


