Abstract
The DNA-encoding alkaline proteinase (AP) of Pseudomonas aeruginosa IFO 3455 was cloned, and its complete nucleotide sequence was determined. When the cloned gene was ligated to pUC18, the Escherichia coli expression vector, the gene-incorporated bacteria expressed high levels of both AP activity and AP antigens. The amino acid sequence deduced from the nucleotide sequence revealed that the mature AP consists of 467 amino acids with a relative molecular weight of 49,507. The amino acid composition predicted from the DNA sequence was similar to the chemically determined composition of purified AP reported previously. The amino acid sequence analysis revealed that both the N-terminal side sequence of the purified AP and several internal lysyl peptide fragments were identical to the deduced amino acid sequences. The percent homology of amino acid sequences between AP and Serratia protease was about 55%. The zinc ligands and an active site of the AP were predicted by comparing the structure of the enzyme with of Serratia protease, thermolysin, Bacillus subtilis neutral protease, and Pseudomonas elastase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsumi Y., Yamamoto S., Morihara K., Fukushima J., Takeuchi H., Mizuki N., Kawamoto S., Okuda K. Cloning and expression of the alkaline proteinase gene from Pseudomonas aeruginosa IFO 3455. J Bacteriol. 1989 Sep;171(9):5173–5175. doi: 10.1128/jb.171.9.5173-5175.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever R. A., Iglewski B. H. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. J Bacteriol. 1988 Sep;170(9):4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Pasloske B. L., Paranchych W. Expression of the Pseudomonas aeruginosa PAK pilin gene in Escherichia coli. J Bacteriol. 1986 Feb;165(2):625–630. doi: 10.1128/jb.165.2.625-630.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- Fukushima J., Yamamoto S., Morihara K., Atsumi Y., Takeuchi H., Kawamoto S., Okuda K. Structural gene and complete amino acid sequence of Pseudomonas aeruginosa IFO 3455 elastase. J Bacteriol. 1989 Mar;171(3):1698–1704. doi: 10.1128/jb.171.3.1698-1704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Brooks J. E. Cloned restriction/modification system from Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1983 Jan;80(2):402–406. doi: 10.1073/pnas.80.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Activation of an elastase precursor by the lasA gene product of Pseudomonas aeruginosa. J Bacteriol. 1987 Oct;169(10):4532–4539. doi: 10.1128/jb.169.10.4532-4539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J., Murgier M., Filloux A., Lazdunski A. Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J Bacteriol. 1990 Feb;172(2):942–948. doi: 10.1128/jb.172.2.942-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Homma J. Y., Abe C., Tanamoto K., Hirao Y., Morihara K., Tsuzuki H., Yanagawa R., Honda E., Aoi Y., Fujimoto Y. Effectiveness of immunization with single and multi-component vaccines prepared from a common antigen (OEP), protease and elastase toxoids of Pseudomonas aeruginosa on protection against hemorrhagic pneumonia in mink due to P. aeruginosa. Jpn J Exp Med. 1978 Apr;48(2):111–133. [PubMed] [Google Scholar]
- INOUE H., NAKAGAWA T., MORIHARA K. Pseudomonas aeruginosa proteinase. II. Molecular weight and molecular dimension. Biochim Biophys Acta. 1963 May 7;73:125–131. doi: 10.1016/0006-3002(63)90427-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lory S., Strom M. S., Johnson K. Expression and secretion of the cloned Pseudomonas aeruginosa exotoxin A by Escherichia coli. J Bacteriol. 1988 Feb;170(2):714–719. doi: 10.1128/jb.170.2.714-719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukac M., Collier R. J. Restoration of enzymic activity and cytotoxicity of mutant, E553C, Pseudomonas aeruginosa exotoxin A by reaction with iodoacetic acid. J Biol Chem. 1988 May 5;263(13):6146–6149. [PubMed] [Google Scholar]
- MORIHARA K. PRODUCTION OF ELASTASE AND PROTEINASE BY PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:745–757. doi: 10.1128/jb.88.3.745-757.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H. PSEUDOMONAS AERUGINOSA PEPTIDE PEPTIDOHYDROLASE. 3. SOME CHARACTERS AS A CA2+-METALLOENZYME. Biochim Biophys Acta. 1964 Nov 22;92:351–360. [PubMed] [Google Scholar]
- MORIHARA K., YOSHIDA N., KURIYAMA K. PSEUDOMONAS AERUGINOSA PEPTIDE PEPTIDOHYDROLASE. IV. OPTICAL ROTATORY DISPERSION AND AMINO ACID COMPOSITION. Biochim Biophys Acta. 1964 Nov 22;92:361–366. doi: 10.1016/0926-6569(64)90194-4. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Minton N. P., Atkinson T., Bruton C. J., Sherwood R. F. The complete nucleotide sequence of the Pseudomonas gene coding for carboxypeptidase G2. Gene. 1984 Nov;31(1-3):31–38. doi: 10.1016/0378-1119(84)90192-6. [DOI] [PubMed] [Google Scholar]
- Morihara K. Comparative specificity of microbial proteinases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):179–243. doi: 10.1002/9780470122860.ch5. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H., Oka T. On the specificity of Pseudomonas aeruginosa alkaline proteinase with synthetic peptides. Biochim Biophys Acta. 1973 Jun 6;309(2):414–429. doi: 10.1016/0005-2744(73)90040-5. [DOI] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Substrate specificity of elastolytic and nonelastolytic proteinases from Pseudomonas aeruginosa. Arch Biochem Biophys. 1966 Apr;114(1):158–165. doi: 10.1016/0003-9861(66)90317-1. [DOI] [PubMed] [Google Scholar]
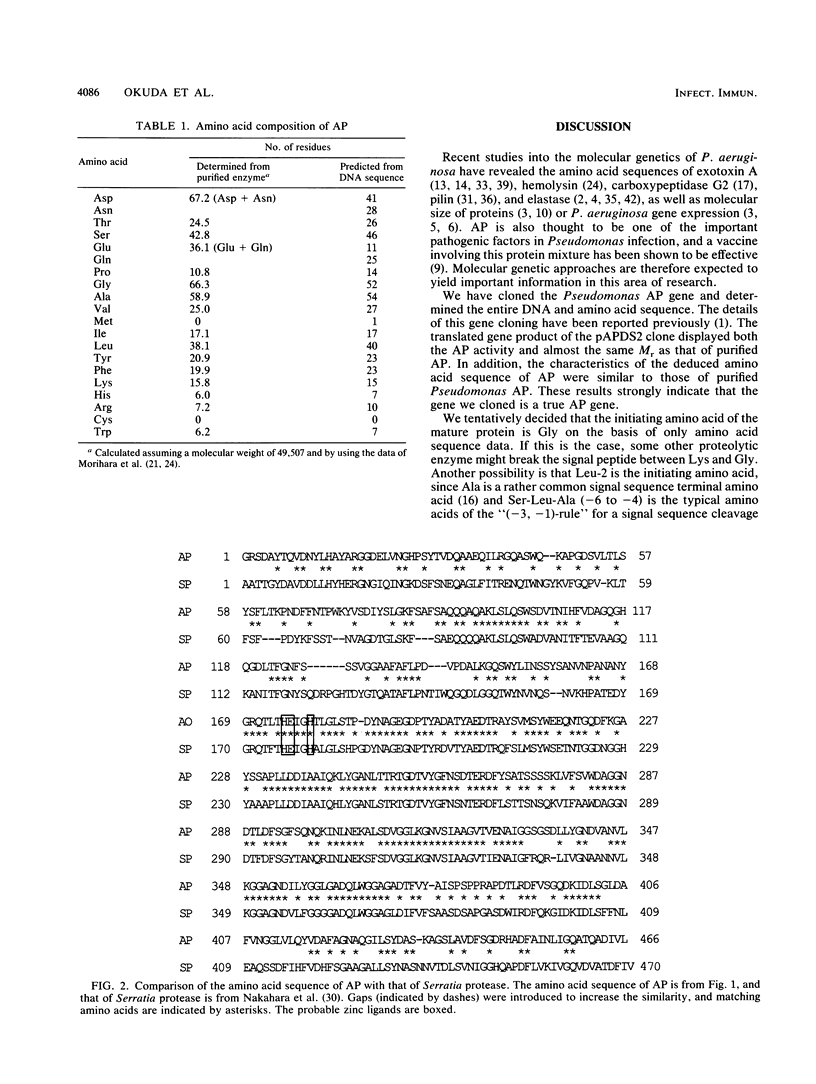
- Nakahama K., Yoshimura K., Marumoto R., Kikuchi M., Lee I. S., Hase T., Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acids Res. 1986 Jul 25;14(14):5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasloske B. L., Finlay B. B., Paranchych W. Cloning and sequencing of the Pseudomonas aeruginosa PAK pilin gene. FEBS Lett. 1985 Apr 22;183(2):408–412. doi: 10.1016/0014-5793(85)80821-8. [DOI] [PubMed] [Google Scholar]
- Pritchard A. E., Vasil M. L. Nucleotide sequence and expression of a phosphate-regulated gene encoding a secreted hemolysin of Pseudomonas aeruginosa. J Bacteriol. 1986 Jul;167(1):291–298. doi: 10.1128/jb.167.1.291-298.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schad P. A., Bever R. A., Nicas T. I., Leduc F., Hanne L. F., Iglewski B. H. Cloning and characterization of elastase genes from Pseudomonas aeruginosa. J Bacteriol. 1987 Jun;169(6):2691–2696. doi: 10.1128/jb.169.6.2691-2696.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986 Feb;165(2):367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Chamberlain C., Grant C. C. Molecular studies of Pseudomonas exotoxin A gene. Infect Immun. 1986 May;52(2):538–548. doi: 10.1128/iai.52.2.538-548.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver L. H., Kester W. R., Matthews B. W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of extended substances. J Mol Biol. 1977 Jul;114(1):119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Fukushima J., Atsumi Y., Takeuchi H., Kawamoto S., Okuda K., Morihara K. Cloning and characterization of elastase structural gene from Pseudomonas aeruginosa IFO 3455. Biochem Biophys Res Commun. 1988 May 16;152(3):1117–1122. doi: 10.1016/s0006-291x(88)80400-5. [DOI] [PubMed] [Google Scholar]
- Yang M. Y., Ferrari E., Henner D. J. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984 Oct;160(1):15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]


