Abstract
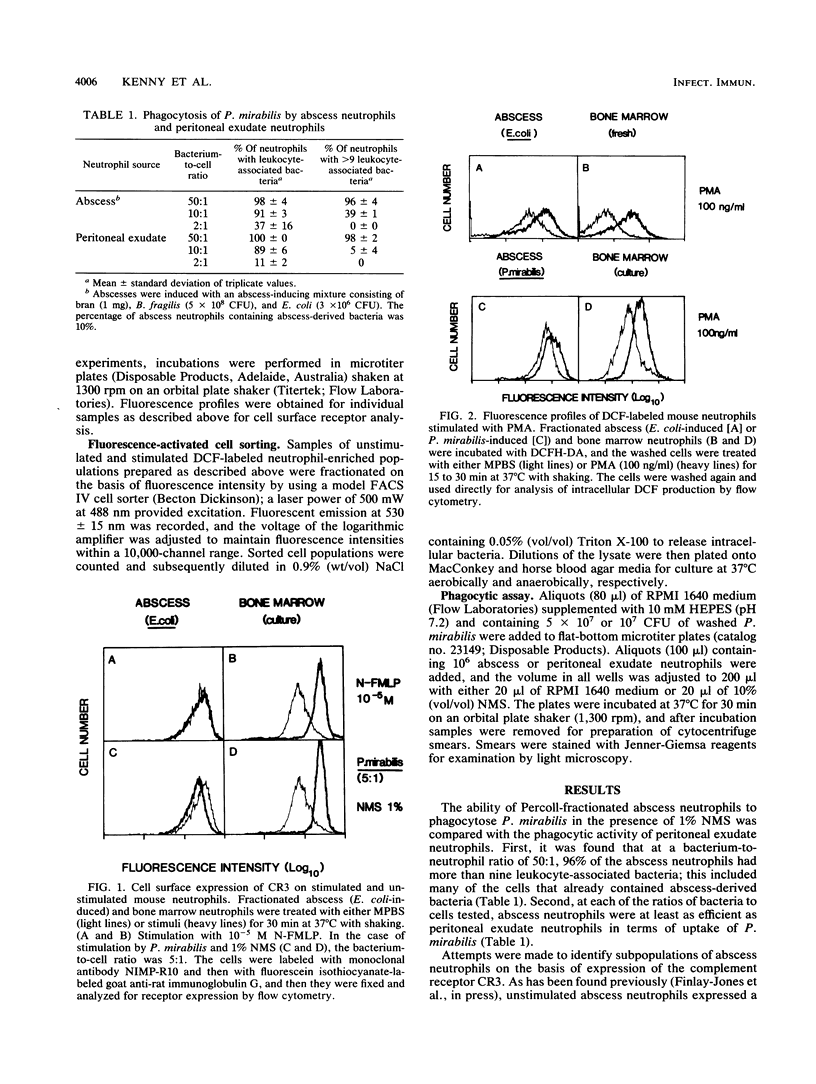
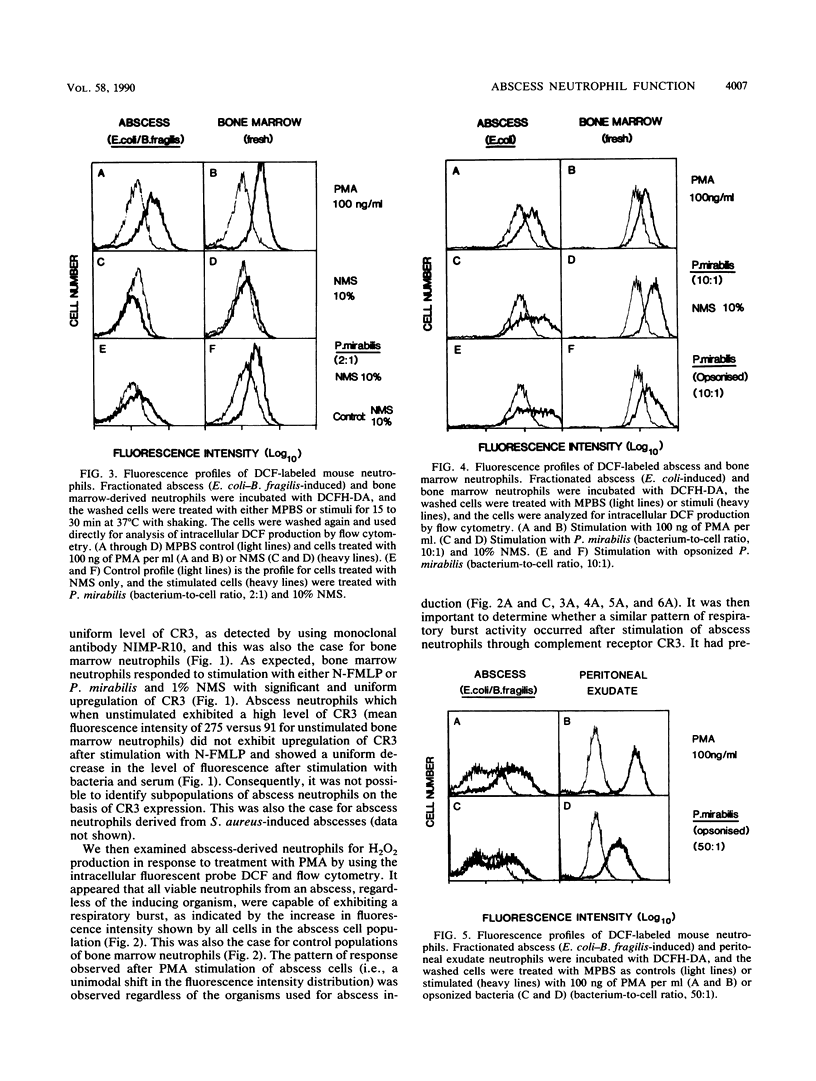
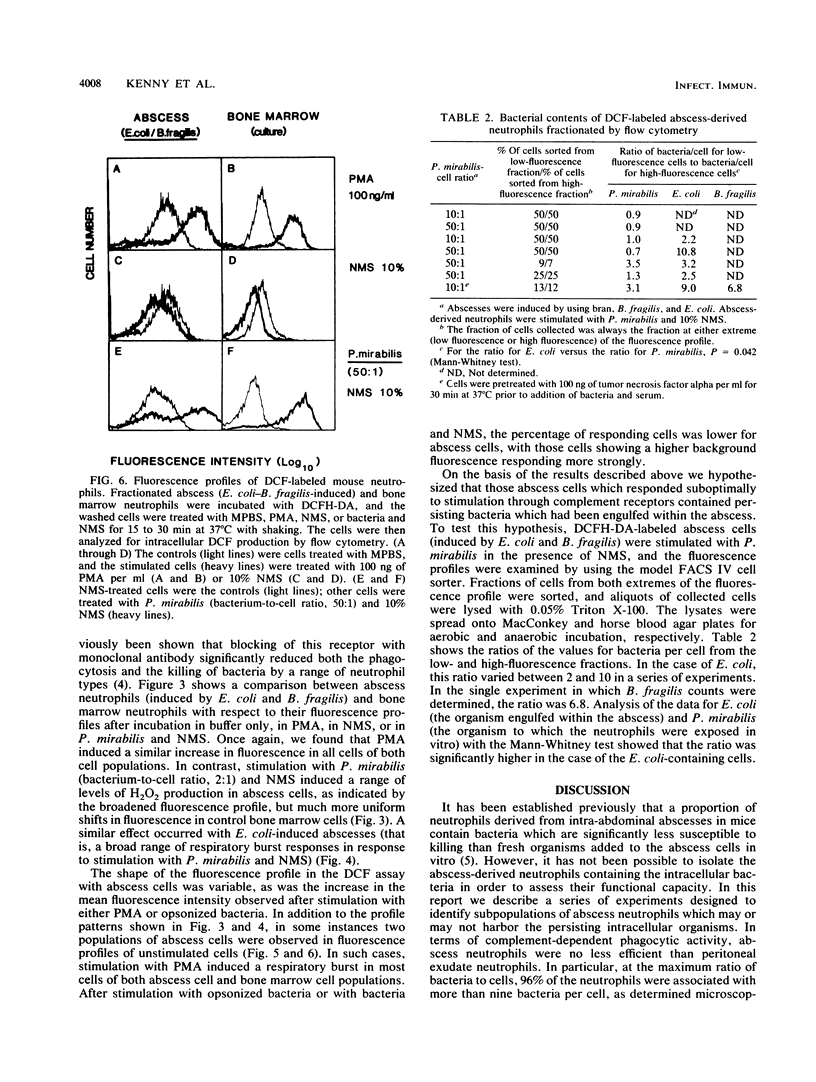
In the absence of antibiotic therapy, viable bacteria can persist within intra-abdominal abscesses in mice for at least 10 weeks. The mechanisms contributing to this survival are unknown, but abscess-derived neutrophils have impaired abilities to kill, in vitro, organisms engulfed in vivo. In order to determine whether subpopulations of abscess neutrophils might be discernible on the basis of phenotypic or functional criteria, cells from murine intra-abdominal abscesses were examined for phagocytic activity, CR3 expression, and H2O2 production in response to soluble and particulate stimuli. With respect to phagocytosis of Proteus mirabilis, abscess cells were no less efficient than peritoneal exudate neutrophils; no significant subpopulation of cells was incapable of phagocytosis in the presence of normal mouse serum. Using flow cytometry to examine abscess neutrophils for CR3 expression, we found that no subpopulations of cells were observed with unstimulated cells or with cells incubated with either phorbol 12-myristate 13-acetate or bacteria and serum. Intracellular H2O2 levels were measured by using the probe 2',7'-dichlorofluorescin diacetate. In general, incubation with phorbol 12-myristate 13-acetate resulted in similar increases in H2O2 production in all cells of the population. However, stimulation with bacteria and serum revealed a variable but consistent, poorly responsive subpopulation of neutrophils in abscess cell populations. Cell-sorting experiments showed that cells from the poorly responsive section of the FACS profile contained significantly higher numbers of abscess-derived bacteria, suggesting the presence of a subpopulation of viable abscess neutrophils harboring persisting viable bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Kenny P. A., Lopez A. F., McDonald P. J., Finlay-Jones J. J. Functional neutrophils from long-term murine bone marrow cell cultures. J Immunol Methods. 1987 Jun 26;100(1-2):223–233. doi: 10.1016/0022-1759(87)90193-1. [DOI] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nikoloutsopoulos A., Lopez A. F., Vadas M. A., McDonald P. J., Finlay-Jones J. J. Role of cell surface receptors in the regulation of intracellular killing of bacteria by murine peritoneal exudate neutrophils. Infect Immun. 1986 Apr;52(1):245–251. doi: 10.1128/iai.52.1.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Spencer L. K., Nulsen M. F., McDonald P. J., Finlay-Jones J. J. Neutrophil activity in abscess-bearing mice: comparative studies with neutrophils isolated from peripheral blood, elicited peritoneal exudates, and abscesses. Infect Immun. 1986 Mar;51(3):936–941. doi: 10.1128/iai.51.3.936-941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Strath M., Sanderson C. J. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984 Jul;57(3):489–494. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Seligmann B., Chused T. M., Gallin J. I. Human neutrophil heterogeneity identified using flow microfluorometry to monitor membrane potential. J Clin Invest. 1981 Nov;68(5):1125–1131. doi: 10.1172/JCI110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. W., Finlay-Jones J. J. Studies on a fractionated murine fibrosarcoma: a reproducible method for the cautious and a caution for the unwary. J Cell Physiol. 1977 Mar;90(3):535–552. doi: 10.1002/jcp.1040900316. [DOI] [PubMed] [Google Scholar]
- Valet G., Raffael A., Moroder L., Wünsch E., Ruhenstroth-Bauer G. Fast intracellular pH determination in single cells by flow-cytometry. Naturwissenschaften. 1981 May;68(5):265–266. doi: 10.1007/BF01047331. [DOI] [PubMed] [Google Scholar]
- Valet G., Raffael A., Rüssmann L. Determination of intracellular calcium in vital cells by flow-cytometry. Naturwissenschaften. 1985 Nov;72(11):600–602. doi: 10.1007/BF00365284. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Meyer B. C. Phorbol esters cause sequential activation and deactivation of complement receptors on polymorphonuclear leukocytes. J Immunol. 1986 Mar 1;136(5):1759–1764. [PubMed] [Google Scholar]


