Abstract
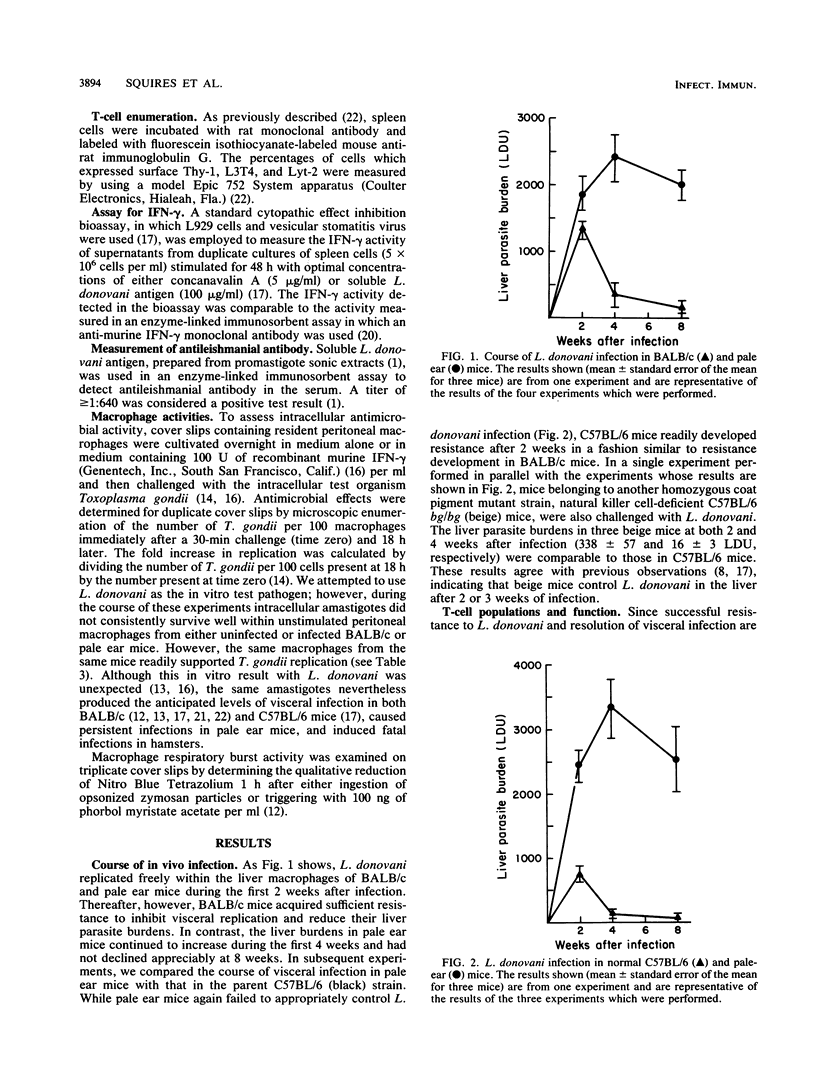
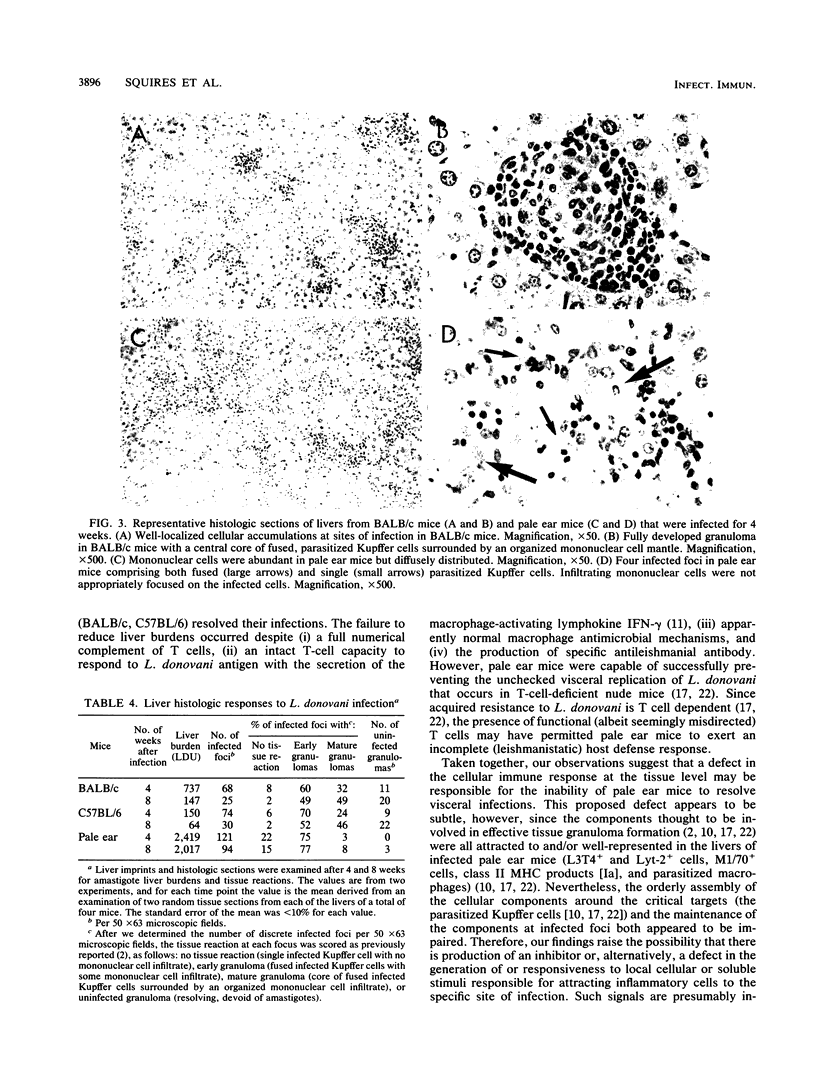
In BALB/c mice, successful defense against visceral leishmaniasis is T cell dependent, expressed by tissue granuloma formation, and probably mediated by macrophages activated by cytokines, including gamma interferon (IFN-gamma). C57BL/6 ep/ep (pale ear) mice, which reportedly exhibit impaired IFN-gamma production, were challenged with Leishmania donovani to determine the outcome of infection in a euthymic host with an apparent defect in lymphokine secretion. In BALB/c and normal C57BL/6 mice, L. donovani liver burdens peaked at 2 weeks and were largely eliminated by 4 weeks. In contrast, in pale ear mice, infection progressed until after 4 weeks and persisted at high levels at 8 weeks. The failure to resolve hepatic infections was not related to deficiencies in (i) Thy-1+, L3T4+, or Lyt-2+ T cells; (ii) IFN-gamma secretion; (iii) liver tissue Ia expression; (iv) macrophage antimicrobial capacity; or (v) antileishmanial antibody production. However, despite the anticipated influx of mononuclear cells into livers, these cells were not properly focused on the parasitized Kupffer cells, the inflammatory infiltrate receded prematurely, and mature granulomas failed to develop. These results suggest that there is a cellular immune defect at the tissue level and emphasize the critical role of granuloma formation in successful resolution of systemic intracellular infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta A. M., Sadigursky M., Santos-Buch C. A. Anti-striated muscle antibody activity produced by Trypanosoma cruzi. Proc Soc Exp Biol Med. 1983 Mar;172(3):364–369. doi: 10.3181/00379727-172-41571. [DOI] [PubMed] [Google Scholar]
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Feng Z. Y., Louis J., Kindler V., Pedrazzini T., Eliason J. F., Behin R., Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol. 1988 Aug;18(8):1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- Hoover D. L., Nacy C. A., Meltzer M. S. Human monocyte activation for cytotoxicity against intracellular Leishmania donovani amastigotes: induction of microbicidal activity by interferon-gamma. Cell Immunol. 1985 Sep;94(2):500–511. doi: 10.1016/0008-8749(85)90274-6. [DOI] [PubMed] [Google Scholar]
- Kelly C. D., Russo C. M., Rubin B. Y., Murray H. W. Antigen-stimulated human interferon-gamma generation: role of accessory cells and their expressed or secreted products. Clin Exp Immunol. 1989 Sep;77(3):397–402. [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. E., Farrell J. P. Leishmaniasis in beige mice. Infect Immun. 1982 Dec;38(3):1208–1216. doi: 10.1128/iai.38.3.1208-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- McElrath M. J., Murray H. W., Cohn Z. A. The dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988 Jun 1;167(6):1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Carriero S. M., Donelly D. M. Presence of a macrophage-mediated suppressor cell mechanism during cell-mediated immune response in experimental visceral leishmaniasis. Infect Immun. 1986 Nov;54(2):487–493. doi: 10.1128/iai.54.2.487-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Squires K. E., Schreiber R. D., McElrath M. J., Rubin B. Y., Anderson S. L., Murray H. W. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defense and tissue granulomatous response. J Immunol. 1989 Dec 15;143(12):4244–4249. [PubMed] [Google Scholar]
- Stern J. J., Oca M. J., Rubin B. Y., Anderson S. L., Murray H. W. Role of L3T4+ and LyT-2+ cells in experimental visceral leishmaniasis. J Immunol. 1988 Jun 1;140(11):3971–3977. [PubMed] [Google Scholar]
- Titus R. G., Sherry B., Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–2104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser W. Y., Van Niel A., Clark S. C., David J. R., Remold H. G. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. doi: 10.1084/jem.166.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Beller D. I., Sypek J. P. Macrophage activation for antileishmanial defense by an apparently novel mechanism. J Immunol. 1987 Feb 15;138(4):1246–1249. [PubMed] [Google Scholar]



