Abstract
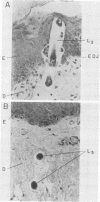
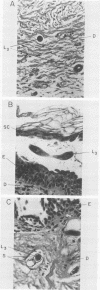
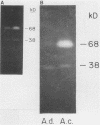
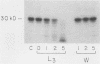
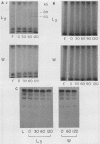
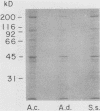
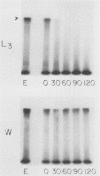
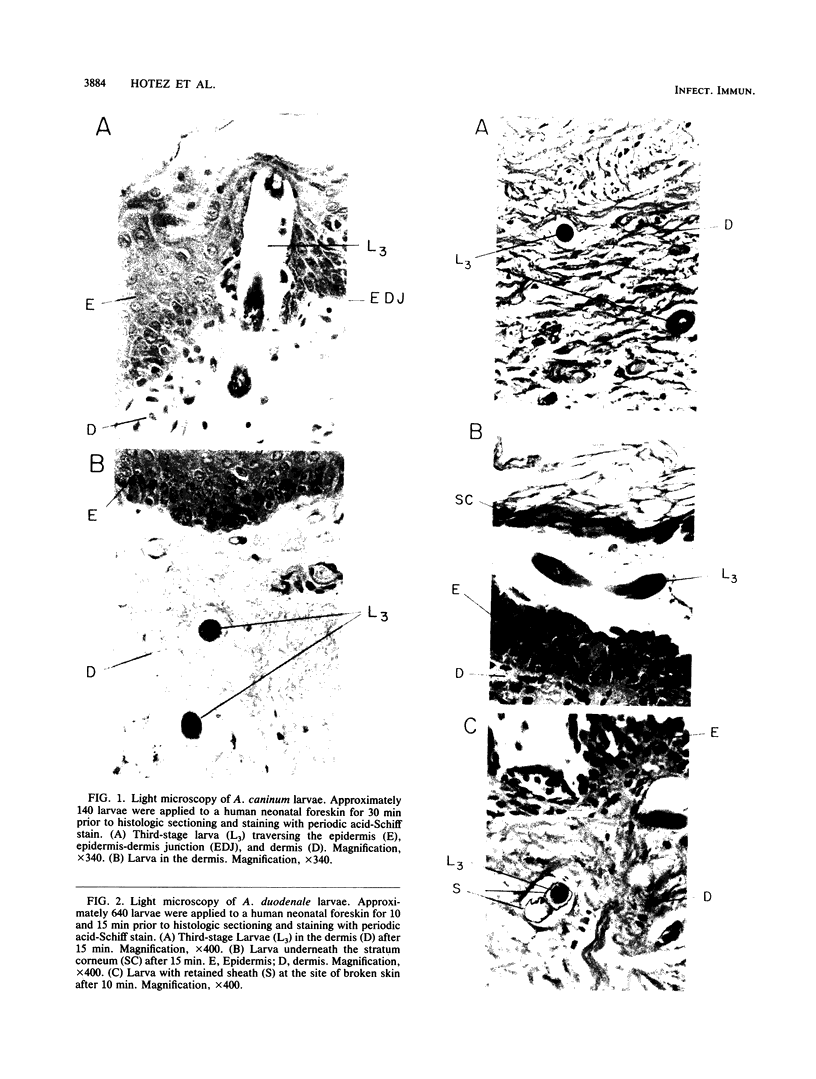
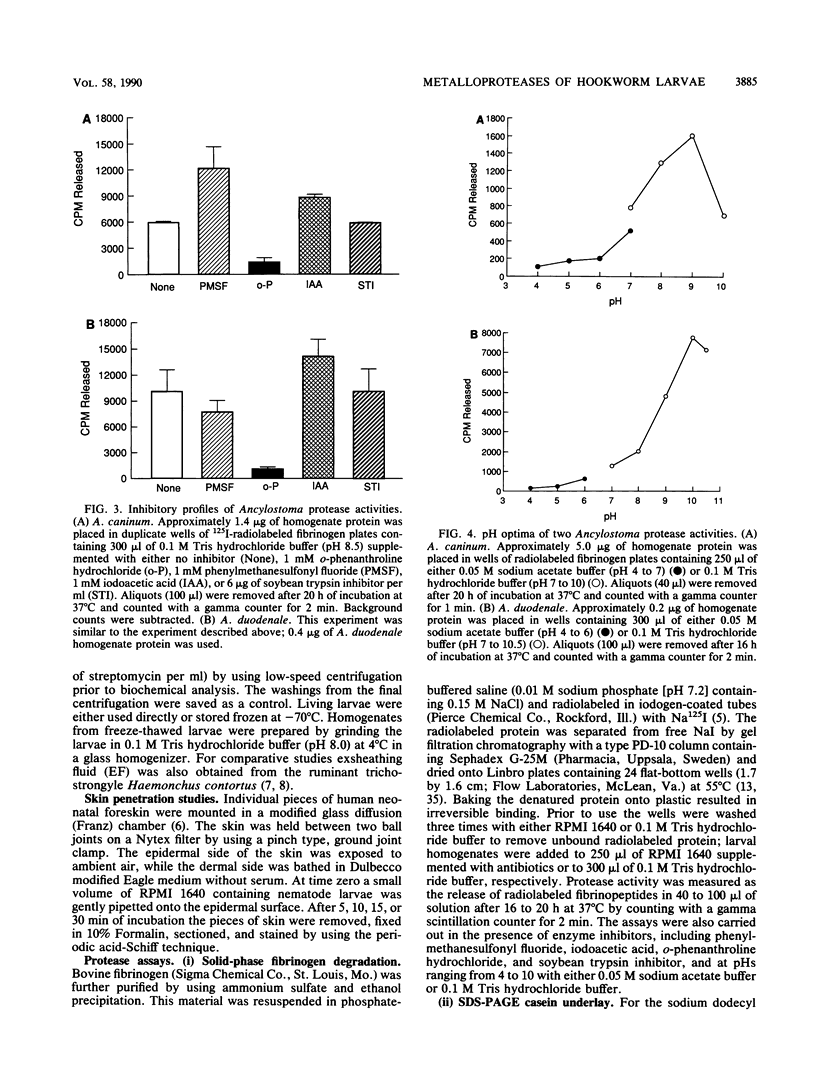
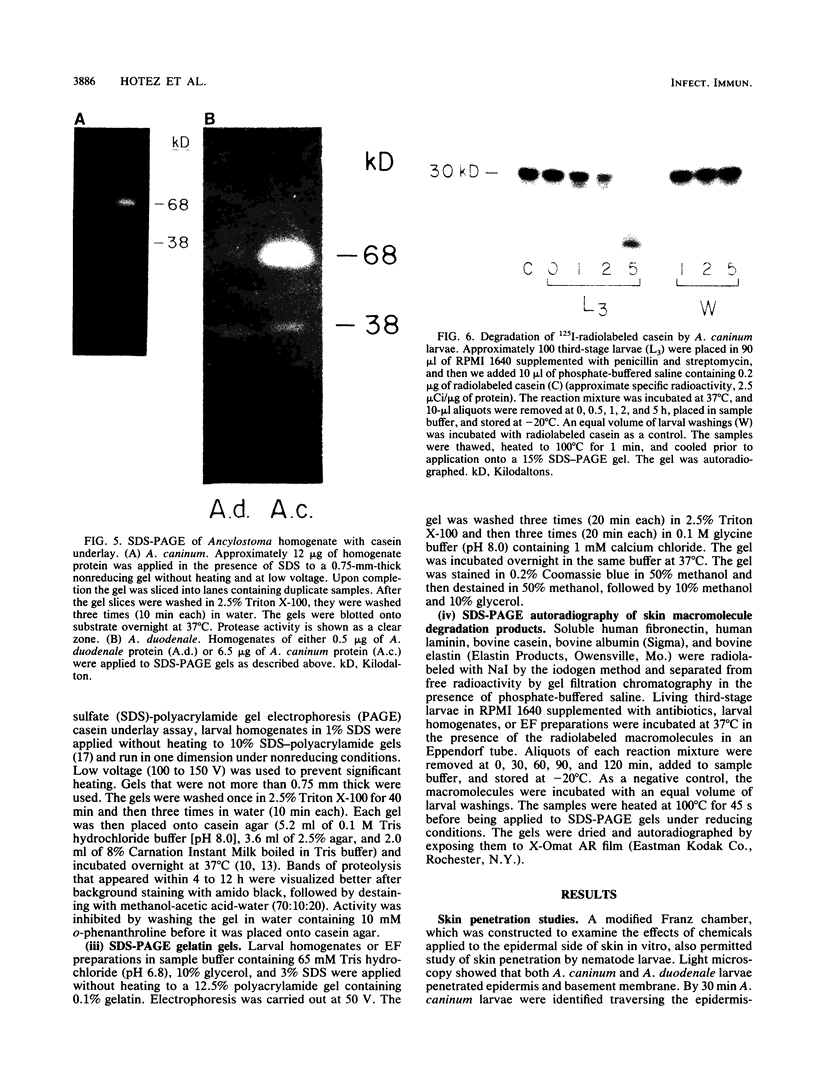
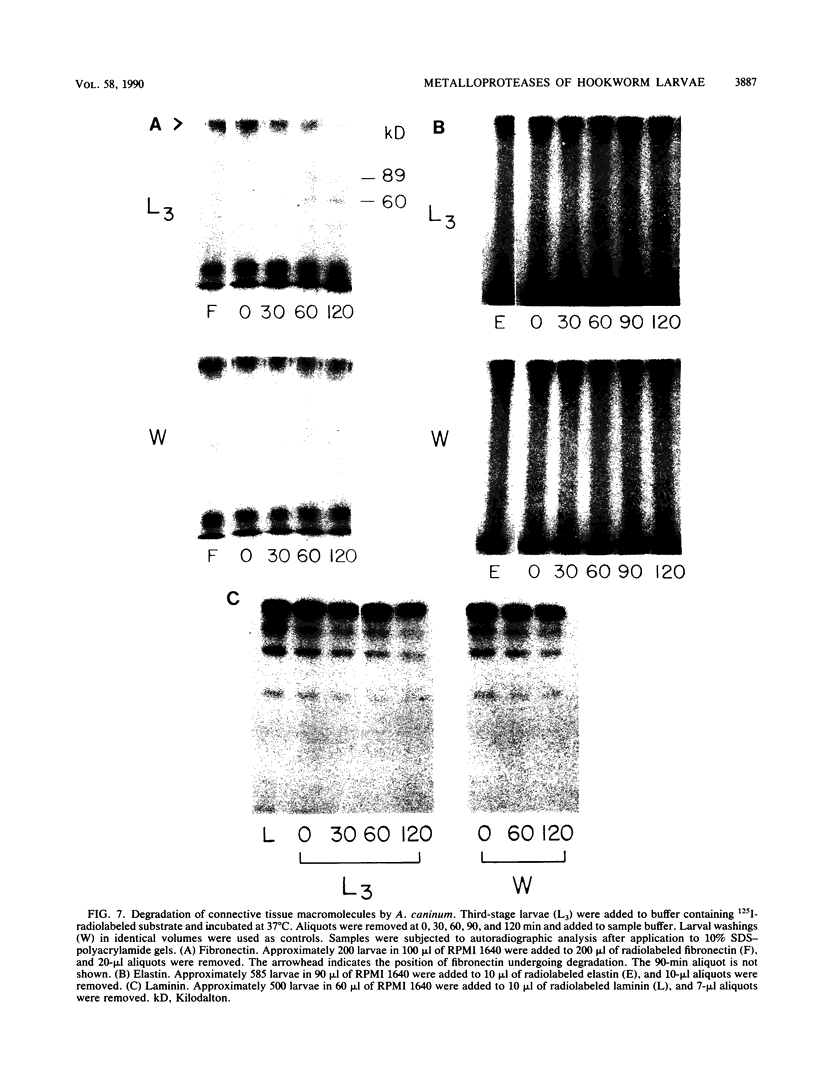
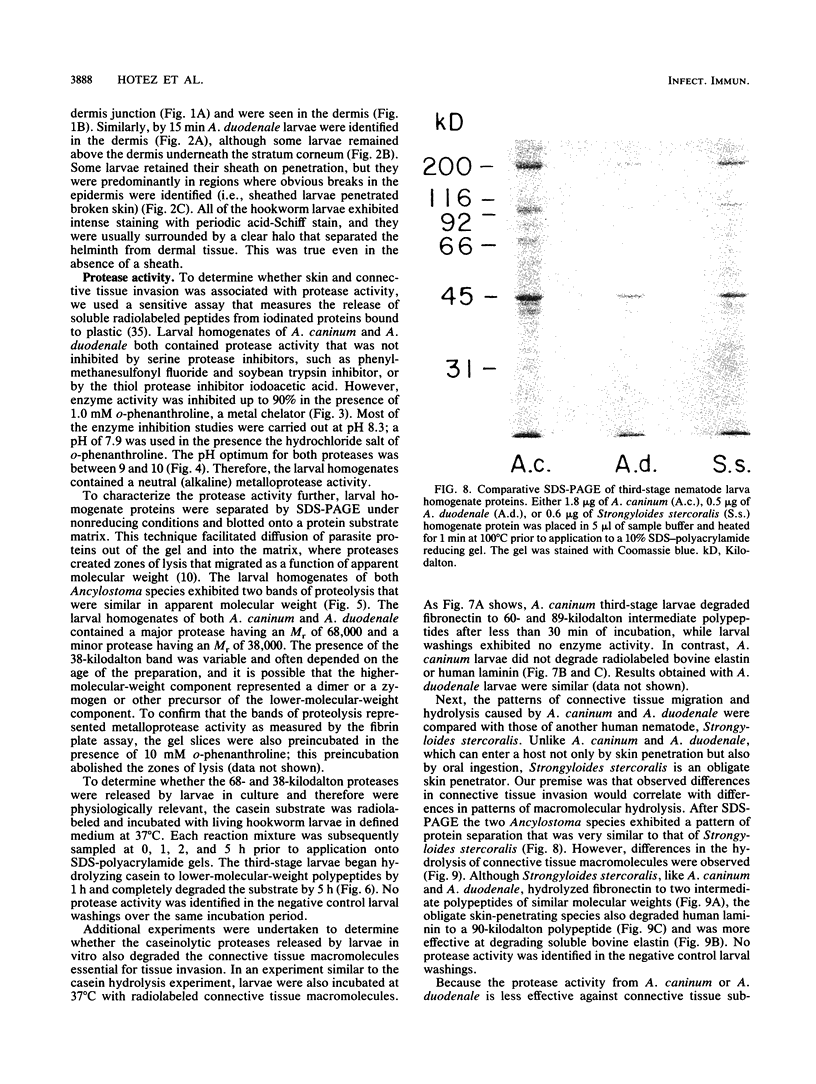
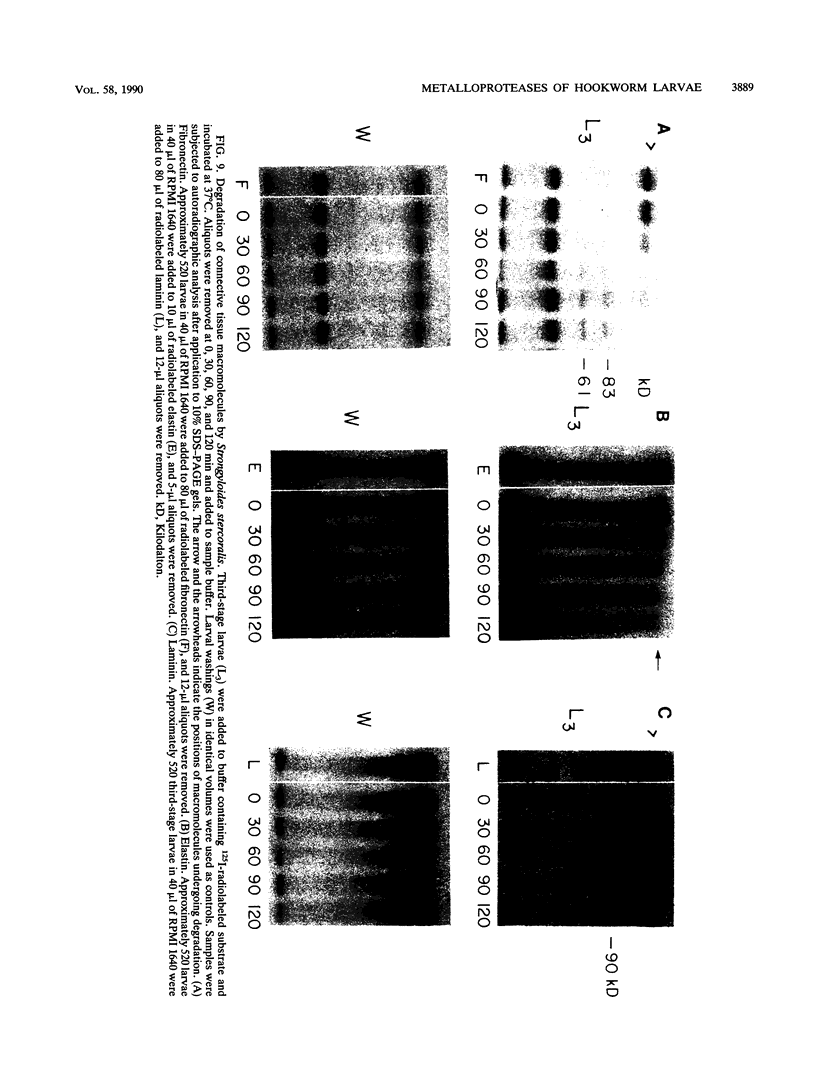
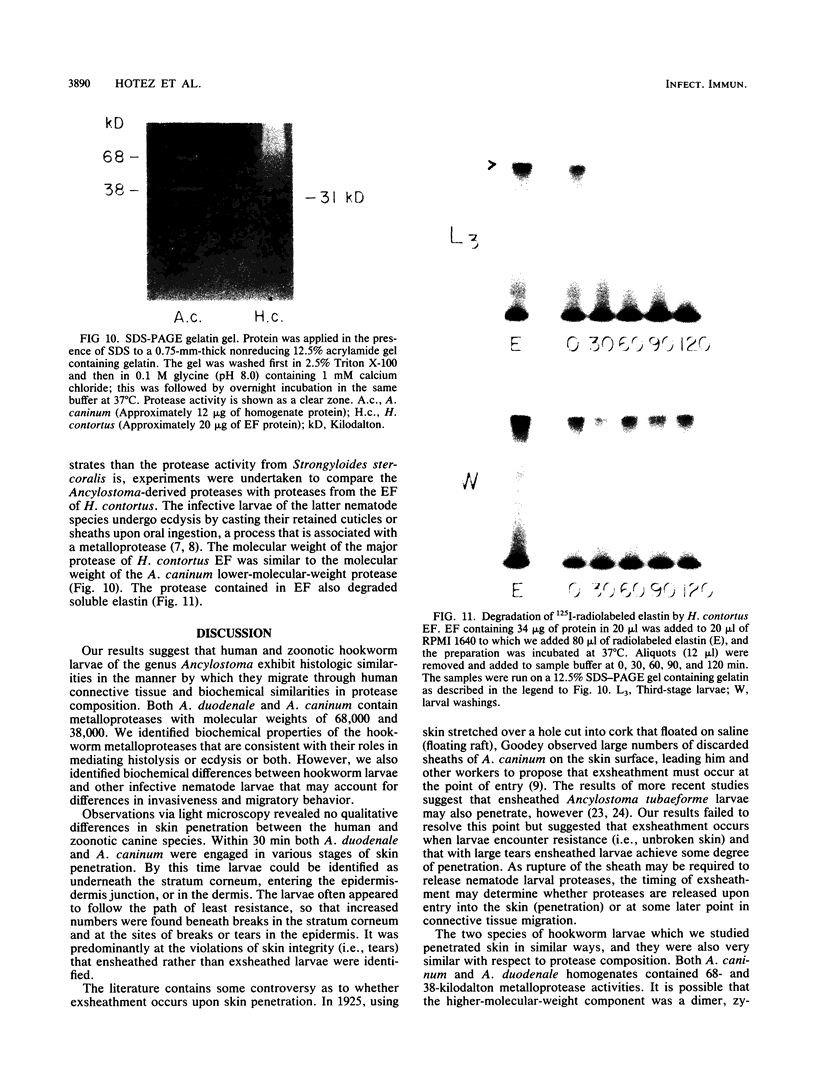
To infect their hosts, hookworm larvae must exsheath and migrate through connective tissue. A modified in vitro skin chamber was used to show that the human hookworm Ancylostoma duodenale and the zoonotic canine hookworm Ancylostoma caninum penetrate epidermis, basement membrane, and dermis in similar ways. These similarities in tissue invasion properties reflect the observed biochemical similarities in parasite protease composition. The larvae of both species contain protease activity that is inhibited by o-phenanthroline; this identifies the proteases as metalloproteases. The enzyme activities exhibit an alkaline pH optimum between pH 9 and 10. During modified sodium dodecyl sulfate-polyacrylamide gel electrophoresis in which a protein substrate (either casein or gelatin) was used, the protease activities resolved into a major band at an Mr of 68,000 and a minor band at an Mr of 38,000. Proteases were released by living A. caninum larvae in vitro and degraded purified and radiolabeled casein to smaller peptides. Motile hookworm larvae were also incubated with purified and radiolabeled connective tissue macromolecules in vitro. Both Ancylostoma species degraded human fibronectin to a 60,000-Mr polypeptide intermediate, but could not degrade solubilized bovine elastin or human laminin. In contrast, the obligate skin-penetrating nematode Strongyloides stercoralis degraded all three substrates. This biochemical difference may explain some observed differences in invasiveness.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEAVER P. C. Larva migrans. Exp Parasitol. 1956 Nov;5(6):587–621. doi: 10.1016/0014-4894(56)90032-7. [DOI] [PubMed] [Google Scholar]
- Beaver P. C. The nature of visceral larva migrans. J Parasitol. 1969 Feb;55(1):3–12. [PubMed] [Google Scholar]
- Croese T. J. Eosinophilic enteritis--a recent north Queensland experience. Aust N Z J Med. 1988 Dec;18(7):848–853. doi: 10.1111/j.1445-5994.1988.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Dresden M. H., Rege A. A., Murrell K. D. Strongyloides ransomi: proteolytic enzymes from larvae. Exp Parasitol. 1985 Apr;59(2):257–263. doi: 10.1016/0014-4894(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Franz T. J. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol. 1975 Mar;64(3):190–195. doi: 10.1111/1523-1747.ep12533356. [DOI] [PubMed] [Google Scholar]
- Gamble H. R., Lichtenfels J. R., Purcell J. P. Light and scanning electron microscopy of the ecdysis of Haemonchus contortus infective larvae. J Parasitol. 1989 Apr;75(2):303–307. [PubMed] [Google Scholar]
- Gamble H. R., Purcell J. P., Fetterer R. H. Purification of a 44 kilodalton protease which mediates the ecdysis of infective Haemonchus contortus larvae. Mol Biochem Parasitol. 1989 Feb;33(1):49–58. doi: 10.1016/0166-6851(89)90041-8. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawdon J. M., Schad G. A. Serum-stimulated feeding in vitro by third-stage infective larvae of the canine hookworm Ancylostoma caninum. J Parasitol. 1990 Jun;76(3):394–398. [PubMed] [Google Scholar]
- Hotez P. J., Cerami A. Secretion of a proteolytic anticoagulant by Ancylostoma hookworms. J Exp Med. 1983 May 1;157(5):1594–1603. doi: 10.1084/jem.157.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P. J. Hookworm disease in children. Pediatr Infect Dis J. 1989 Aug;8(8):516–520. doi: 10.1097/00006454-198908000-00009. [DOI] [PubMed] [Google Scholar]
- Hotez P. J., Trang N. L., McKerrow J. H., Cerami A. Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum. J Biol Chem. 1985 Jun 25;260(12):7343–7348. [PubMed] [Google Scholar]
- KALMON E. H. Creeping eruption associated with transient pulmonary infiltrations. Radiology. 1954 Feb;62(2):222–226. doi: 10.1148/62.2.222. [DOI] [PubMed] [Google Scholar]
- LEE C. L., LEWERT R. M. Quantitative studies of the collagenase-like enzymes of cercariae of Schistosoma mansoni and the larvae of Strongyloides ratti. J Infect Dis. 1956 Jul-Aug;99(1):1–14. doi: 10.1093/infdis/99.1.1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leiby D. A., el Naggar H. M., Schad G. A. Thirty generations of Ancylostoma duodenale in laboratory-reared beagles. J Parasitol. 1987 Aug;73(4):844–848. [PubMed] [Google Scholar]
- Lepage T., Gache C. Purification and characterization of the sea urchin embryo hatching enzyme. J Biol Chem. 1989 Mar 25;264(9):4787–4793. [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Little M. D., Halsey N. A., Cline B. L., Katz S. P. Ancylostoma larva in a muscle fiber of man following cutaneous larva migrans. Am J Trop Med Hyg. 1983 Nov;32(6):1285–1288. doi: 10.4269/ajtmh.1983.32.1285. [DOI] [PubMed] [Google Scholar]
- MUHLEISEN J. P. Demonstration of pulmonary migration of the causative organism of creeping eruption. Ann Intern Med. 1953 Mar;38(3):595–600. doi: 10.7326/0003-4819-38-3-595. [DOI] [PubMed] [Google Scholar]
- Matthews B. E. Invasion of skin by larvae of the cat hookworm, Ancylostoma tubaeforme. Parasitology. 1972 Dec;65(3):457–467. doi: 10.1017/s0031182000044085. [DOI] [PubMed] [Google Scholar]
- Matthews B. E. Mechanism of skin penetration by Ancylostoma tubaeforme larvae. Parasitology. 1975 Feb;70(1):25–38. doi: 10.1017/s0031182000048836. [DOI] [PubMed] [Google Scholar]
- Matthews B. E. Skin penetration by Necator americanus larvae. Z Parasitenkd. 1982;68(1):81–86. doi: 10.1007/BF00926660. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H., Brindley P., Brown M., Gam A. A., Staunton C., Neva F. A. Strongyloides stercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol. 1990 Feb;70(2):134–143. doi: 10.1016/0014-4894(90)90094-s. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H. Parasite proteases. Exp Parasitol. 1989 Jan;68(1):111–115. doi: 10.1016/0014-4894(89)90016-7. [DOI] [PubMed] [Google Scholar]
- McKerrow J. H., Pino-Heiss S., Lindquist R., Werb Z. Purification and characterization of an elastinolytic proteinase secreted by cercariae of Schistosoma mansoni. J Biol Chem. 1985 Mar 25;260(6):3703–3707. [PubMed] [Google Scholar]
- Miller T. A. Hookworm infection in man. Adv Parasitol. 1979;17:315–384. doi: 10.1016/s0065-308x(08)60552-7. [DOI] [PubMed] [Google Scholar]
- Molla A., Tanase S., Hong Y. M., Maeda H. Interdomain cleavage of plasma fibronectin by zinc-metalloproteinase from Serratia marcescens. Biochim Biophys Acta. 1988 Jun 29;955(1):77–85. doi: 10.1016/0167-4838(88)90181-1. [DOI] [PubMed] [Google Scholar]
- Norris D. E. The migratory behavior of the infective-stage larvae of Ancylostoma braziliense and Ancylostoma tubaeforme in rodent paratenic hosts. J Parasitol. 1971 Oct;57(5):998–1009. [PubMed] [Google Scholar]
- Ossowski L., Unkeless J. C., Tobia A., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. II. Mammalian fibroblast cultures transformed by DNA and RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):112–126. doi: 10.1084/jem.137.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad G. A. Ancylostoma duodenale: maintenance through six generations in helminth-native pups. Exp Parasitol. 1979 Apr;47(2):246–253. doi: 10.1016/0014-4894(79)90077-8. [DOI] [PubMed] [Google Scholar]
- Schad G. A., Hellman M. E., Muncey D. W. Strongyloides stercoralis: hyperinfection in immunosuppressed dogs. Exp Parasitol. 1984 Jun;57(3):287–296. doi: 10.1016/0014-4894(84)90103-6. [DOI] [PubMed] [Google Scholar]