Abstract
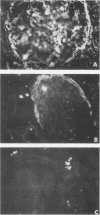
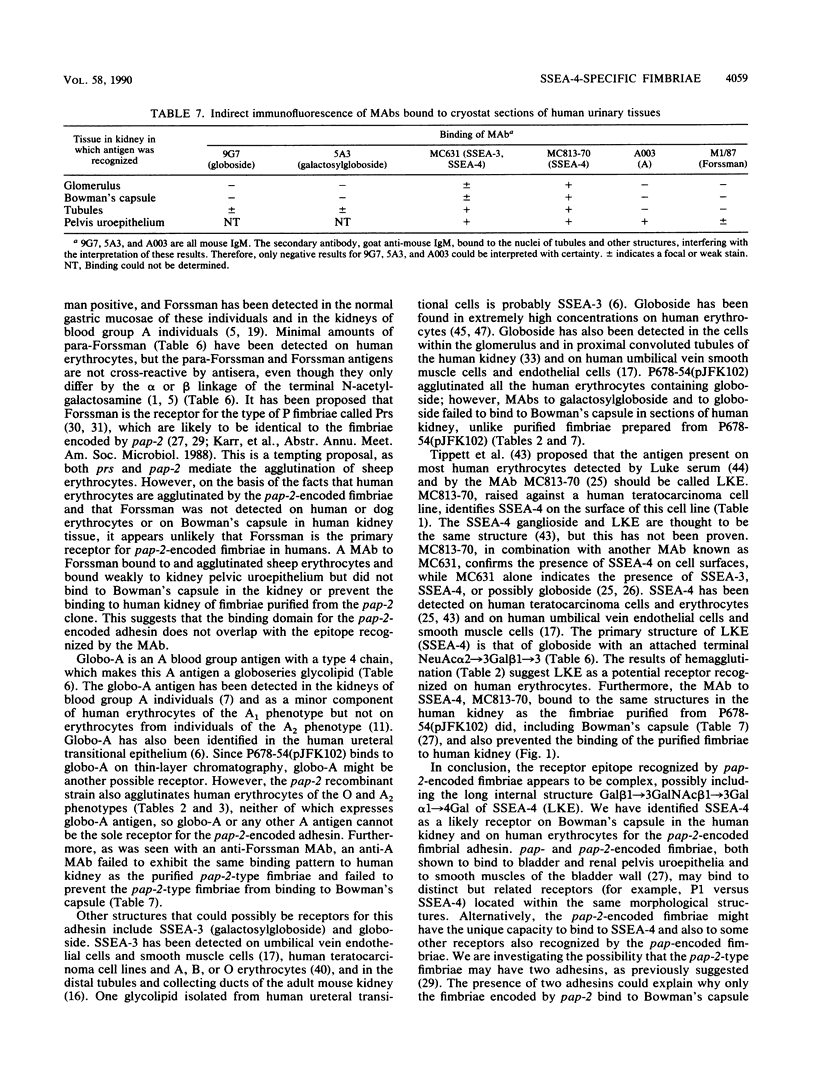
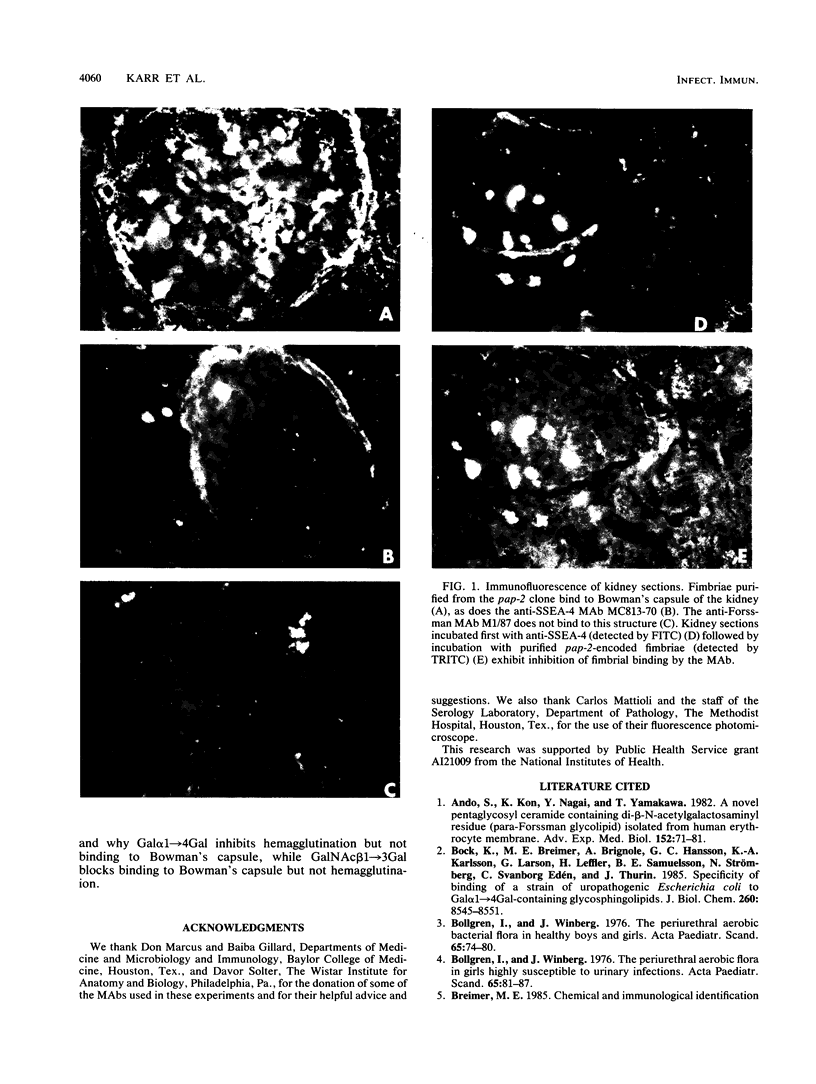
A subtype of P fimbriae, encoded by the pap-2 gene cluster, has been analyzed for agglutination of erythrocytes and for binding to cryostat sections of the human kidney. We have demonstrated that pap-2-encoded fimbriae are capable of binding to erythrocytes from some animal species and to human erythrocytes which express globoside and the LKE (stage-specific embryonic antigen 4 [SSEA-4]) antigen. The pap-2 fimbriae bind to Bowman's capsule in the human kidney. Monoclonal antibodies directed against glycosphingolipids were used for the detection of specific P blood group-related antigens in the human kidney and on erythrocytes. Preincubation of kidney sections with monoclonal antibody MC813-70, which binds to the SSEA-4 antigen, inhibited adherence of purified pap-2-encoded fimbriae to Bowman's capsule. We suggest that one receptor for pap-2-encoded fimbriae is the antigen known as LKE (Luke) on human erythrocytes or SSEA-4 in the tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Kon K., Nagai Y., Yamakawa T. A novel pentaglycosyl ceramide containing di-beta-N-acetylgalactos-aminyl residue (Para-Forssman glycolipid) isolated from human erythrocyte membrane. Adv Exp Med Biol. 1982;152:71–81. [PubMed] [Google Scholar]
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Bollgren I., Winberg J. The periurethral aerobic bacterial flora in healthy boys and girls. Acta Paediatr Scand. 1976 Jan;65(1):74–80. doi: 10.1111/j.1651-2227.1976.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Bollgren I., Winberg J. The periurethral aerobic flora in girls highly susceptible to urinary infections. Acta Paediatr Scand. 1976 Jan;65(1):81–87. doi: 10.1111/j.1651-2227.1976.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Leffler H. The specific glycosphingolipid composition of human ureteral epithelial cells. J Biochem. 1985 Nov;98(5):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135383. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Jovall P. A. Structural characterization of a blood group A heptaglycosylceramide with globo-series structure. The major glycolipid based blood group A antigen of human kidney. FEBS Lett. 1985 Jan 1;179(1):165–172. doi: 10.1016/0014-5793(85)80213-1. [DOI] [PubMed] [Google Scholar]
- Bruce M., Watt A., Gabra G. S., Mitchell R., Lakhesar D., Tippett P. LKE red cell antigen and its relationship to P1 and Pk: serological study of a large family. Vox Sang. 1988;55(4):237–240. doi: 10.1111/j.1423-0410.1988.tb04704.x. [DOI] [PubMed] [Google Scholar]
- Chen H. T., Kabat E. A. Immunochemical studies on blood groups. The combining site specificities of mouse monoclonal hybridoma anti-A and anti-B. J Biol Chem. 1985 Oct 25;260(24):13208–13217. [PubMed] [Google Scholar]
- Chen H. T., Kabat E. A., Lundblad A., Ratcliffe R. M. Nucleotide and translated amino acid sequences of cDNA coding for the variable regions of the light and heavy chains of mouse hybridoma antibodies to blood group A and B substances. J Biol Chem. 1987 Oct 5;262(28):13579–13583. [PubMed] [Google Scholar]
- Clausen H., Watanabe K., Kannagi R., Levery S. B., Nudelman E., Arao-Tomono Y., Hakomori S. Blood group A glycolipid (Ax) with globo-series structure which is specific for blood group A1 erythrocytes: one of the chemical bases for A1 and A2 distinction. Biochem Biophys Res Commun. 1984 Oct 30;124(2):523–529. doi: 10.1016/0006-291x(84)91585-7. [DOI] [PubMed] [Google Scholar]
- Cox C. E. The urethra and its relationship to urinary tract infection: the flora of the normal female urethra. South Med J. 1966 May;59(5):621–626. doi: 10.1097/00007611-196605000-00027. [DOI] [PubMed] [Google Scholar]
- Dakour J., Lundblad A., Zopf D. Separation of blood group A-active oligosaccharides by high-pressure liquid affinity chromatography using a monoclonal antibody bound to concanavalin A silica. Anal Biochem. 1987 Feb 15;161(1):140–143. doi: 10.1016/0003-2697(87)90663-4. [DOI] [PubMed] [Google Scholar]
- Fihn S. D., Johnson C., Stamm W. E. Escherichia coli urethritis in women with symptoms of acute urinary tract infection. J Infect Dis. 1988 Jan;157(1):196–199. doi: 10.1093/infdis/157.1.196. [DOI] [PubMed] [Google Scholar]
- Fowler J. E., Jr, Stamey T. A. Studies of introital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J Urol. 1977 Apr;117(4):472–476. doi: 10.1016/s0022-5347(17)58501-8. [DOI] [PubMed] [Google Scholar]
- Fox N., Shevinsky L., Knowles B. B., Solter D., Dawjanov I. Distribution of murine stage-specific embryonic antigens in the kidneys of three rodent species. Exp Cell Res. 1982 Aug;140(2):331–339. doi: 10.1016/0014-4827(82)90122-7. [DOI] [PubMed] [Google Scholar]
- Gillard B. K., Jones M. A., Marcus D. M. Glycosphingolipids of human umbilical vein endothelial cells and smooth muscle cells. Arch Biochem Biophys. 1987 Aug 1;256(2):435–445. doi: 10.1016/0003-9861(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S., Wang S. M., Young W. W., Jr Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with "A-like antigen" in human cancer. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3023–3027. doi: 10.1073/pnas.74.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Hull S. I., Falkow S. Frequency of gene sequences necessary for pyelonephritis-associated pili expression among isolates of Enterobacteriaceae from human extraintestinal infections. Infect Immun. 1984 Mar;43(3):1064–1067. doi: 10.1128/iai.43.3.1064-1067.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Cochran N. A., Ishigami F., Hakomori S., Andrews P. W., Knowles B. B., Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Levery S. B., Ishigami F., Hakomori S., Shevinsky L. H., Knowles B. B., Solter D. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983 Jul 25;258(14):8934–8942. [PubMed] [Google Scholar]
- Karr J. F., Nowicki B., Truong L. D., Hull R. A., Hull S. I. Purified P fimbriae from two cloned gene clusters of a single pyelonephritogenic strain adhere to unique structures in the human kidney. Infect Immun. 1989 Nov;57(11):3594–3600. doi: 10.1128/iai.57.11.3594-3600.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Baker N., Falk P., Hull R., Hull S., Karr J., Leffler H., Svanborg Edén C., Larson G. Binding specificities of wild-type and cloned Escherichia coli strains that recognize globo-A. Infect Immun. 1989 Nov;57(11):3389–3394. doi: 10.1128/iai.57.11.3389-3394.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B., Marklund B. I., Strömberg N., Lindberg F., Karlsson K. A., Normark S. Uropathogenic Escherichia coli can express serologically identical pili of different receptor binding specificities. Mol Microbiol. 1988 Mar;2(2):255–263. doi: 10.1111/j.1365-2958.1988.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Gilbert S., Sekine M., Suzuki A. Monoclonal antibodies that bind to galactosylgloboside (SSEA-3 antigen). Arch Biochem Biophys. 1988 May 1;262(2):620–625. doi: 10.1016/0003-9861(88)90414-6. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Janis R. Localization of glycosphingolipids in human tissues by immunofluorescence. J Immunol. 1970 Jun;104(6):1530–1539. [PubMed] [Google Scholar]
- Marre R., Hacker J. Role of S- and common-type I-fimbriae of Escherichia coli in experimental upper and lower urinary tract infection. Microb Pathog. 1987 Mar;2(3):223–226. doi: 10.1016/0882-4010(87)90023-4. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Swantee C. A., Hartlen M. Aerobic and anaerobic urethral flora of healthy females in various physiological age groups and of females with urinary tract infections. J Clin Microbiol. 1980 Jun;11(6):654–659. doi: 10.1128/jcm.11.6.654-659.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Svanborg-Edén C., Hull R., Hull S. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Truong L., Moulds J., Hull R. Presence of the Dr receptor in normal human tissues and its possible role in the pathogenesis of ascending urinary tract infection. Am J Pathol. 1988 Oct;133(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- O'Grady F. W., Mcherry M. A., Richards B., Cattell W. R., O'Farrell S. M. Introital enterobacteria, urinary infection, and the urethral syndrome. Lancet. 1970 Dec 12;2(7685):1208–1210. doi: 10.1016/s0140-6736(70)92177-x. [DOI] [PubMed] [Google Scholar]
- Plos K., Carter T., Hull S., Hull R., Svanborg Edén C. Frequency and organization of pap homologous DNA in relation to clinical origin of uropathogenic Escherichia coli. J Infect Dis. 1990 Mar;161(3):518–524. doi: 10.1093/infdis/161.3.518. [DOI] [PubMed] [Google Scholar]
- Shevinsky L. H., Knowles B. B., Damjanov I., Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982 Oct;30(3):697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- TIPPETT P., SANGER R., RACE R. R., SWANSON J., BUSCH S. AN AGGLUTININ ASSOCIATED WITH THE P AND THE ABO BLOOD GROUP SYSTEMS. Vox Sang. 1965 May-Jun;10:269–280. doi: 10.1111/j.1423-0410.1965.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Tippett P., Andrews P. W., Knowles B. B., Solter D., Goodfellow P. N. Red cell antigens P (globoside) and Luke: identification by monoclonal antibodies defining the murine stage-specific embryonic antigens -3 and -4 (SSEA-3 and SSEA-4). Vox Sang. 1986;51(1):53–56. doi: 10.1111/j.1423-0410.1986.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Borne A. E., Bos M. J., Joustra-Maas N., Tromp J. F., van't Veer M. B., van Wijngaarden-du Bois R., Tetteroo P. A. A murine monoclonal IgM antibody specific for blood group P antigen (globoside) Br J Haematol. 1986 May;63(1):35–46. doi: 10.1111/j.1365-2141.1986.tb07492.x. [DOI] [PubMed] [Google Scholar]