Abstract
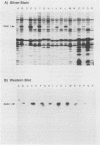
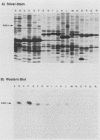
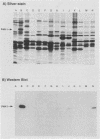
The Pan 1 protein of Neisseria gonorrhoeae is a novel 54-kDa outer membrane protein expressed only when gonococci are grown in the absence of oxygen. It is a major antigen recognized by sera from patients with gonococcal infection. We raised mouse monospecific polyclonal antiserum to gel-purified Pan 1 from gonococcal strain F62. The antiserum was broadly cross-reactive among gonococcal strains; all strains tested reacted in immunoblot analysis proportionate to the amount of Pan 1 visible in silver-stained sodium dodecyl sulfate (SDS)-polyacrylamide gels. In immunoblot experiments, N. lactamica and N. cinerea reacted very strongly to the anti-Pan 1 antiserum, whereas N. sicca, N. flava, and N. mucosa did not react at all. The other commensals tested, N. subflava and N. perflava, exhibited only a minor reaction. These results correlated with the apparent amount of Pan 1 seen on SDS-polyacrylamide gels of outer membranes. SDS-polyacrylamide gel analysis of six meningococcal strains revealed no visible anaerobically induced outer membrane proteins, and the subsequent immunoblots showed only slight or no reaction to the anti-Pan 1 antibody. In the four meningococcal strains that did react slightly with the antiserum, a Pan 1-like protein was seen only in anaerobically grown cells. Thus, meningococci did not express Pan 1 at levels comparable to that found in gonococci; however, when Pan 1 was expressed in meningococcal strains, it was oxygen regulated. This is the first example of a protein found in the gonococcal outer membrane that, under identical growth conditions, is not expressed at similar levels in the meningococcus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow A. K., Heckels J. E., Clarke I. N. The class 1 outer membrane protein of Neisseria meningitidis: gene sequence and structural and immunological similarities to gonococcal porins. Mol Microbiol. 1989 Feb;3(2):131–139. doi: 10.1111/j.1365-2958.1989.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Barritt D. S., Schwalbe R. S., Klapper D. G., Cannon J. G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987 Sep;55(9):2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Cannon J. G. Cloning of the gene for the common pathogenic Neisseria H.8 antigen from Neisseria gonorrhoeae. Infect Immun. 1985 Jan;47(1):322–325. doi: 10.1128/iai.47.1.322-325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Wetzler L. M., Gotschlich E. C., Rice P. A. Protein III: structure, function, and genetics. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S60–S63. doi: 10.1128/cmr.2.suppl.s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S1–S4. doi: 10.1128/cmr.2.suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonetti N. H., Sparling P. F. Molecular cloning and characterization of the structural gene for protein I, the major outer membrane protein of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9084–9088. doi: 10.1073/pnas.84.24.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987 Jun;55(6):1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Knapp J. S., Thompson S., Klimpel K. W. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb Pathog. 1988 Nov;5(5):381–390. doi: 10.1016/0882-4010(88)90038-1. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Blake M. S., Koomey J. M., Seiff M., Derman A. Cloning of the structural genes of three H8 antigens and of protein III of Neisseria gonorrhoeae. J Exp Med. 1986 Sep 1;164(3):868–881. doi: 10.1084/jem.164.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M. E., Blake M. S., Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8135–8139. doi: 10.1073/pnas.84.22.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. Protein I: structure, function, and genetics. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S41–S48. doi: 10.1128/cmr.2.suppl.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawula T. H., Aho E. L., Barritt D. S., Klapper D. G., Cannon J. G. Reversible phase variation of expression of Neisseria meningitidis class 5 outer membrane proteins and their relationship to gonococcal proteins II. Infect Immun. 1988 Feb;56(2):380–386. doi: 10.1128/iai.56.2.380-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Deoxyribonucleic acid homologies among species of the genus Neisseria. J Bacteriol. 1967 Oct;94(4):870–874. doi: 10.1128/jb.94.4.870-874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Clark V. L. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect Immun. 1984 Oct;46(1):176–181. doi: 10.1128/iai.46.1.176-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Falkow S. Nucleotide sequence homology between the immunoglobulin A1 protease genes of Neisseria gonorrhoeae, Neisseria meningitidis, and Haemophilus influenzae. Infect Immun. 1984 Jan;43(1):101–107. doi: 10.1128/iai.43.1.101-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Billyard E., Haas R., Storzbach S., So M. Pilus genes of Neisseria gonorrheae: chromosomal organization and DNA sequence. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzner T. A., Barnes R. C., JeanLouis Y. A., Shafer W. M., Morse S. A. Distribution of an antigenically related iron-regulated protein among the Neisseria spp. Infect Immun. 1986 Jan;51(1):60–68. doi: 10.1128/iai.51.1.60-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall WJ5th, Mail L. B., Wilde C. E., 3rd, Jones R. B. Purification and antigenic relatedness of proteins II of Neisseria gonorrhoeae. Infect Immun. 1985 Sep;49(3):576–580. doi: 10.1128/iai.49.3.576-580.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts W. J., Saunders J. R. Nucleotide sequence of the structural gene for class I pilin from Neisseria meningitidis: homologies with the pilE locus of Neisseria gonorrhoeae. Mol Microbiol. 1988 Sep;2(5):647–653. doi: 10.1111/j.1365-2958.1988.tb00073.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Clark V. L. Genetic loci and linkage associations in Neisseria gonorrhoeae and Neisseria meningitidis. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S92–103. doi: 10.1128/cmr.2.suppl.s92. [DOI] [PMC free article] [PubMed] [Google Scholar]