Abstract
In the present study, the apoptotic effect of cordycepin on MA-10 cells, a mouse Leydig tumor cell line, was investigated. Results demonstrated that the number of rounding-up cell increased by cordycepin (10 μM to 5 mM for 24 h), and cells with plasma membrane blebbing could be observed by 100 μM cordycepin. In viability test, MA-10 cell surviving rate significantly decreased as the dosage (10 μM to 5 mM) and duration (3–24 h) of cordycepin treatment increased (P < 0.05). Cordycepin at 100 μM and 1 mM for 24 h treatment induced significant DNA fragmentation (P < 0.05). In addition, the percentage of G1 and G2/M phase cell significantly declined by cordycepin (100 μM and 1 mM) for 24 h treatment, while the percentages of subG1 phase cell increased by 100 μM and/or 1 mM cordycepin in 6, 12 and 24 h treatments (P < 0.05), respectively, which highly suggested that cordycepin induced MA-10 cell apoptosis. In mechanism study with the treatments of caspases, c-Jun NH2 terminal kinase (JNK) or reactive oxygen species (ROS) inhibitors plus cordycepin for 24 h, only caspases inhibitor suppressed subG1 phase in MA-10 cells. Moreover, western blotting results showed that cordycepin induced caspase-9, -3 and -7 protein expressions, but not caspase-8, in time- and dose-dependent manners. In conclusion, cordycepin induced apoptosis in MA-10 mouse Leydig tumor cells through a caspase-9 and -3 and -7 dependent pathway.
1. Introduction
Cordycepin (3′deoxyadenosine), the analog of adenosine, is a constituent from the mycelia of Cordyceps sinensis (CS), and was considered as an active component with effects altering cytokines secretion, improving lung function, increasing energy levels and sex drive [1–3]. It has been demonstrated that cordycepin has anti-tumor effect on mouse melanoma and lung carcinoma cells and human oral cancer cells [4, 5]. Moreover, cordycepin could inhibit polyadenylate polymerase (PAP) or inactivate mRNA polyadenylation to induce tumor cell apoptosis, which is characterized by the cellular rounding-up, cytoplasmic contraction, plasma membrane blebbing, chromatin condensation, DNA fragmentation and many biochemical characteristics [6–11]. However, the molecular mechanisms regarding apoptotic signal pathways remain elusive.
The activation of cystein aspartic-specific proteases (caspases) is commonly thought to be one of the earliest points in the no-return pathway of apoptosis. Caspases are broadly categorized into upstream regulatory caspases and downstream effector caspases [12]. The upstream caspases, such as caspase-8 (death receptor pathway) and caspase-9 (mitochondria pathway), typically have a long N-terminal prodomain that facilitates interaction with and recruitment of proapoptotic proteins, including other caspases [13]. The downstream caspases, such as caspase-3, -6, and -7, typically have short prodomains that primarily cleave protein, which is important for cellular functions, and results in cell apoptosis [9, 14–16]. Moreover, some investigations have indicated that c-Jun NH2 terminal kinase (JNK) pathway also participates in apoptosis. JNK, a stress-activated protein kinase, is a subgroup of the MAPK superfamily, which can be activated by cell stress such as ultraviolet, TNF and interleukin-1 [17, 18]. Furthermore, reactive oxygen species (ROS), molecules possessing an odd of electrons, could induce various biological responses, including cell growth, arrest and/or cell damage [19, 20]. Excess ROS would cause damage to cellular component such as lipid membranes, protein, and DNA, leading to apoptosis [21–23].
We have previously demonstrated that CS could induce MA-10 cell apoptosis [24]. It is possible that cordycepin, the pure substance from C. sinensis, induce MA-10 cell death. Although some reports have demonstrated that cordycepin possesses anti-tumor action, limited reports about cordycepin-mediated apoptosis in testicular tumor cells have been illustrated. In addition, it has been demonstrated that between 1973 and 1995 the incidence of testicular cancer in the United States increases 51% (1% of total cancer cases annually), and orchidectomy is the common protocol to treat testicular and Leydig cell cancers [25, 26]. Thus, rather than orchidectomy, the exploration of cordycepin-induced cell death with the study of mechanism will be valuable to design more effective chemotherapy agents on testicular tumor cells.
2. Materials and Methods
2.1. Chemicals
Cordycepin, bovin serum albumin (BSA), Waymouth MB 752/1 medium, 3-ethyl-5-benzyl-2-methyl-4-phenylethylethynyl-6-phenyl-1,4-dihydropyridine-3,5-dicarboxylate (MRS 1191), methylthiazolecterazolium (MTT), SP600125, RNase A, propidium iodide and mercaptoethanol were purchased from Sigma Chemical (St Louis, MO, USA). Fetal bovine serum (FBS), Dulbecco's phosphate buffered saline, lyophilized trypsin-EDTA and gentamicin sulfate were purchased from Gibco (Grand Island, NY, USA). Sodium hydroxide, hydrochloric acid, sodium dodecyl sulfate (SDS), sucrose, EDTA, isopropyl alcohol, chloroform and Tween 20 were purchased from Merck (Darmstadt, Germany). Tris base and general caspase inhibitor (zVAD-fmk) were purchased from Calbiochem (San Diego, CA, USA). Acrylamide was purchased from JT Baker (Phillipsburg, NJ, USA). HEPES was purchased from Mallinckrodt Baker, Inc. (Paris, Kentucky, USA). Tissue culture grade sodium bicarbonate, sodium carbonate, sodium chloride, sodium dihydrogen phosphate and potassium chloride were purchased from Riedel-deHaen (Seelze, Germany). Donkey anti-rabbit IgG conjugated with horseradish peroxidase were purchased from Amersham International (Arlington Heights, IL, USA). Antibody against β-actin and the monoclonal antibody against caspase-3, -7, and -9 were purchased from Cell Signaling (Beverly, MA, USA). The polyclonal antibody against caspase-8 was purchased from Santa Cruz (Santa Crus, CA, USA).
2.2. Cell Culture
The MA-10 cell line was a gift from Dr Mario Ascoli (University of Iowa, Iowa City, IA, USA), and was maintained with the standard technique [27]. Cells were maintained in Waymouth medium containing 15% FBS and incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37°C.
2.3. Morphology Observation
MA-10 cells (6 × 105) were seeded in 6-cm Petri dish (Techno Plastic Products AG, Trasadingen, Switzerland) with 2 mL serum medium. After 70–80% confluence, cells were treated without or with 10 μM, 100 μM, 1 mM, 2 mM and 5 mM cordycepin for 24 h. Cell morphology was then observed and recorded under light microscopy (Olympus, CK 40). Apoptosis is characterized by the loss of cellular contact with the matrix and the appearance of plasma membrane blebbing [9].
2.4. DNA Fragmentation Assay
In order to investigate if cordycepin could induce cell apoptosis, DNA fragmentation was determined first. After treatment, MA-10 cells (1 × 106) were lysed in a 0.6 mL cell lysis solution containing 20 mM Tris–HCl, 10 mM EDTA, pH 8.0 and 0.3% Triton X-100. DNA was extracted with 0.6 mL phenol/chloroform (1 : 1), and the mixture was centrifuged at 12 500 r.p.m. for 10 min. DNA in the aqueous phase was extracted with phenol/chloroform (1 : 1) again. The aqueous phase with DNA was mixed with isopropanol at −20°C overnight. After centrifugation, DNA pellets were washed with 70% ethanol and air-dried. DNA pellets were dissolved in TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 8.0), and RNase A (3 mg/mL) was added to remove RNA at 37°C for 30 min. DNA electrophoresis was carried out in 2% agarose gel. The gel was stained with ethidium bromide. DNA fragments were visualized under UV light and photographed.
2.5. MTT Cytotoxicity Test
Methylthiazoletetrazolium test was used to determine cell viability with the treatment of cordycepin [28]. MA-10 cells were seeded in 96-well plate (Techno Plastic Products AG, Trasadingen, Switzerland) containing 2 × 104 cells with 100 μL serum medium in each well. After 70–80% confluence, cells were treated without or with 10 μM, 100 μM, 1 mM, 2 mM and 5 mM cordycepin for 3, 6, 12, and 24 h, respectively. MTT was added at different time points with the final concentration of 0.5 mg/mL, and then incubated at 37°C for 4 h. The medium was removed and DMSO (50 μL) was added into each well to dissolve the crystals by shaking the plate weakly for 20 min in dark. The OD values in each treatment were then determined at λ = 590 nm by an ELISA reader (Opsy MR, Dynex, USA).
2.6. Flow Cytometry Analysis
To further confirm whether cordycepin could induce cell apoptosis, the redistribution of cell cycle by flow cytometric analysis was used with propidium iodine stain [29, 30]. MA-10 cells (6 × 105) were seeded in 6-cm dish with 2 mL serum medium. After 70–80% confluence, cells were treated with free medium containing various concentrations of cordycepin for 3, 6, 12, and 24 h, respectively. Cordycepin-treated cells were harvested with trypsin, washed with PBS, and mixed in 75% ethanol for at least 2 h at −20°C. After fixation, cells were washed with cold PBS and then collected by centrifugation, mixed with 100 μg/mL RNase, and stained with PI (propidium iodide) solution containing 40 μg/mL in PBS. The stained cells were analyzed using a fluorescence activated cell sorter (FACScan, Becton-Dickinson, Mountain View, CA, USA) at λ = 488 nm using Cell-Quest software (Becton-Dickinson, Mountain View, CA, USA). The DNA content distribution of normal growing cells is characterized by two peaks G1/G0 and G2/M phase. G1/G0 phase possesses normal functioning and resting state of cell cycle with most diploid DNA content, while the DNA content in G2/M phase are more than diploid. Cells in subG1 phase have least DNA content in cell cycle distribution, called hypodiploid. The hypoploid DNA contents represent the DNA fragmentation [30].
2.7. Immunoblotting Analysis
Cells (6 × 106) were seeded in 6-cm dish. After treatment, cells were rinsed with cold PBS. Cells were then harvested by 100 μL lysis buffer (50 mM Tris-base, 150 mM NaCl, 1% NP40, 0.1% SDS, 0.5% deoxychloride acid and 1 mM PMSF). Cell lysate was centrifuged at 32 000 r.p.m. for 10 min at 4°C. The pellet was collected by 10 μL lysis buffer and was centrifuged again at 12 000 g. The supernatant, which contains total protein, was collected and stored at −20°C. Protein concentration of the cell lysates were determined by the Lowry method [31]. Cell proteins (40–60 μg) were separated in 12% SDS–polyacrylamide gel, that performed at 100 V for 2.5 h using standard running buffer (24 mM Tris–HCl, 0.19 M glycine, 0.5% SDS, pH 8.3), and electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane at 400 mA for 4 h in transfer buffer (20 mM Tris–HCl, 150 mM glycine, 10% methanol, 0.01% SDS). The membranes were blocked with 5% nonfat milk, washed by TBST (20 mM Tris-base, 137 Mm NaCl, 0.1% Tween 20, pH 7.6), and subsequently incubated with primary caspase-3 or -7 antibodies at 1 : 4000 dilutions and caspase-8 or -9 antibodies at 1 : 2000 dilutions overnight at 4°C. After washing, the membrane was incubated with horseradish peroxidase-conjugated sheep anti-mouse antibody or donkey anti-rabbit antibody, and then visualized by enhanced chemiluminescence (ECL) detection kit (Amersharn-Pharmacia International PLC, UK). The optical density of each protein band was quantitated by a Quantity One (PDI, Huntington Station, NY, USA) computer-assisted image analysis system [32]. The amount of β-actin in each lane was also detected as a control to correct the expression of caspases proteins.
2.8. Statistic Analysis
Each data point in the figures represents the mean ± SEM of three separate experiments. Statistically significant differences between treatments and controls were determined by one-way ANOVA and then Least Significance Difference (LSD) comparison procedure. Statistical significance was set at P < .05.
3. Results
3.1. Cordycepin-Induced Morphological Change and DNA Fragmentation in MA-10 Cells
MA-10 cells were treated without or with cordycepin (10 μM, 100 μM, 1 mM, 2 mM, and 5 mM) for 24 h, and morphological changes were observed under light microscopy. Cells without cordycepin treatment showed polygonal shape with healthy appearances, blurred outline and firm attachment, which is normal cell growth phenomenon (Figure 1(a)). Cells appeared rounded-up phenomenon but still adherent to the ground matrix with 10 μM cordycepin (Figure 1(b). Cordycepin at 100 μM caused more cells rounded-up, and some cells expressed plasma membrane blebbings (Figure 1(c)). More adherent cells appeared membrane blebbings with more floating cells under 1, 2, and 5 mM cordycepin treatments, and the number of attached cells reduced (Figures 1(d)–1(f)). These phenomena suggest that cordycepin might induce apoptotic cell death in MA-10 cells.
Figure 1.
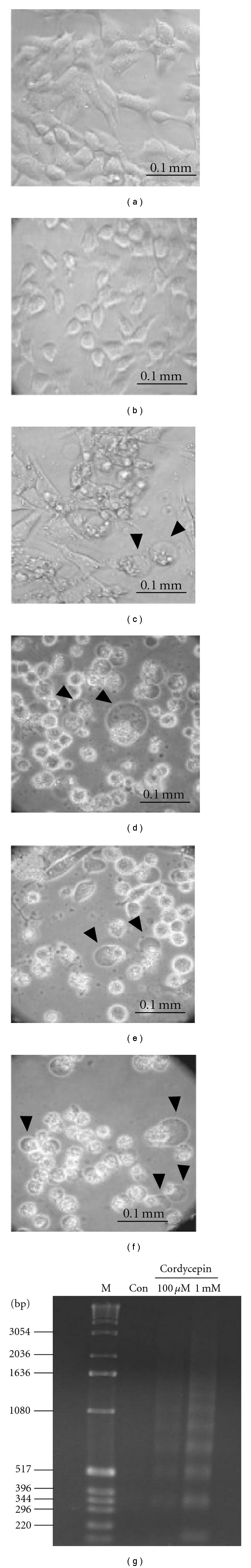
The effect of cordycepin on morphological change and DNA fragmentation in MA-10 cells. MA-10 cells were treated without (a), or with 10 μM (b), 100 μM (c), 1 mM (d), 2 mM (e) and 5 mM (f) cordycepin for 24 h. Morphological changes of cells were examined under light microscopy (bar: 0.1 mm; arrow heads: membrane blebbed cells). Also, gel electrophoresis of a 1 kb DNA ladder marker (lane M) or DNA isolated from MA-10 cells that were cultured for 24 h in the presence of medium alone, 100 μ or 1 mM cordycepin (g). DNA was visualized by ethidium bromide staining and photographed under UV illumination. Experiments were performed three times with similar results (Con = control).
Previous results illustrated that cordycepin would cause cell death in MA-10 cells. DNA fragmentation laddering assay and flow cytometry analysis were further used to confirm apoptotic effect of cordycepin on MA-10 cells. The occurrence of DNA fragmentation is a characteristic event of cell apoptosis with genomic DNA degrading into multiples of 180–200 bp units producing a ladder feature on agarose gel electrophoresis. Figure 1(g) notably illustrates that DNA fragmentations were observed in MA-10 cells with 24 h cordycepin treatment at 100 μM and 1 mM.
3.2. Decreased MA-10 Cell Viability by Cordycepin
The morphological changes suggested the involvement of cell death induced by cordycepin in MA-10 cells. Thus, MTT test was used to investigate the effect of cordycepin on cell viability in MA-10 cells. Cells were treated without or with various concentrations (10 μM to 5 mM) of cordycepin for different time points (3, 6, 12, and 24 h). MTT tests showed that cordycepin induced death effect on MA-10 cells in a time- and dose-dependent manner (Figure 2).
Figure 2.
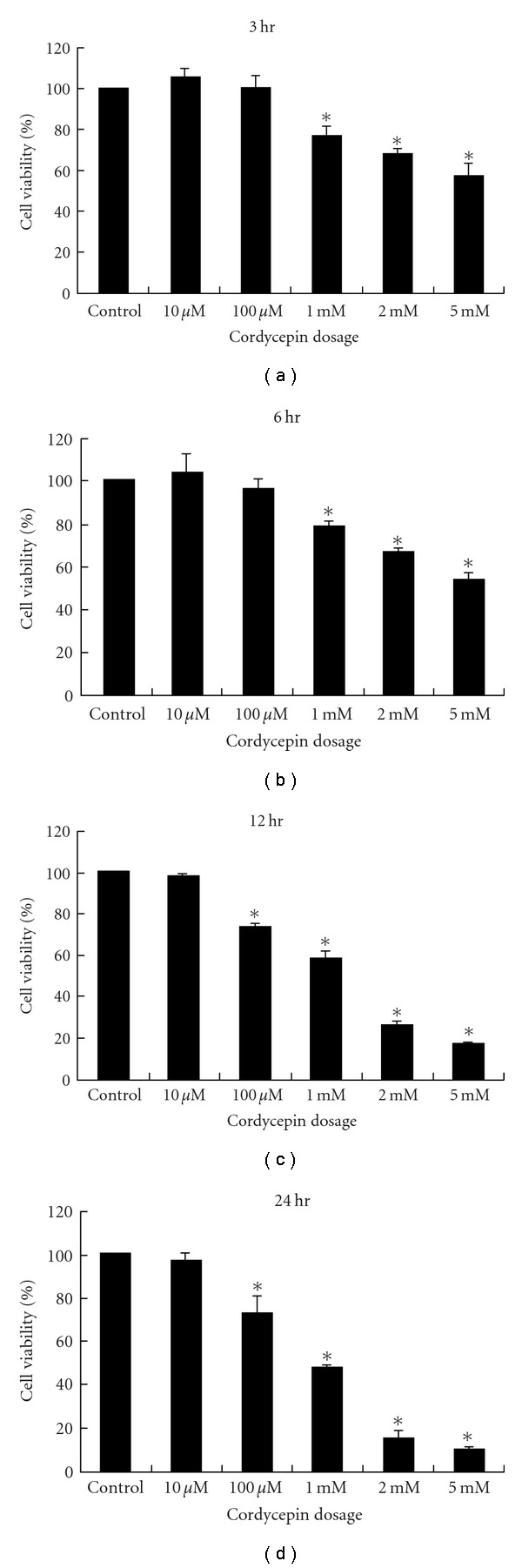
Effects of cordycepin on cell viability of MA-10 cells. Cells (5000 cells/well) were treated with different concentrations of cordycepin (0.01–5 mmol/L) for 3 h (a), 6 h (b), 12 h (c) and 24 h (d), respectively, and cell viability was quantified by MTT test. Results are expressed as percentages of cell growth relative to initial number of viable cells in controls (as 100%). Data represent the mean ± SEM of four separate experiments. *Groups differing significantly from control (P < .05).
Figure 2(a) illustrates that 3-h treatment of 1, 2, and 5 mM cordycepin significantly reduced cell viability to 76.8 ± 4.3%, 67.9 ± 2.5% and 57.2 ± 5.7% (P < .05), respectively, in MA-10 cells. After 6 h treatment with 1, 2, and 5 mM cordycepin, cell viability reduced to 78.8 ± 2.6%, 66.6 ± 2.6%, and 53.9 ± 3.4%, respectively (P < .05) (Figure 2(b)). Treatment with 100 μM, 1, 2, and 5 mM cordycepin after 12 h caused a reduction in cell viability to 73.0 ± 2.3%, 58.1 ± 3.8%, 25.7 ± 2.3% and 16.2 ± 0.6%, respectively (P < 0.05) (Figure 2(c)). Moreover, treatment with 100 μM, 1, 2 and 5 mM cordycepin as 24 h reduced cell viability to 73.1 ± 7.9%, 47.8 ± 1.6%, 15.0 ± 3.9% and 10.1 ± 1.2%, respectively (P < .05) (Figure 2(d)). The effective cordycepin concentration for 50% inhibition (EC50) on MA-10 cell viability after 24 h was 1 mM. Thus, 100 μM and 1 mM were chosen for the subsequent experiments.
3.3. Cordycepin-Induced Cell Cycle Redistribution in MA-10 Cells
Moreover, in flow cytometry analysis, DNA content in each individual cell was determined. Figure 3 illustrates the distributions of PI stained MA-10 cells treated with various concentrations of cordycepin (control, 10 μM, 100 μM, 1 mM, 2 mM, or 5 mM) for 3 h (Figure 3(a)), 6 h (Figure 3(b)), 12 h (Figure 3(c)) and 24 h (Figure 3(d)), respectively. The increases of subG1 phase in cell cycle distribution could be observed at 3 and 6 h treatments by 100 μM cordycepin, and 12 and 24 h by 100 μM and 1 mM cordycepin, respectively. Meanwhile, the decreases of cell cycle distribution could only be observed in G1 phase by cordycepin at 100 μM and 1 mM, or in G2/M phase by cordycepin at 1 mM for 24 h treatment, respectively.
Figure 3.
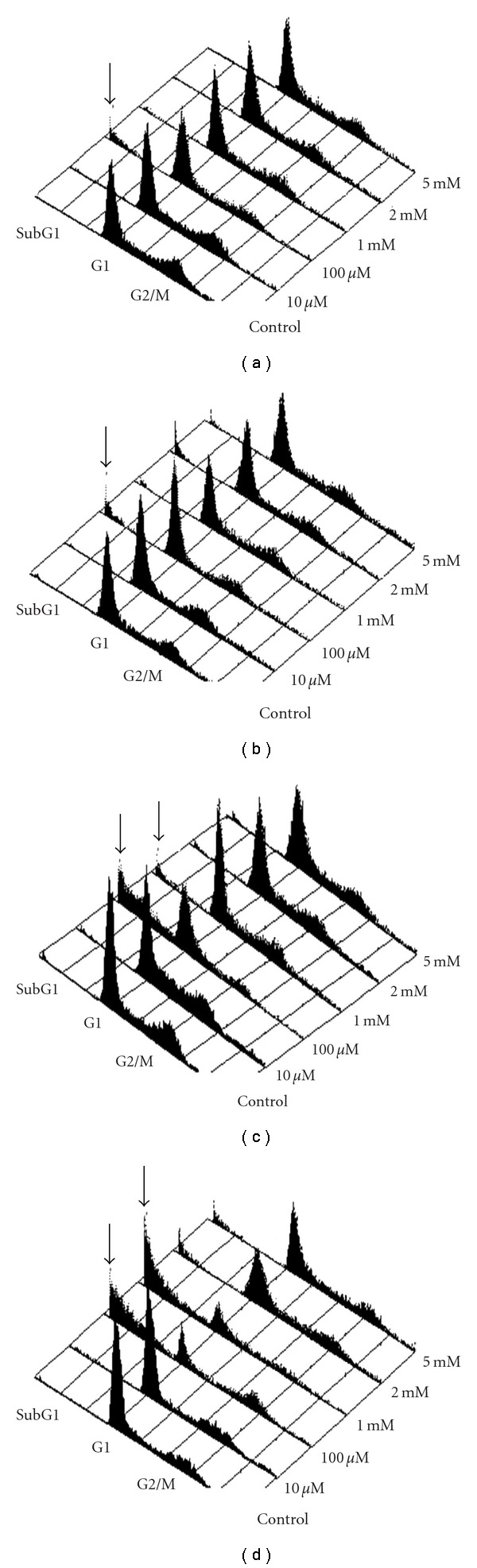
Cordycepin increased subG1 cell cycle phase in MA-10 cell line. The 3D histogram plot of flow cytometry analysis in MA-10 cells treated without or with cordycepin (10 μM to 5 mM) for 3 h (a), 6 h (b), 12 h (c) and 24 h (d), respectively. At the proper time points, cells were fixed, stained with propidium iodide, and analyzed of cell cycle progression by flow cytometry as described in Section 2. SubG1 = cells with less than normal amount of DNA content; G1, cells in G1 cell cycle phase; G2/M, cells in G2/M cell cycle phase.
3.4. The Tendency and Analysis of Cell Cycle under Cordycepin Influence in MA-10 Cells
Statistical analysis from three independent experiments of Figure 3 with different concentration of cordycepin after 3, 6, 12, or 24 h treatments regarding the change of subG1, G1, and G2/M phases of cell cycle in percentages was analyzed and illustrated in Figure 4. There was no significant difference regarding the change of subG1 phase after 3 h cordycepin treatment (Figure 4(a)). However, after 6 h treatment, the subG1 phase significantly increased from 1.27% in control group to 7.64% in 100 μM cordycepin treatment group (Figure 4(b)) (P < .05). After 12 h treatment, subG1 phase significantly increased from 2.8% in control group to 23.1 and 11.4% in 100 μM and 1 mM cordycepin treatment groups, respectively (Figure 4(c) (P < .05). After 24 h treatment, subG1 phase significantly increased from 4.4% in control group to 38.2 and 63.1% in 100 μM and 1 mM cordycepin treatment groups, respectively (Figure 4(d) (P < .05). In G1 phase, there were no significant differences by different dosages of cordycepin after 3, 6, and 12 h treatments (Figures 4(e)–4(g)) (P < .05). 100 μM and 1 mM cordycepin treated MA-10 cells for 24 h did significantly decrease G1 phase from 56.3% in control group to 23.1 and 18.1% in 100 μM and 1 mM cordycepin treatment groups, respectively (Figure 4(h)) (P < .05). Moreover, only cordycepin at 1 mM treatment for 24 h could significantly decrease G2/M phase from 39.4% in control group to 18.7% in cordycepin treatment group (Figure 4(l)) (P < .05).
Figure 4.
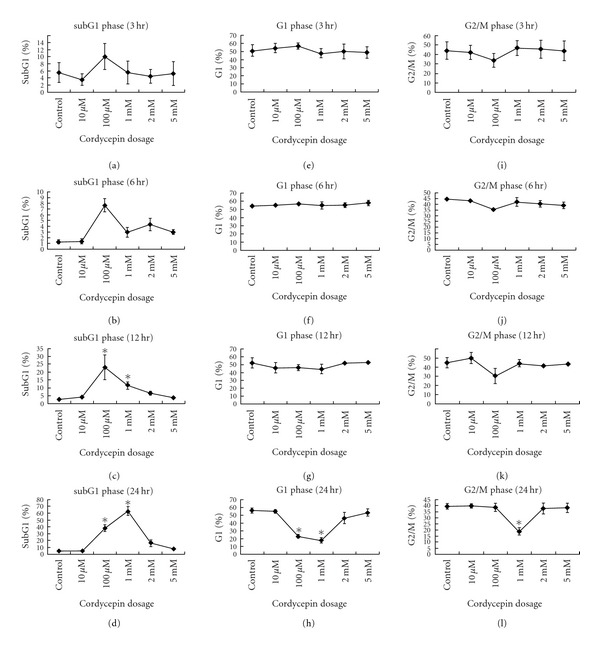
Quantification in percentage of subG1 phase cell number. Statistical analysis from three independent experiments of Figure 3 with different concentrations of cordycepin (10 μM to 5 mM) regarding the change of cell cycle in percentages of subG1 phase for 3 h (a), 6 h (b), 12 h (c), and 24 h (d); in percentages of G1 phase for 3 h (e), 6 h (f), 12 h (g) and 24 h (h); and in percentages of G2/M phase for 3 h (i), 6 h (j), 12 h (k), and 24 h (l) was analyzed and illustrated. Data represent the mean ± SEM of three separate experiments. *Groups differing significantly from control (P < .05).
3.5. The Involvement of Cordycepin on ROS, JNK and/or Caspase Pathways Related to Apoptosis in MA-10 Cells
To investigate possible pathway that cordycepin might activate to induce MA-10 cell apoptosis, cells were co-treated with 100 μM cordycepin plus 1 μM JNK inhibitor (SP600125), 1 mM ROS inhibitor (NAC) or 100 μM general caspase inhibitor (zVAD-fmk) for 24 h. The change of subG1 ratios determined by flow cytometry was then analyzed. Figure 5(a) illustrates that subG1 ratio increased from 2.97% in control group to 39.82% in 100 μM cordycepin treatment after 24 h (P < .05). JNK and ROS inhibitors could not suppress the subG1 ratio (40.76 and 40.05%, resp.) back to control levels (P > .05). In caspase inhibitor experiment (Figure 5(b)), the subG1 ratio increased from 1.78% in control group to 14.66% in cordycepin treatment after 24 h (P < .05). Interestingly, general caspase inhibitor significantly decreased subG1 ratio to 9.26% (P < .05). These results indicated that caspase activation/pathway played roles in cordycepin-induced apoptosis in MA-10 cells.
Figure 5.
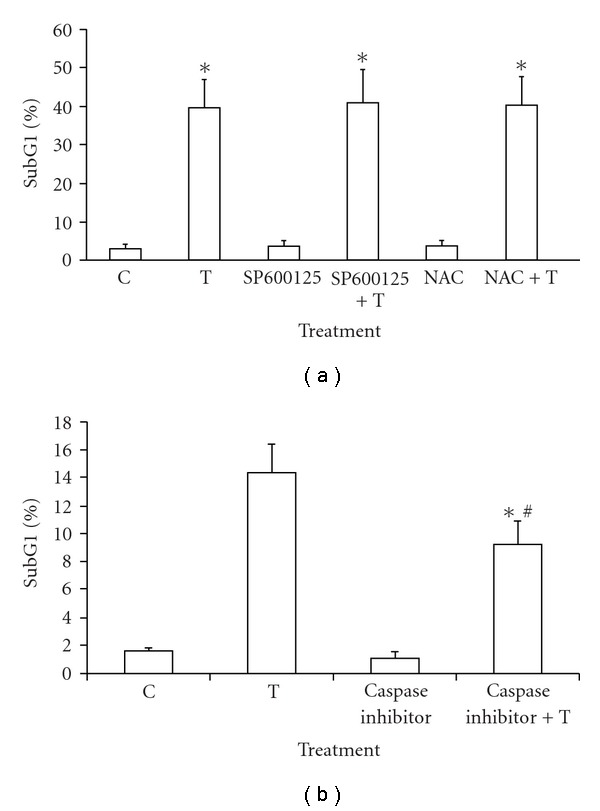
Participation of JNK, ROS and caspases in cordycepin-induced apoptosis. MA-10 cells were incubated with or without 100 μM cordycepin in the presence or absence of 1 μM JNK inhibitor (SP600125 which abbreviated as SP) and 1 mM ROS inhibitor (NAC) (a), or 100 μM caspases inhibitor (b), respectively, for 24 h, and the change of cell cycle in percentage of subG1 phase was analyzed and illustrated. Data represent the mean ± SEM of three separate experiments. *Groups differing significantly from control (P < .05). #Groups differing significantly from 100 μM cordycepin treatment.
3.6. Cordycepin-Induced Differential Caspases Proteins Expression Related to Apoptosis in MA-10 Cells
Previous data implied that caspase protein was involved in cordycepin-activated apoptosis in MA-10 cells. We further determined whether cordycepin could induce caspase expression. Figure 6(a) demonstrates immunoblotting results of caspase-8, -9, -3 and -7 with cordycepin treatments (control, 100 μM and 1 mM) for 0, 1, 3, 6, 12, and 24 h, respectively. Figure 6(b) illustrates no significant differences among cordycepin treatments in each time point of caspase-8 expression (P > .05). However, 1 mM cordycepin (12 h) significantly induced caspase-9 expression (P < .05) (Figure 6(c)), 100 μM cordycepin (6 h) and 1 mM cordycepin (24 h) induced caspase-3 expression (P < .05) (Figure 6(d)) and 100 μM cordycepin (12 h) significantly induced caspase-7 expression (Figure 6(e)) (P < .05). These results indicate that cordycepin induced caspase-9, 3-, and -7 expressions, but not caspase-8, to activate MA-10 cell apoptosis.
Figure 6.
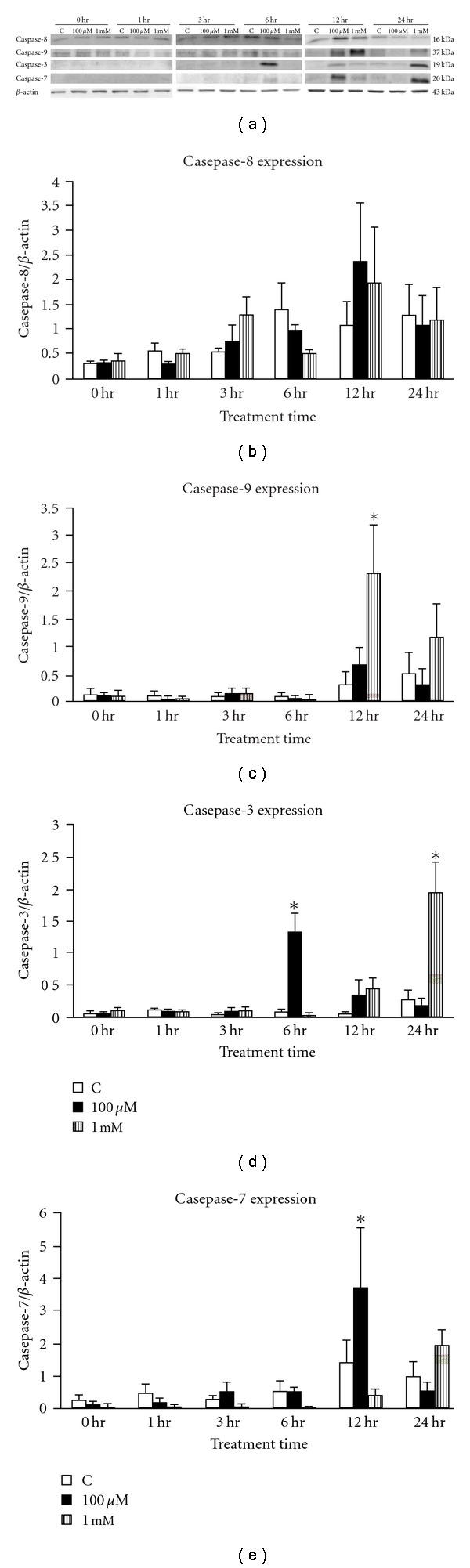
The expression of effector caspase-3 in cordycepin-induced apoptosis. Cells were treated without or with 100 μM and 1 mM cordycepin for different time (0, 1, 3, 6, 12, and 24 h, resp.). Caspase-8 (16 kDa), caspase-9 (37 kDa), caspase-3 (19 kDa) and caspase-7 (20 kDa) specific bands were detected by Western blot. Immunoblot represents the observations from one single experiment repeated three times (a). The integrated optical densities (IOD) of caspase-8, -9, -3, and -7 proteins after normalization with β-actin (43 kDa) in each lane using PDI image system were demonstrated in (b), (c), (d), and (e), respectively. Each data point in the figure represents the mean ± SEM of three separate experiments. *Statistical difference compared to control (P < .05).
4. Discussion
In the present study, cordycepin would induce MA-10 cell rounded-up with blebbed membrane, and reduced MA-10 cell viability with apparent DNA fragmentation. It is well known that internucleosomal DNA fragmentation displays as a biochemical hallmark during apoptosis [33]. The induction of DNA fragmentation phenomenon by cordycepin at 100 μM for 24 h correlated with the increase of subG1 phase cell number of cytometry analysis, which confirms that cordycepin could induce apoptosis in MA-10 cells. Meanwhile, there were significant decreases of G1 and G2/M phase cells. Moreover, caspases inhibitor, but not JNK and ROS inhibitors, decreased subG1 ratio, implying that caspase pathway played role in cordycepin-induced apoptosis. Furthermore, cordycepin induced caspase-9, -3 and -7 expressions, but not caspase-8, to activate MA-10 cell apoptosis.
Hyperphosphorylation of PAP from p34 cyclin B kinase during mitosis will lead to the inactivation of PAP, which contributes to the reduction of mRNA and protein synthesis during the M phase of the cell cycle [34]. Also, PAP activity levels are significantly elevated at the G1/S phase of the cell cycle, along with the increased rate of mRNA polyadenylation and accumulation in the cytoplasm [35]. In the present study, treatment of MA-10 cells with cordycepin lead to the decreases of G1 and G2/M phases. It is possible that cordycepin inhibited polyadenylation, which was necessary at the G1/S phase of the cell cycle in order for all the required mRNAs to be polyadenylated, transferred to the cytoplasm and translated, in order for mitosis to take place. As a result, mitosis will not finish, and cells will arrest at the onset of apoptosis, since not all necessary proteins are translated. Indeed, the decreases of G1 and G2/M phases may imply that MA-10 cells were very sensitive to cordycepin since there was no G2/M phase arrest.
Many evidences have demonstrated that excess ROS in cell could induce cell apoptosis [22, 23, 36, 37]. An increase in ROS is also associated with the activation of redox sensitive JNK/MAPK signaling pathway, which is often involved in the transcriptional activation of genes and posttranslational modifications of proteins necessary for apoptosis [23, 38]. Hence, in the present study, both JNK and ROS inhibitors should be able to inhibit apoptotic effect. Interestingly, JNK inhibitor and ROS inhibitor could not decrease the subG1 ratio, which indicated that both JNK and ROS do not play any role in cordycepin-induced apoptosis in MA-10 cells. However, caspase inhibitor did suppress the subG1 ratio, which highly suggested that caspase pathway was involved in the cordycepin-induced apoptosis in MA-10 cells. Many investigations have illustrated that caspases play essential role in apoptosis [39]. Thus, our observations are not unprecedented.
In the present study, 100 μM and 1 mM cordycepin behaved differently to induce temporal expression of caspases. In 100 μM cordycepin treatment, the maximal expression of caspase-3 and -7 were at 6 and 12 h, respectively. Although there were increasing trends of caspases-8 and -9 expressions at 12 h, no significances were observed. This phenomenon illustrated that there was no caspase initiator to activate effector caspase-3 and -7. It has been shown that other initiators, such as caspase-2 and/or caspase-10 can activate effector caspases [40, 41]. It is possible that 100 μM cordycepin could induce caspase-2 and/or caspase-10 to activate caspase-3 and -7 expressions for cell apoptosis. In 1 mM cordycepin treatment, caspase-9 expression (the initiator) significantly increased first at 12 h, and then caspase-3 (the effector) increased at 24 h, which illustrated a logical temporal phenomenon to induce MA-10 cell apoptosis. The possible mechanism of how different dosages of cordycepin regulating MA-10 cell apoptosis will be interesting to be further investigated.
In general, the induction of caspase-9 is due to the activation of mitochondrial apoptotic pathway, which can be activated by cytochrome c, ROS and JNK pathway and/or caspase-2 to induce cell apoptosis [42, 43]. In the present study, we observed that caspase-9 was activated without any involvement of ROS and JNK. It is possible that cordycepin could induce cytochrome c, caspase-2 or other alternative pathways to induce caspase-9 expression in MA-10 cells. This indecision will be further examined.
It has been shown that cordycepin is an analog of adenosine. The possibility of adenosine receptor involved in apoptotic effect on some types of cancer cell is also proposed [44]. Thus, it is possible that cordycepin might associate with adenosine receptor to activate apoptosis in MA-10 cells. Future works regarding the investigation on the relations to adenosine receptor in MA-10 cells will be valuable.
In conclusion, cordycepin could induce the decrease of G1 and G2/M cell numbers and the increase of subG1 cell number, followed by significant apoptotic cell death in MA-10 mouse Leydig cell line. The expressions of caspases also demonstrate that cordycepin did induce apoptosis on MA-10 mouse Leydig tumor cell line. Many Chinese herbs and its pure compounds have been demonstrated with the anti-cancer effects [45, 46]. This promising observation about the apoptotic effect of cordycepin on testicular cancer cell may be considered as a good approach in search and development of new anti-cancer drug.
Funding
National Science Council (NSC 96-2320-B-006-059-MY3 to B.-M. Huang), Taiwan, Republic of China.
Acknowledgments
C.-Y. Jen and C.-Y. Lin contribute equally to this work.
References
- 1.Cunningham KG, Manson W, Spring FS, Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of cordyceps militaris (Linn.) Link. Nature. 1950;166(4231):p. 949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- 2.Cui XM. Artificial culture of Cordyceps sinensis . Asia Pacific Biotech News. 1999;3:333–337. [Google Scholar]
- 3.Zhou X, Meyer CU, Schmidtke P, Zepp F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. European Journal of Pharmacology. 2002;453(2-3):309–317. doi: 10.1016/s0014-2999(02)02359-2. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura K, Yoshikawa N, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumor effect of cordycepin (3′-deoxyadenosine) on mouse melanoma and lung carcinoma cells involves adenosine A3 receptor stimulation. Anticancer Research. 2006;26(1):43–47. [PubMed] [Google Scholar]
- 5.Wu W-C, Hsiao J-R, Lian Y-Y, Lin C-Y, Huang B-M. The apoptotic effect of cordycepin on human OEC-M1 oral cancer cell line. Cancer Chemotherapy and Pharmacology. 2007;60(1):103–111. doi: 10.1007/s00280-006-0354-y. [DOI] [PubMed] [Google Scholar]
- 6.Thomadaki H, Tsiapalis CM, Scorilas A. Polyadenylate polymerase modulations in human epithelioid cervix and breast cancer cell lines, treated with etoposide or cordycepin, follow cell cycle rather than apoptosis induction. Biological Chemistry. 2005;386(5):471–480. doi: 10.1515/BC.2005.056. [DOI] [PubMed] [Google Scholar]
- 7.Lallas GC, Courtis N, Havredaki M. K562 cell sensitization to 5-fluorouracil- or interferon-alpha-induced apoptosis via cordycepin (3′-deoxyadenosine): fine control of cell apoptosis via poly(A) polymerase upregulation. International Journal of Biological Markers. 2004;19(1):58–66. doi: 10.1177/172460080401900108. [DOI] [PubMed] [Google Scholar]
- 8.Cory S. Cell death throes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12077–12079. doi: 10.1073/pnas.95.21.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S. Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sciences. 2001;69(25-26):2957–2964. doi: 10.1016/s0024-3205(01)01404-7. [DOI] [PubMed] [Google Scholar]
- 10.Thomadaki H, Tsiapalis CM, Scorilas A. The effect of the polyadenylation inhibitor cordycepin on human Molt-4 and Daudi leukaemia and lymphoma cell lines. Cancer Chemotherapy and Pharmacology. 2008;61(4):703–711. doi: 10.1007/s00280-007-0533-5. [DOI] [PubMed] [Google Scholar]
- 11.Thomadaki H, Scorilas A, Tsiapalis CM, Havredaki M. The role of cordycepin in cancer treatment via induction or inhibition of apoptosis: implication of polyadenylation in a cell type specific manner. Cancer Chemotherapy and Pharmacology. 2008;61:251–265. doi: 10.1007/s00280-007-0467-y. [DOI] [PubMed] [Google Scholar]
- 12.Cryns V, Yuan J. Proteases to die for. Genes and Development. 1998;12(11):1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 14.Bauer MKA, Wesselborg S, Schulze-Osthoff K. The Caenorhabditis elegans death protein Ced-4 contains a motif with similarity to the mammalian ’death effector domain’. FEBS Letters. 1997;402(2-3):256–258. doi: 10.1016/s0014-5793(96)01497-4. [DOI] [PubMed] [Google Scholar]
- 15.Srinivasula SM, Ahmad M, MacFarlane M, et al. Generation of constitutively active recombinant caspases-3 and -6 by rearrangement of their subunits. The Journal of Biological Chemistry. 1998;273(17):10107–10111. doi: 10.1074/jbc.273.17.10107. [DOI] [PubMed] [Google Scholar]
- 16.Van de Craen M, Van Loo G, Declercq W, et al. Molecular cloning and identification of murine caspase-8. Journal of Molecular Biology. 1998;284(4):1017–1026. doi: 10.1006/jmbi.1998.2226. [DOI] [PubMed] [Google Scholar]
- 17.Weston CR, Davis RJ. The JNK signal transduction pathway. Current Opinion in Cell Biology. 2007;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nature Cell Biology. 2002;4(5):E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 19.Davies KJA. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999;48(1):41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 20.Matés JM, Sánchez-Jiménez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. International Journal of Biochemistry and Cell Biology. 2000;32(2):157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 21.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radical Biology and Medicine. 2000;28(10):1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 22.Carmody RJ, Cotter TG. Signalling apoptosis: a radical approach. Redox Report. 2001;6(2):77–90. doi: 10.1179/135100001101536085. [DOI] [PubMed] [Google Scholar]
- 23.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resistance Updates. 2004;7(2):97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Yang H-Y, Leu S-F, Wang Y-K, Wu C-S, Huang B-M. Cordyceps sinensis mycelium induces MA-10 mouse leydig tumor cell apoptosis by activating the caspase-8 pathway and suppressing the NF-κB pathway. Archives of Andrology. 2006;52(2):103–110. doi: 10.1080/01485010500315818. [DOI] [PubMed] [Google Scholar]
- 25.McKiernan JM, Goluboff ET, Liberson GL, Golden R, Fisch H. Rising risk of testicular cancer by birth cohort in the United States from 1973 to 1995. Journal of Urology. 1999;162(2):361–363. [PubMed] [Google Scholar]
- 26.Tortosa E, Avila J, Pérez M. Acetylsalicylic acid decreases tau phosphorylation at serine 422. Neuroscience Letters. 2006;396(1):77–80. doi: 10.1016/j.neulet.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 27.Ascoli M. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology. 1981;108(1):88–95. doi: 10.1210/endo-108-1-88. [DOI] [PubMed] [Google Scholar]
- 28.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival—modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 29.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. Journal of Immunological Methods. 1991;139(2):271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 30.Wang B-J, Won S-J, Yu Z-R, Su C-L. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food and Chemical Toxicology. 2005;43(4):543–552. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Chen Y-C, Huang Y-L, Huang B-M. Cordyceps sinensis mycelium activates PKA and PKC signal pathways to stimulate steroidogenesis in MA-10 mouse Leydig tumor cells. International Journal of Biochemistry and Cell Biology. 2005;37(1):214–223. doi: 10.1016/j.biocel.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 34.Colgan DF, Murthy KGK, Zhao W, Prives C, Manley JL. Inhibition of poly(A) polymerase requires p34(cdc2)/cyclin B phosphorylation of multiple consensus and non-consensus sites. The EMBO Journal. 1998;17(4):1053–1062. doi: 10.1093/emboj/17.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martincic K, Campbell R, Edwalds-Gilbert G, Souan L, Lotze MT, Milcarek C. Increase in the 64-kDa subunit of the polyadenylation/cleavage stimulatory factor during the G0 to S phase transition. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(19):11095–11100. doi: 10.1073/pnas.95.19.11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLean L, Soto U, Agama K, et al. Aminoflavone induces oxidative DNA damage and reactive oxidative species-mediated apoptosis in breast cancer cells. International Journal of Cancer. 2008;122(7):1665–1674. doi: 10.1002/ijc.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Cheung H-Y, Zhang Z, Chan GKL, Fong W-F. Andrographolide induces cell cycle arrest at G2/M phase and cell death in HepG2 cells via alteration of reactive oxygen species. European Journal of Pharmacology. 2007;568(1–3):31–44. doi: 10.1016/j.ejphar.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Sinha-Hikim I, Braga M, Shen R, Sinha Hikim AP. Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: role of testosterone. Apoptosis. 2007;12(11):1965–1978. doi: 10.1007/s10495-007-0120-6. [DOI] [PubMed] [Google Scholar]
- 39.Turk B, Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Letters. 2007;581(15):2761–2767. doi: 10.1016/j.febslet.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 40.Filomenko R, Prévotat L, Rébé C, et al. Caspase-10 involvement in cytotoxic drug-induced apoptosis of tumor cells. Oncogene. 2006;25(58):7635–7645. doi: 10.1038/sj.onc.1209733. [DOI] [PubMed] [Google Scholar]
- 41.Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304(5672):843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 42.Guerrero AD, Chen M, Wang J. Delineation of the caspase-9 signaling cascade. Apoptosis. 2008;13(1):177–186. doi: 10.1007/s10495-007-0139-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang CCC, Chiang Y-M, Sung S-C, Hsu Y-L, Chang J-K, Kuo P-L. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Letters. 2008;259(1):82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa N, Nakamura K, Yamaguchi Y, Kagota S, Shinozuka K, Kunitomo M. Antitumour activity of cordycepin in mice. Clinical and Experimental Pharmacology ’ Physiology. 2004;31(supplement 2):S51–S53. doi: 10.1111/j.1440-1681.2004.04108.x. [DOI] [PubMed] [Google Scholar]
- 45.Salvioli S, Sikora E, Cooper EL, Franceschi C. Curcumin in cell death processes: a challenge for CAM of age-related pathologies. Evidence-Based Complementary and Alternative Medicine. 2007;4(2):181–190. doi: 10.1093/ecam/nem043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Firenzuoli F, Gori L, Lombardo G. The medicinal mushroom Agaricus blazei murrill: review of literature and pharmaco-toxicological problems. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):3–15. doi: 10.1093/ecam/nem007. [DOI] [PMC free article] [PubMed] [Google Scholar]


