Abstract
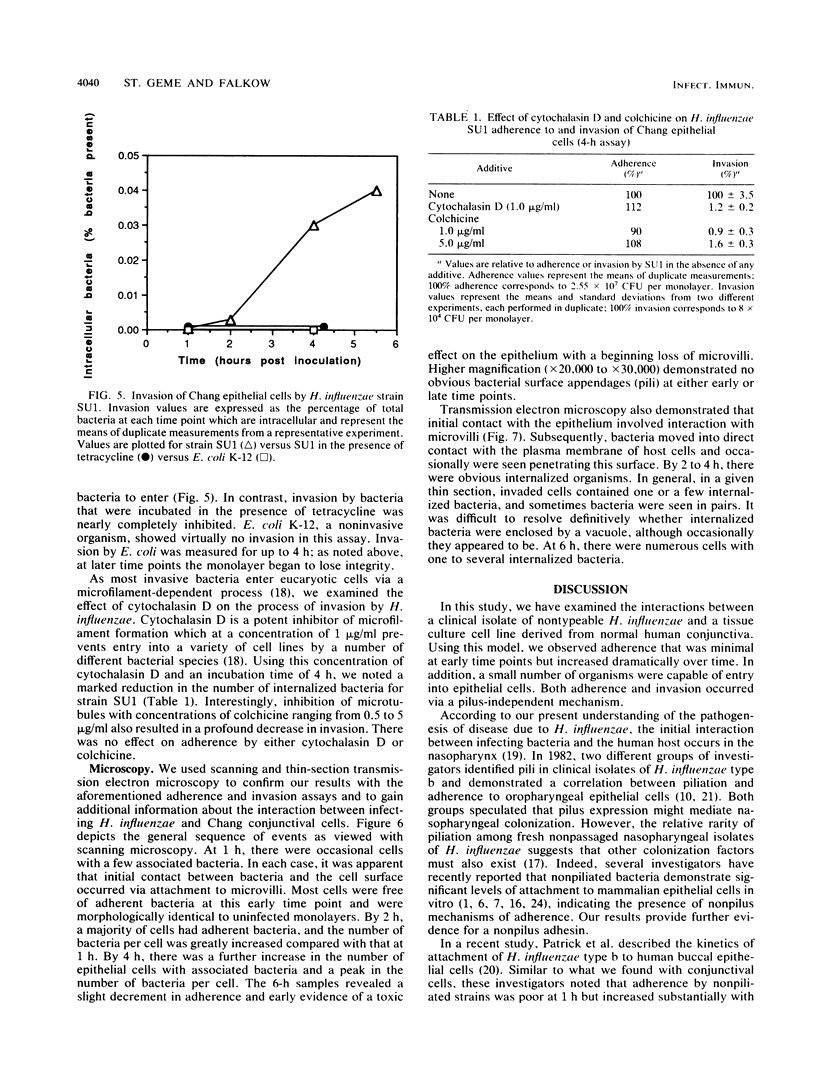
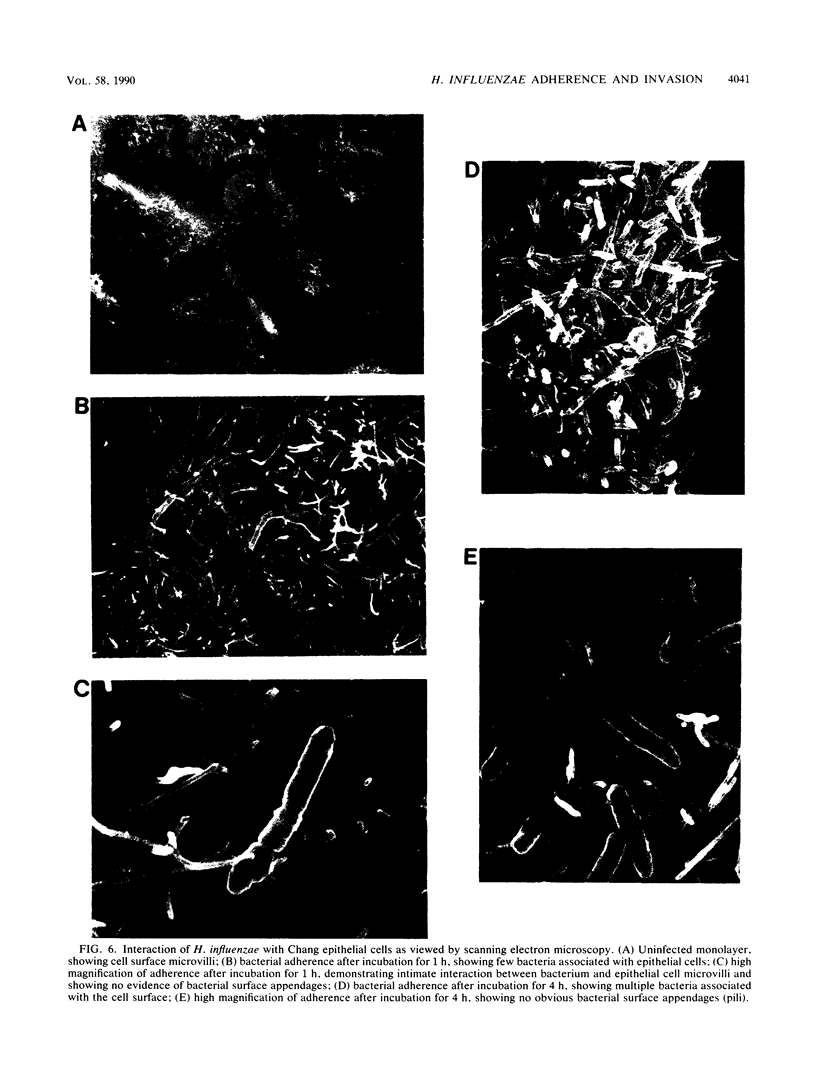
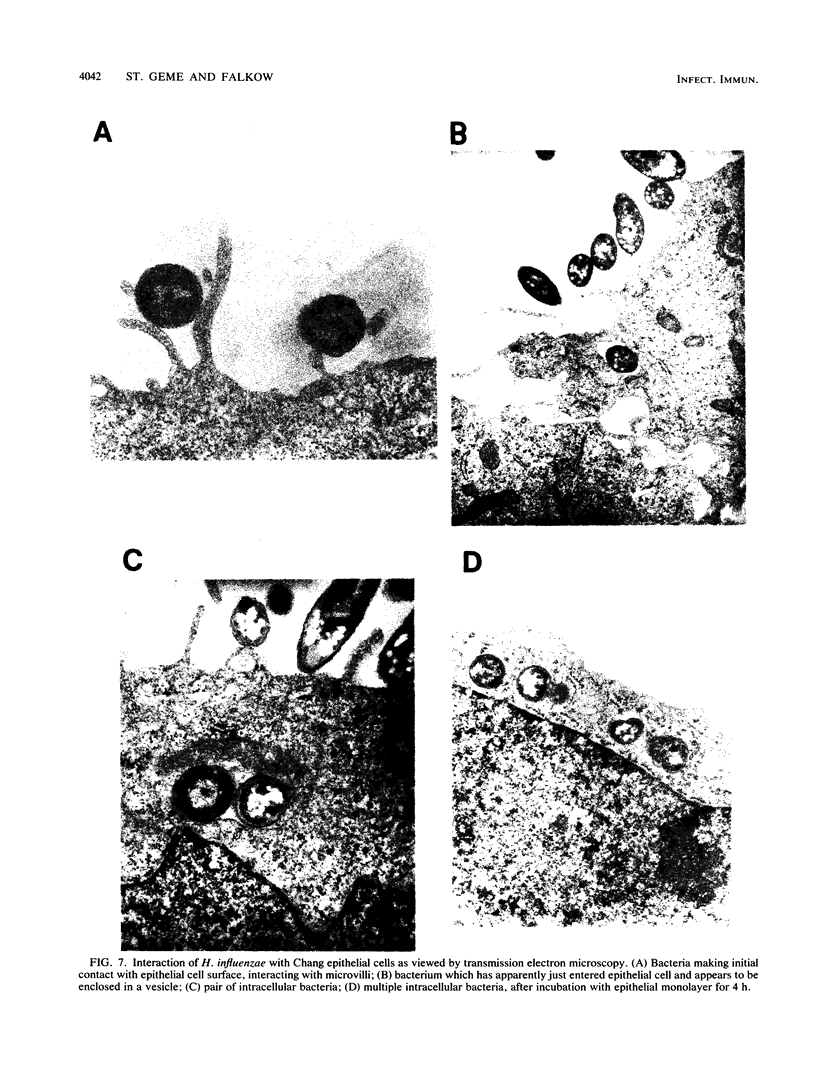
Haemophilus influenzae is a common commensal organism of the human respiratory tract that initiates infection by colonizing the nasopharyngeal epithelium. In some individuals, colonization is followed by localized respiratory tract or systemic disease. To gain insight into the mechanisms by which H. influenzae attaches to and persists within the nasopharynx, we examined the interactions between a nonpiliated clinical isolate of H. influenzae and human epithelial cells. We noted substantial adherence that occurred independently of pili and required viable bacteria capable of de novo protein synthesis. Comparison of profiles of outer membrane proteins synthesized during incubation with epithelial cells for adherent and nonadherent bacteria identified several candidate adhesin molecules. In addition, a small number of adherent bacteria were capable of entering epithelial cells in a process that was inhibited by cytochalasin D and colchicine. The suggestion from our studies is that one or more of several newly synthesized nonpilus bacterial proteins are required for maximal in vitro adherence and invasion. We speculate that H. influenzae entry into epithelial cells may provide a mechanism for evasion of host defenses, thereby allowing persistence in the nasopharynx.
Full text
PDF



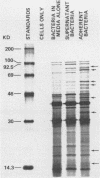
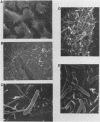
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakaletz L. O., Tallan B. M., Hoepf T., DeMaria T. F., Birck H. G., Lim D. J. Frequency of fimbriation of nontypable Haemophilus influenzae and its ability to adhere to chinchilla and human respiratory epithelium. Infect Immun. 1988 Feb;56(2):331–335. doi: 10.1128/iai.56.2.331-335.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG R. S. M. Continuous subcultivation of epithelial-like cells from normal human tissues. Proc Soc Exp Biol Med. 1954 Nov;87(2):440–443. doi: 10.3181/00379727-87-21406. [DOI] [PubMed] [Google Scholar]
- Carlone G. M., Thomas M. L., Rumschlag H. S., Sottnek F. O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986 Sep;24(3):330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor E. M., Loeb M. R. A hemadsorption method for detection of colonies of Haemophilus influenzae type b expressing fimbriae. J Infect Dis. 1983 Nov;148(5):855–860. doi: 10.1093/infdis/148.5.855. [DOI] [PubMed] [Google Scholar]
- Donnenberg M. S., Donohue-Rolfe A., Keusch G. T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- Farley M. M., Stephens D. S., Kaplan S. L., Mason E. O., Jr Pilus- and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990 Feb;161(2):274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- Farley M. M., Stephens D. S., Mulks M. H., Cooper M. D., Bricker J. V., Mirra S. S., Wright A. Pathogenesis of IgA1 protease-producing and -nonproducing Haemophilus influenzae in human nasopharyngeal organ cultures. J Infect Dis. 1986 Nov;154(5):752–759. doi: 10.1093/infdis/154.5.752. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Langermann S., Clegg H. W., Kessler T. W., Goldman D. A., Gilsdorf J. R. Adherence of piliated Haemophilus influenzae type b to human oropharyngeal cells. J Infect Dis. 1982 Oct;146(4):564–564. doi: 10.1093/infdis/146.4.564. [DOI] [PubMed] [Google Scholar]
- HERS J. F., MULDER J. The mucosal epithelium of the respiratory tract in muco-purulent bronchitis caused by Haemophilus influenzae. J Pathol Bacteriol. 1953 Jul;66(1):103–108. doi: 10.1002/path.1700660114. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Andåker L. Inability of gentamicin and fosfomycin to eliminate intracellular Enterobacteriaceae. J Antimicrob Chemother. 1985 Jun;15(6):723–728. doi: 10.1093/jac/15.6.723. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Connor E., Penney D. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988 Feb;56(2):484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason E. O., Jr, Kaplan S. L., Wiedermann B. L., Norrod E. P., Stenback W. A. Frequency and properties of naturally occurring adherent piliated strains of Haemophilus influenzae type b. Infect Immun. 1985 Jul;49(1):98–103. doi: 10.1128/iai.49.1.98-103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C. C., Patrick G. S., Kaplan S. L., Barrish J., Mason E. O., Jr Adherence kinetics of Haemophilus influenzae type b to eucaryotic cells. Pediatr Res. 1989 Nov;26(5):500–503. doi: 10.1203/00006450-198911000-00028. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Richardson W. P., Sadoff J. C. Induced engulfment of Neisseria gonorrhoeae by tissue culture cells. Infect Immun. 1988 Sep;56(9):2512–2514. doi: 10.1128/iai.56.9.2512-2514.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Jacobs R. F., Haas J. E., Smith A. L. Adherence of Haemophilus influenzae to monkey respiratory tissue in organ culture. J Gen Microbiol. 1984 Jun;130(6):1437–1447. doi: 10.1099/00221287-130-6-1437. [DOI] [PubMed] [Google Scholar]
- Sable N. S., Connor E. M., Hall C. B., Loeb M. R. Variable adherence of fimbriated Haemophilus influenzae type b to human cells. Infect Immun. 1985 Apr;48(1):119–123. doi: 10.1128/iai.48.1.119-123.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter H. B., Rees D. A. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987 Jul;105(1):507–515. doi: 10.1083/jcb.105.1.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984 Aug;18(1):1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]