Abstract
Hedgehog is a ligand-activated signaling pathway that regulates Gli-mediated transcription. Although most noted for its role as an embryonic morphogen, hyperactive hedgehog also causes human skin and brain malignancies. The hedgehog-related gene anomalies found in these tumors are rarely found in prostate cancer. Yet surveys of human prostate tumors show concordance of high expression of hedgehog ligands and Gli2 that correlate with the potential for metastasis and therapy-resistant behavior. Likewise, prostate cancer cell lines express hedgehog target genes, and their growth and survival is affected by hedgehog/Gli inhibitors. To date, the preponderance of data supports the idea that prostate tumors benefit from a paracrine hedgehog microenvironment similar to the developing prostate. Uncertainty remains as to whether hedgehog’s influence in prostate cancer also includes aspects of tumor cell autocrine-like signaling. The recent findings that Gli proteins interact with the androgen receptor and affect its transcriptional output have helped to identify a novel pathway through which hedgehog/Gli might affect prostate tumor behavior and raises questions as to whether hedgehog signaling in prostate cancer cells is suitably measured by the expression of Gli target genes alone.
Keywords: androgen signaling, cyclopamine, Gli, hedgehog signaling, prostate cancer, Smoothened
Hedgehog is a cell signaling pathway that is most noted for its involvement in embryogenesis. Increasingly, however, inappropriate hedgehog signaling activity is viewed as a factor in the development of human malignancy or as a factor involved in the acquisition of aggressive behaviors of already established tumors. Here, we review the putative role(s) of hedgehog signaling in prostate cancer. Prostate cancer is a challenging disease. Aside from the fact that it is the most common malignancy in males [201], it poses a considerable dilemma for public health policy with regards to screening and treatment issues. For example, even though prostate tumors are highly invasive, the majority of afflicted men experience prostate cancer as an indolent disease with a relatively slow growth rate [1]. Since it is usually diagnosed in men older than 60 years of age, the predominance of indolent prostate cancers raises questions regarding the effectiveness of prostate cancer screening efforts that are thought to identify large numbers of patients for whom the treatment may be more problematic than the tumor itself [2–4]. These facts highlight the need to understand the etiology that underlies the widespread occurrence of this disease and to develop a means of selectively diagnosing those individuals with aggressive form(s).
Second, despite the abundance of indolent disease, owing to its overall high incidence, prostate cancer remains a leading cause of deaths from cancer in males [201]. This fact underscores the urgent need for better treatments for aggressive disease to reduce mortality. Finally, prostate cancer, in contrast to other human tumors, is distinguished by a remarkable dependency on androgenic steroids. Prostate cancer only arises in androgenically intact males, and, when it has spread beyond the confines of the prostate, is commonly treated by hormone therapies that deplete the patient’s circulating androgenic steroid levels [5,6]. Acutely, androgen-deprivation therapies can be very effective and can shrink both primary and metastatic tumors while slowing the growth of residual tumor cells. With chronic use, however, hormone therapies usually prove to be only palliative; patients often recur with more aggressive, therapy-resistant disease referred to as castration-recurrent prostate cancer (CRPC). Here the tumor cells are able to grow in a seemingly androgen-independent (AI) fashion, and this is the form of disease that is overwhelmingly associated with mortality from prostate cancer. Despite the behavior of CRPC tumor cells, whose ability to grow in castrated patients mimics that of tumor cells that are completely independent of androgens, there is extensive evidence that CRPC cells continue to utilize their endogenous androgen signaling system to drive their growth. Enigmatically, CRPC cells are believed to have acquired the means to maintain androgen signaling even though the systemic milieu of androgens in hormone-treated patients remains at castrate levels [7–10]. Since CRPC cells remain dependent on androgen signaling to grow, this dilemma creates the need to understand the molecular process(es) that enables androgen receptors (ARs) in the CRPC cell to continue to function in the castrate state. With this understanding, one might be able to conceive novel therapies to block the aberrant androgen signaling in CRPC cells and extend the effectiveness of hormone therapies in prostate cancer patients.
The focus here on hedgehog signaling in prostate cancer is driven by a growing body of literature that addresses various aspects of the signaling pathway in prostate tumors or in prostate cancer cells. This literature is plagued by contradictions and controversies, yet, despite these problems, many investigators continue to view the outcomes of their studies as evidence for involvement of hedgehog signaling in prostate cancer development or in progression of prostate tumors to aggressive or therapy-resistant states. In addition, the outcomes of some preclinical studies that showed some striking effects of hedgehog-blocking drugs in animal-based prostate cancer models give strong reason to consider whether these types of therapies might have value for prostate cancer patients, especially those with advanced or therapy-resistant disease.
Abnormal (hyperactive) hedgehog signaling is already established as being a causative factor for the development of certain types of human skin, brain or cartilage-derived tumors (discussed later). Likewise, published literature supports the potential for the involvement of particular aspects of the hedgehog/Gli signaling pathway in other types of solid human tumors [11–16]. Here we will first address the nature of hedgehog signaling in normal and malignant cells and then describe the literature that suggests that hedgehog contributes to human prostate cancer. We will address the controversy as to whether hedgehog acts in prostate cancer exclusively through a paracrine response pathway that mimics hedgehog’s involvement in normal prostate development or whether there is any evidence to support a role for a tumor cell-autonomous hedgehog signaling process similar to that found in basal cell carcinoma and medulloblastoma. We will also propose that hedgehog may have an especially important role in promoting progression of prostate cancer to CRPC, at least partly through Gli support of abnormal androgen signaling in tumors of patients subsequent to hormone therapy. While the validation of any potential relationship between prostate cancer and hedgehog signaling or between the aggressive behavior of the CRPC cell and hedgehog/Gli might provide insights leading to improved diagnosis or prognostication of disease behavior, the availability of several small-molecule inhibitors that target hedgehog/Gli at different parts of the signaling pathway suggests that the most useful benefit in exploring this relationship lies in the possibility of using hedgehog-/Gli-blocking drugs to treat patients with advanced or hormone therapy-resistant disease who currently have a very poor prognosis.
Overview of the hedgehog signaling pathway
Hedgehog is considered to be one of the primal cell signaling pathways that regulates cell fate during embryonic development (along with Wnt and Notch) [17–19]. Originally discovered in Drosophila, this signaling pathway acquired its name from the distinctive morphology of certain mutant larvae that were characteristically short and stubby with clustered, spine-like denticles that occurred as a consequence of disruption of the normal anterior–posterior segmental pattern formation during embryogenesis [20]. This developmental anomaly was then attributed to a mutation in a drosophila gene termed ‘hedgehog’ that encodes a secreted polypeptide (ligand) that can initiate hedgehog signaling in receptive drosophila cells [21]. We now know that some form of hedgehog signaling is evolutionarily conserved throughout metazoans and that hedgehog is an important tissue morphogen that participates in the establishment of embryonic polarity and the early patterning of tissues that sets the stage for acquisition of adult tissue structure and function.
Canonical hedgehog signaling is initiated by peptide ligands that are still referred to as hedgehogs, and it serves, at the end point, to activate transcription from the Gli family of transcription factors in responsive cells. Humans have three gene homologs that encode hedgehog ligands (Sonic [Shh], Indian [Ihh] or Desert [Dhh] hedgehog) [22,23]. Shh is the most well studied and is predominant with regards to its more widespread expression throughout different tissues of the body, although all can similarly engage with receptor to initiate the signaling process. Shh is synthesized as a propolypeptide that is processed by a unique autocatalytic reaction in which the C-terminal domain catalyzes a cholesterol-dependent internal cleavage of the pro-form that simultaneously attaches a cholesterol moiety to the cleaved N-terminal domain [24]. The autocatalysis is not sufficient for secretion of the mature ligand; this requires the action of an independent membrane protein referred to as Dispatched [25]. Cholesterol-modified mature Shh is inherently highly hydrophobic and this can limit its diffusion away from the cells that secrete it. The short-acting nature of the hedgehog signaling process in early development helps to promote the formation of patterns in tissues that are based upon ligand diffusion gradients that restrict ligand access to target cells more distal from the hedgehog-secreting cells.
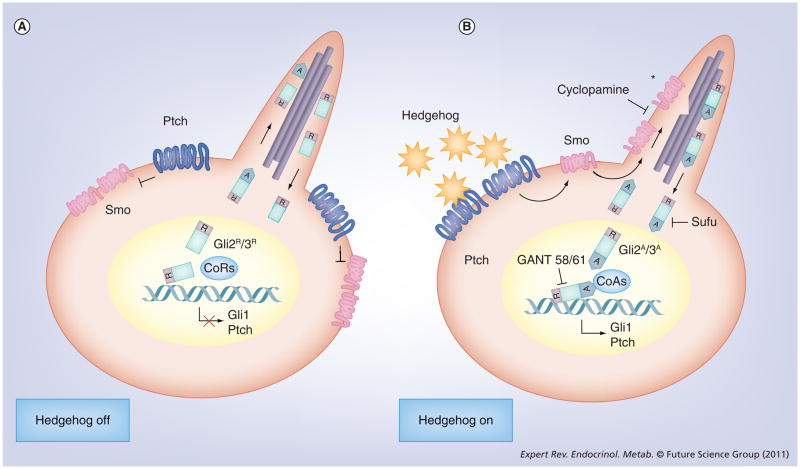
The signaling process proceeds when the mature ligand engages a receptor on a target cell and, for hedgehog, proteins of the Patched (Ptch) family serve this purpose. Ptch proteins are large, 12-pass membrane proteins, and humans encode two homologs [26], Ptch1 and Ptch2, with differing affinities for hedgehog ligands and differential expression in various tissues of the body. A diagram of the general intracellular process that accompanies hedgehog signaling is shown in Figure 1. It should be noted that the brief schema described here is specific for vertebrate-derived cells as evolution from invertebrates was accompanied by modifications that tether the proximal stage of hedgehog signal processing to the subcellular organelle referred to as the primary cilia [27,28]. The integration of hedgehog signaling into the primary cilia provides vertebrate cells with unique opportunities to regulate the signaling process, but the linkage also has some important implications for our understanding of hedgehog action in human tumors, as will be discussed later. Likewise, vertebrates have a more complex end-response to hedgehog signaling through evolutionary divergence of the function of the invertebrate Ci transcription factor that is activated by hedgehog onto three different Gli proteins (Gli1, 2 and 3) in vertebrates [29,30]. Since the topic of this treatise is human prostate cancer, hereafter our discussion will focus on the signaling pathway as it is known to function in higher vertebrates (mouse through humans).
Figure 1. Schematic of the hedgehog signaling process in a target cell.
(A) Hedgehog-off. In the absence of hedgehog ligand, Ptch gates the movement of Smo into the primary cilia and prevents its activation. Without activated Smo, Gli2/3 cilia where they are processed to remove the C-terminal activation domain. Lacking this domain, the truncated Gli proteins exit the cilia and migrate into the nucleus where they bind to Gli-response elements on DNA and attract a transcription corepressor protein complex that blocks transcription of Gli target genes. (B) Hedgehog-on. Hedgehog ligand binds to Ptch and enable Smo to traffic into cilia where it becomes activated (*). With activated Smo in the primary cilia, Gli protein processing to the repressor Gli2/3 proteins exit the primary cilia with an activation domain intact and they can enter the nucleus, bind to Gli-response elements on DNA and attract a transcription coactivator protein complex that enables transcription of Gli target genes.
CoA: Coactivator; CoR: Corepressor; GANT: Gli antagonist; GliA: Activated Gli; GliR: Repressed Gli; Ptch: Patched; Smo: Smoothened; Sufu: Suppressor of fused.
Ligand engagement of Ptch relieves repression of the Smoothened (Smo) protein that is required for further signaling. Smo, a seven-pass transmembrane protein of the extended G-protein-coupled receptor family, has an active and an inactivate state that appears to be defined both by its location within the cell (inside or outside of the primary cilia) [31] and by other modifications that may include its ability to capture oxysterols at an active site [32,33]. Smo activation requires two steps that were operationally defined by certain low-molecular-weight compounds that disrupt the activation process [34]. The first step involves the movement of Smo proteins from the plasma membrane and endoplasmic vesicles into primary cilium and here unliganded Ptch acts as a gatekeeper that restricts access of Smo to the primary cilium. Ptch action in this regard is mimicked by the drug, SANT-1, which similarly suppresses ciliary accumulation of Smo, even in the presence of ligand [35,36]. Once in the primary cilia, however, Smo activation requires a secondary step that is also regulated by Ptch, and this activation step is operationally defined by inhibition with cyclopamine or derivatives that allow Smo ciliary accumulation but prevent any further downstream signaling activities. The nature of the secondary Smo activation event remains enigmatic, although it probably involves a conformational shift and/or a change in Smo interaction with other ciliary proteins that are involved in hedgehog signal processing. Regardless of our understanding of this particular event, the presence of active Smo within primary cilia induces a functional change in the organelle that fundamentally alters the manner in which the two dominant Gli proteins, Gli2 and Gli3, are post-translationally processed.
As transcription factors with shared function, all Gli proteins have a homologous internal DNA-binding domain that recognizes and binds a cis-regulatory consensus motif on DNA: G–A–C–C–A–C–C–A [37]. The lack of this consensus sequence within or near any given gene does not preclude regulation by Gli since functional nonconsensus binding sites are also described [38]. Given their nature as transcription factors, all Gli proteins also possess activation domains within their C-terminal region that interact with other transcriptional accessory proteins needed for the chromatin remodeling involved in active transcription. Outside of this organizational similarity, however, there are distinct differences between the three homologs that provide the basis for separation of functions in the Gli-mediated transcription process. For one, the proteins encoded by Gli2 and 3 also possess repressor domains within their N-terminus that can preferentially attract corepressor protein complexes to the DNA-binding sites when the activation domain is proteolytically removed [39,40]. It is the relative efficiency with which these two Gli forms are specifically proteolyzed that distinguishes the inactive versus the active hedgehog signaling state. In the absence of activated Smo, Gli2 and 3 proteins traffic into primary cilium where they are modified into repressor forms [41]. This process is initiated by a series of sequential phosphorylations, initiated by protein kinase A and then followed by glycogen synthase kinase-3-β and casein kinase 1. Following phosphorylation, the Gli2/3 repressor forms are generated by proteolysis that may be guided by site-directed ubiquitylation under the control of SCF-βTRCP [42]. The Gli2/3 modification and proteolytic process also requires the presence of certain ciliary kinesin motor proteins to shepherd Glis through the primary cilium and to scaffold the modification complex during the process [27]. The Gli2/3 proteins are also distinguished by their differing contributions to the repressive or activated Gli state of a cell. Whereas native Gli2 is a more avid transcriptional activator than native Gli3, cleaved Gli3 is a stronger transcriptional repressor when compared with cleaved Gli2, so the intensity of the response to hedgehog signaling in a target cell also depends upon the relative expression levels of the two different proteins in that cell. Gli proteins are also targeted for ubiquitylation by the SPOP ubiquitin ligase [43] but it is unclear whether proteasomal degradation under this element is involved in the specific generation of repressor forms rather than their generalized degradation along with Gli1 [44]. In summary, the presence of activated Smo within the primary cilium suppresses the generation of the Gli2/3 repressor forms so they accumulate within the primary cilium in this state. They are also much more likely to exit the cilium with an intact C-terminal domain that is able to enter the nucleus, bind to Gli response elements and capture the chromatin accessory proteins required for an active transcription complex.
Given the importance of hedgehog/Gli signaling for vertebrate development and cancers, there is considerable interest in the targets of active Gli-mediated transcription. Here, it is somewhat ironic that the most well-recognized targets of active Gli transcription include Gli1 and the Ptch genes that are mechanistically involved in the signaling process [45]. The nature of the Gli1 protein, which lacks a repressor form, and its short-lived character suggests that it functions mainly as a means for amplifying the output of the hedgehog signaling process once it is initiated. Indeed, this function is consistent with lack of an overt phenotype in Gli1-knockout mice, whereas Gli2- or Gli3-knockouts are more severely affected [46,47]. By contrast, Ptch upregulation by active hedgehog provides a means to eventually diminish the activity of the signaling process once initiated, so this action appears to be part of a negative-feedback loop controlling hedgehog activity in any given target cell. Other genes reported to be hedgehog targets include hedgehog-interacting protein (HIP), whose gene product also feeds back to diminish local signaling activity; cell cycle regulators, including N-myc, cyclin D1 and D2, which may partially explain hedgehog effects on cell growth; effectors of other developmental signaling pathways including Wnt and Notch ligands and other gene products (bcl-2, FOX transcription factors, bone morphogenetic proteins and follistatin) (Table 1) that are probably associated with differentiated states. In summary, the spectrum of known hedgehog target genes reveals the autoregulating nature of the signaling pathway and explains its obvious involvement in developmental organization of tissues, cell growth and differentiation.
Table 1.
Genes that are known to be regulated by Gli binding.
| Gli-regulated genes | Ref. |
|---|---|
| GLI1 | [113] |
| PTCH1 | [114] |
| HHIP | [115] |
| CDKN2A/p16 | [116] |
| CCND2/cyclin D2 | [117] |
| MYCN/N-myc | [118] |
| CDK2 | [119] |
| FOXA2 | [120] |
| FOXM1 | [121] |
| FOXE1 | [122] |
| JUN | [123] |
| NKX2–1/Nkx2.1 | [124] |
| NKX2–2/Nkx2.2 | [124] |
| EGR2/Krox20 | [125] |
| PRDM1/Blimp1 | [126] |
| IGFBP3 | [127] |
| IGFBP6 | [117] |
| SFRP1 | [128] |
| FST | [129] |
| SPP1/OPN | [117] |
| RAB34 | [124] |
| RGS4 | [127] |
| BCL2 | [130] |
| EDN2/ET-2 | [131] |
| JUP/PKGB | [117] |
| FBN2 | [127] |
The complex and unique characteristics of the basic hedgehog signaling process, described in the previous section, allows for its regulation at many alternative steps. These include interference with hedgehog ligand processing, release or receptor binding by effectors of sterol biosynthesis [32] or direct interference with mature ligand function by the presence of the HIP protein that binds to ligands and prevents their interaction with receptors [48]. For the target cell, hedgehog signaling can be facilitated by the presence of heparin proteoglycans and lower affinity hedgehog coreceptor proteins that include CDON and BOC [49]. Further downstream, integration of vertebrate hedgehog signaling into the primary cilium means that signal processing requires the activities of numerous ciliar transport proteins to shuttle Gli proteins into and out of the cilium [41,50,51]. Genetic ablation of individual ciliar transport proteins in mice confers phenotypes that are reiterative of mutations in the primary hedgehog regulatory genes. End-stage Gli transcriptional activity is also affected by acetylation or sumoylation of the Gli proteins [52,53]. Finally, Gli transcriptional function is tempered by the presence of the multifunctional SuFu protein that can bind and sequester Gli active forms in the cytoplasm or attract transcriptional corepressors to activator Gli complexes already bound to chromatin [54,55]. The multiplicity of alternative regulatory sites along the hedgehog signaling cascade provides copious opportunities for signal facilitation or interference and it complicates attempts to understand the reason why hedgehog signaling abnormalities strongly underlie certain types of developmental defects or malignancies but not others.
Another notable aspect of hedgehog signaling is its remarkably sensitivity to small-molecule manipulation. This is mainly attributable to the unique nature of the Smo molecule, whose activity is strongly influenced by its association with sterols or other low-molecular-weight compounds. Sterol-like compounds, such as Smo agonist [35] or purmorphomine [56], promote the activated Smo state and these molecules provide an alternative means of antagonizing hedgehog for experimental purposes. By contrast, sterols modeled after the phyto-derived jerveratrum alkaloid, cyclopamine, strongly inhibit Smo activation and these drugs are frequently used experimentally to antagonize hedgehog signaling [57]. The evidence that hyperactive hedgehog signaling plays a role in human cancers has been a tremendous impetus for the discovery of novel compounds that might be used for the purpose of therapeutics and these efforts have resulted in the identification of numerous other low-molecular-weight compounds that can antagonize hedgehog or block Gli action. Since many of these newer compounds are being considered for clinical utilization in oncology, we will assess the spectrum of potential hedgehog/Gli-targeting agents in a later section of this article.
Hedgehog in prostate development
Hedgehog’s importance as a developmental morphogen for vertebrates is established by the striking developmental anomalies that are associated with abrogation of pathway activity. Loss of Shh, Gli2 or Gli3 function in mutant or knockout mice can be embryonically lethal or result in the death of the neonate shortly after birth associated with developmental defects that include holoproencephaly/cyclopism [58], spinal cord anomalies and other neuronal deficits [59], defects in the formation of the axial skeleton and limbs [60], underdeveloped lungs, and anorectal malformations that include persistent cloaca [61], depending on the severity of the pathway ablation. For males, sexual accessory tissue development is also affected by hedgehog deficiencies and this effect includes hypodevelopment of the prostate gland.
The prostate gland is derived from the embryonic urogenital sinus (UGS) and Shh is expressed in rodent and human UGS and in the buds and ducts that outgrow from it during the process of prostate organogenesis and maturation [62]. Embryonic male mice that lack functional Shh as a consequence of homozygous mutation fail to show the early inductive budding from the UGS that initiates prostate formation [63,64]. However, it is remarkable that inductive budding can be restored simply by supplementing testosterone to the female mouse (in vivo) or to isolated mutant male UGS tissues (in vitro) [63]. These observations are highly consistent with a requirement of hedgehog for embryonic testicular steroidogenesis and fetal androgenization that guides the inductive phase of male sexual accessory tissue development [65] and they are inconsistent with the idea that any prostate-autonomous hedgehog activity is required for initial organogenesis. Despite the evidence that prostate-autonomous Shh is unnecessary for UGS inductive budding, later embryonic ductal branching and neonatal maturation of the rodent prostate gland is markedly hampered by the lack of Shh, even when supplemental testosterone is provided. Thus, the secondary budding and ductal extension associated with late embryonic and neonatal prostate development is dependent upon prostate-autonomous hedgehog signaling. This developmental situation may be analogous to the regrowth of the regressed prostate in chronically castrated adult rodents that occurs subsequent to testosterone replenishment. Here, cyclopamine treatment was shown to block the androgen-stimulated regrowth of the regressed adult mouse prostate associated with testosterone replacement and this outcome suggests that testosterone replacement induces hedgehog expression needed for prostate ductal expansion in adults [66].
With regards to the nature of the hedgehog signaling process in the developing prostate, in situ hybridization and immunohistochemical analyses of embryonic or neonatal mouse and rat tissues tends to localize expression of Shh to the epithelium of the rodent UGS and to the growing tips of the prostate epithelial buds as they invade into the surrounding mesodermally derived mesenchyme [67–70]. By contrast, Ptch and Gli1 (the surrogate Gli target gene) were found to be mainly expressed by UGS mesenchyme or stromal cells adjacent to buds of the developing prostate gland that also stain positive for smooth muscle actin. The striking juxtaposition of ligand expression restricted to the developing prostate epithelium with receptor and target gene expression that is mainly found in the adjacent mesenchyme shows that hedgehog encompasses a typical paracrine signaling process in the developing prostate that is characteristic of the hedgehog signaling paradigm in other types of developing tissues. There are, however, some reports that also find reduced expression of Ptch1 and Gli1 in the epithelium at bud tips [67] and these findings raise questions that extend to human prostate cancer tissue studies as to whether there may be some autocrine-like hedgehog activity in prostate epithelial cells that manifests exclusively under conditions of rapid growth.
Hedgehog & human cancers
Genetically manipulated mouse models have established an oncogenic role for hedgehog signaling in certain tissues that is remarkably predictive of the occurrence of proven hedgehog-driven tumors in humans. Mice with haploinsufficieny of Ptch1 [71,72], or those with haploinsufficiency of SuFu when combined with p53 haploinsufficiency [73], develop a common spectrum of cutaneous, brain and cartilaginous tumors that corresponds to the specific types of gene anomalies found in basal cell (skin) carcinoma (BCC), medulloblastomas or rhabdomyosarcomas in humans [74]. These types of tumors often have reduced Ptch1 expression associated with loss of heterozygosity at 9q22 (the Ptch1 locus), which may or may not be associated with a mutation in the remaining Ptch allele [75]. Likewise, inactivating mutations in Ptch or SuFu underlie the Gorlin syndrome that predisposes to the development of BCC and/or medulloblastoma [76,77]. Conversely, mutations in the Smo gene that confer gain-of-function to the encoded protein are also found in human BCCs and, rarely, in medulloblastomas [78], but exogenous targeted expression of a mutant human Smo gene from BCC in transgenic mice similarly induces cutaneous carcinomas, medulloblastomas and rhabdomyosarcomas. Collectively, the reiteration of tumor development in mice by the same genetic aberrations that are found in human tumors of the same class validates the oncogenic nature of unrestricted hedgehog/Gli signaling in this limited subset of tissues. Although these types of genetic lesions confer the appearance of ‘autocrine-like’ autonomous hedgehog signaling activity in the tumor cell, the abnormal activity is independent of the presence of hedgehog ligands in the tumor microenvironment.
Despite the lack of prevailing evidence for the occurrence of genetic lesions of the type previously described in most other types of solid human tumors, considerable interest remains in the potential roles of hedgehog or Gli, especially for lung, breast, pancreas, colon and prostate carcinoma [12,13,66,79,80]. As will be discussed for prostate cancer, the evidence for association usually encompasses findings of high expression of ligand and/or hedgehog target genes in tumor cells or findings that hedgehog/Gli inhibition, usually by cyclopamine or via Gli expression knockdown, suppresses cell growth in vitro or in vivo as tumor xenografts in mice. The outcomes of these experiments are often used to support the idea that some form of autocrine-like hedgehog signaling is constitutively active in these other types of solid tumor cells. Unfortunately, much less effort is made to establish whether, indeed, any or all of these tumors demonstrate any actual autonomous hedgehog signaling activity, and experimental evidence more strongly implicates that these tumor systems are more influenced through paracrine hedgehog [81], much like in the tissues from which these tumors develop. The situation for tumors other than BCC, medulloblastoma or rhabdomyosarcoma is especially complicated by observations that Gli expression can be regulated independently of hedgehog signaling. TGF-β-, β-catenin- and hyperactive RAS/RAF/MEK/ERK-mediated signaling upregulates Gli expression/activity in tumor cells independent of the presence of hedgehog ligand [70,82,83] and hyperactivity of these alternate cell signaling pathways is known to occur in many different types of cancer. Given the existence of alternative pathways to Gli expression, one should certainly consider whether simple overexpression of Gli, when combined with post-translational processing deficits that fail to generate Gli repressor forms, would be sufficient to explain Gli involvement in them without invoking further upstream hedgehog activities. This is a paradox that we will explore in our focus on prostate cancer.
Finally, the requirement for the primary cilium to process canonical hedgehog signaling in normal cells raises other questions regarding the existence of active hedgehog signaling in cancers that may lack hedgehog-activating mutations since primary cilia are mainly formed on growth-arrested cells whereas cancer cells, especially in culture, usually lack these organelles [84]. The apparent absence of primary cilium in dividing cancer cells then raises critical questions as to how Smo might transition to the active form in cancer cells without activating mutations or evidence of other hedgehog signaling anomalies, and this is an area of research in which we hope to have advances in the coming years.
For those tumor systems that are commonly associated with hyperactive Smo function (due to loss of Ptch function or Smo mutations), there is good reason to consider the testing and use of Smo-targeting agents as anticancer therapeutics. Whereas there was some initial interest in the use of cyclopamine in clinical practice, this agent has critical attributes that make it unfavorable for this purpose and these include its poor availability through nonvenous routes, as well as concerns that it has off-target effects, especially at higher doses [85]. Nonetheless, the remarkable sensitivity of Smo to small-molecule inhibition has encouraged discovery efforts to identify agents that act in a similar way to cyclopamine (by inhibiting Smo activation) with more favorable clinical profiles. Two contemporary Smo-targeting agents, GDC-0449 and IPI-926, are already subject to clinical testing in human patients [86–88]. Use of GDC-0449 alone in Phase I testing has already demonstrated evidence of objective responses for some cancers [88] and investigators are already considering the possible benefit of combining Smo-targeting drugs with other targeted therapeutics for cancers [89] to improve the response. Considering the evidence that many solid tumors benefit from a paracrine hedgehog signaling environment, Smo-targeting drugs could provide an adjuvant therapy to suppress the hedgehog signaling microenvironment of the tumor and open clinical trials for GDC-0049 are actively accruing patients with these alternate solid tumors. Similar effects might be afforded by agents that target hedgehog ligand processing and interaction with receptors. Robotnikinin, a drug that blocks the interaction of Shh with receptors [90], is of this class. Further down the pathway, the knowledge that Gli activity may be an important factor in tumor biology, independent of hedgehog signaling, has also driven discovery efforts to identify drugs that can block this activity, and the Gli antagonists (GANTs; -58 and -61) [91], and, more recently, the HPI class of drugs [92] that interfere with Gli trafficking and transcription, may have clinical applicability. Finally, the actions of arsenic trioxide, which is being tested as a solid tumor therapeutic [93], may also include the inactivation of Gli function in cancer cells [94,95] so this drug may provide an alternative option for hedgehog targeting in cancers.
Overview of hedgehog/Gli in prostate cancer
The involvement of hedgehog signaling in prostate development forms a foundation for considering whether hedgehog/Gli might have some role in prostate malignancy. This concept received substantial impetus from two early reports of cyclopamine- or Shh antibody-mediated suppression of prostate cancer cell growth in vitro and in vivo [66,96], and the outcomes of these experimental studies were viewed as evidence for an active auto-crine-like hedgehog signaling process in these cell lines. This conclusion should now be reconsidered, especially in light of the concerns discussed previously. A review of relevant literature on this topic with these new perspectives shows remarkable weaknesses in the argument that autocrine hedgehog has an important role in the development of prostate cancer. For one, the genetically altered mouse models that were so useful for establishing a relationship between abnormally hyperactive hedgehog signaling and the development of skin and brain malignancies have not shown any evidence that such aberrations lead to the development of prostate neoplasia or malignancy. It is especially notable that even mice with a prostate (epithelial cell)-specific knock-in of gain-of-function mutated Smo gene that is oncogenic when expressed in skin, brain or cartilage, demonstrated no evidence for any type of prostatic pathology [97]. In fact, at this time, the only report of an animal (mouse) model that develops prostate cancer from a hedgehog manipulation involves the direct introduction of a constitutive Shh expression vector into mouse prostate by tissue electroporation [98]. These adult mice uniformly developed prostate intraepithelial neoplasia that rapidly progressed to metastatic prostate adenocarcinoma over time. While this outcome is remarkable and does support the potential for unrestricted hedgehog in prostate cancer development, the electroporation technique lacks the cell-targeting specificity to show that overexpression of Shh in the tissue was acting through any autonomous effect on the prostate epithelium and the outcome could easily be a consequence of an unrestricted hedgehog stimulation of the prostate stroma that destabilizes the tissue, leading to cancer.
With regards to actual human prostate tumors or prostate cancer cell lines, there are no studies identifying abnormalities in Ptch or Smo genes similar to those found in BCC or medulloblastoma. Allelic loss of 9q22 and/or Ptch mutations are not described for this disease, and reports of Smo mutations are similarly lacking, although there is no reason to believe that a screening effort to identify the presence of Smo gene lesions was ever suitably undertaken for prostate cancers. Perhaps the only description of hedgehog-related gene aberrations in prostate cancer involves the finding of two prostate tumors with loss-of-function mutations in the SuFu gene [99]. These mutations were found in a small cohort of tumors in which SuFu immunostaining was also notably reduced. Of further note, the human SuFu gene lies in a chromosomal region (10q24.32) that encompasses an area of frequent allelic loss in prostate cancer. While these coincidences are insufficient to establish a more widespread pattern involving loss of SuFu in prostate cancer development or progression, they do at least establish precedence to seek further evidence that changes in the SuFu gene or in reduced expression of the encoded protein may be a factor in the disease.
Given the paucity of evidence for disruption of genes encoding intermediate hedgehog signaling elements in prostate tumors, what can be learned regarding hedgehog involvement in prostate cancer from gene-expression studies of human prostate tumor specimens? Unfortunately, varied outcomes from the numerous published efforts that describe and quantify expression of hedgehog-related genes in prostate tumors challenge efforts to provide consensus on this issue. There are general concerns that the so-called ‘normal’ regions of human prostate specimens that are available for study might be affected by the common prostate benign disease states that might also invoke abnormal hedgehog responses [100] and this raises questions regarding the establishment of normal prostate basal expression levels for any of these genes. Approaches that assess RNA levels by in situ hybridization are complicated by the uneven cellular architecture of a prostate tumor (in which the cellularity of the stroma can appear sparse compared with the adjacent epithelium) and this might account for the conflicting findings of Gli1 RNAs localized to benign and malignant prostate epithelium in one study [96] versus selective expression in the stroma around tumors in another [100]. Likewise, quantitative reverse-transcriptase PCR approaches that involve bulk extraction from tumor tissues are complicated by the comixtures of tumor and benign stromal cells in the specimens that complicate analysis, so it is difficult to comment on observations based on this approach. In situ immunohistochemical approaches using antibodies against hedgehog-related proteins offer the potential for higher detection specificity, with appropriately validated antibodies, but this approach suffers from a diminished ability to quantify outcomes.
With these considerations, the observations of Azoulay et al., who evaluated hedgehog ligand expressions in a cohort of 231 different prostate tumors, some of which were obtained from patients treated with hormone therapies, were remarkable [101]. They described a significant correlation between high(er) expression of Shh in malignant epithelium with tumor grade or metastasis to lymph nodes. Sheng et al. evaluated 55 different tumors for multiple parameters, including Shh, Ptch1 and HIP expression (the latter being surrogate Gli targets) [99]. Here, the investigators described elevated immunostaining for Shh in malignant epithelium compared with benign epithelium, with increased Ptch1 and HIP expression in tumor cells that correlated with tumor grade. Narita et al. characterized Gli2 expression in 21 localized prostate tumors from androgenically intact patients compared with 14 benign prostatic hyperplasia specimens and described a significant increase in Gli2 immunostaining in the malignant compared with the benign epithelium [102]. Overall, the most validated studies appear to support that expression of Shh in prostate tumor cells tends to increase as a function of tumor grade (and potential for metastasis), that prostate tumor cells tend to show higher Gli2 expression and productive Gli transcriptional activity compared with their benign counterparts, and that Gli2 expression rises further in therapy-resistant tumor cells. These outcomes then suggest that a more active hedgehog signaling microenvironment around a prostate tumor in conjunction with increased tumor cell Gli activity is associated with aggressive cancer cell behaviors that include potential for metastasis and therapy resistance. The outcomes do not, however, sufficiently establish that there is any direct association between the overexpression of hedgehogs in more aggressive prostate tumor cells and the enhanced Gli expression/activity that is also reported to be found in prostate tumor cells.
What can be learned from study of human prostate cancer cell lines? Use of some of the lines as xenografts in mice has revealed additional features of hedgehog effects that provide insight into the in vivo situation. For one, overexpression of the ligand (Shh) in LNCaP cells significantly increased the in vivo tumor growth rate of tumor xenografts compared with control xenografted LNCaP cells [100]. This indicates that the higher expression of Shh found in prostate tumors of higher grade has the potential to impact on prostate tumor growth rates. The fact that similar tumor growth acceleration can also be achieved by co-mixing unmodified LNCaP cells with UGS mesenchymal cells lacking Gli3 repressor (Gli3−/−) [103] certainly shows that signaling action through the paracrine pathway, at least has the potential to significantly contribute to the hedgehog-mediated tumor growth acceleration effect. Finally, observations that the treatment of mice with Shh-targeting antibodies, cyclopamine, Gli2-targeting antisense oligotides [102] or Gli-blocking drugs of the GANT class significantly inhibits the growth of prostate tumor cell xenografts (CWR22rv1 or PC3 cells) identify the potential for use of hedgehog-/Gli-suppressive therapeutics for prostate cancer treatment, although, to date, no actual clinical trials using hedgehog-blocking approaches for prostate cancer patients have been reported.
Evaluation of prostate cancer cell lines in a culture setting provides a means of testing for the presence of any autocrine-like hedgehog signaling activities in the cells and whether activation or interference at various sites of the signaling pathway affects hedgehog target genes or cell growth outside the influence of a paracrine signaling environment. For the most commonly utilized human prostate cancer cell lines (LNCaP and derivatives, DU145, PC3 or CWR22rv1) grown in culture, Shh, Gli1/2 and other key hedgehog target genes (Ptch1, Gli1 and HIP) are, in general, reported to be expressed, although there is wide variability in individual levels among the different lines. The most comprehensive survey for basal expression of hedgehog effector genes (mRNAs) in the common prostate cancer cell lines was published by Zhang et al. [104] and this survey showed no overt concordance between the expression of hedgehog ligands (Shh or Ihh) and the basal expression of hedgehog surrogate targets (Gli1 and Ptch1), except for HIP; no concordance in the expression of the different hedgehog target genes in any of the lines; and, finally, no concordance between the expression of any of the Gli RNAs with Ptch or HIP expression. Likewise, the common prostate cancer cell lines were shown to be refractory to treatment with recombinant Shh protein or to adenoviral transduction of a mutated Smo gene [104]. Collectively, these findings do not lend support to the presence of a basally active or even an accessible endogenous hedgehog signaling process in any of the cell lines evaluated based upon the idea that the activity of the pathway is solely indicated by expression levels of known Gli target genes. Conceptually, the lack of evidence for intermediate hedgehog signaling activity in prostate cancer cell lines based upon these considerations then challenges the idea that cyclopamine treatment, which invariably affects the growth of these cells in vitro, is functionally targeting an active hedgehog signaling process guided by Smo activation. Here again, the failure of cyclopamine to suppress expression of hedgehog target genes (Ptch1, Gli1 or hedgehog reporter) in the cultured prostate cancer cell lines [104,105] provides additional support for the lack of intermediate signaling pathway activity in the cancer cell lines, as long as one can be reassured that pathway activity is exclusively reflected by the relative expression levels of Gli target genes. As we will discuss later, this may not always be the case, at least in prostate cancer cells that express the AR protein. Regardless of these concerns, there are prominent indications that Gli proteins, at least, play some role in the growth potential of prostate cancer cells. Suppression of Gli1 or Gli2 expression using gene-specific si-/shRNAs or antisense oligonucleotides significantly reduced their in vitro growth rate and invasiveness [102,106,107] and increased the propensity for apoptosis. The mechanism supporting the presence of active Gli in these cells remains uncertain.
Hedgehog/Gli & androgen cross-talk in prostate cancer
The androgen signaling pathway that is so central to prostate cancer is remarkably interactive with other cell-signaling pathways. These interactions often occur at the level of the AR protein where AR activity can be increased under stimulation of signal-activated protein kinases [108] or by interaction with other pathway-regulated transcription factors, as is exemplified by β-catenin in the Wnt signaling pathway [109]. These signaling interactions are especially notable when they support promiscuous androgen signaling under low androgen conditions, as this allows for the possibility that the secondary signaling pathway is a druggable target for suppression of CRPC. Recently, we learned of a unique bidirectional interaction between androgen and hedgehog signaling in prostate cancer cells. The nature of this interaction is defined by the androgenic milieu of the prostate cancer cell and it appears to have the potential to produce a more active paracrine hedgehog microenvironment of a tumor in hormone-treated patients and, at the same time, promote promiscuous activity of the tumor cell AR that enables androgen-independent growth.
The nature of this interaction is first defined by evidence that hedgehog ligands are androgen-repressed genes in prostate cancer cells. Using the example of cultured prostate cancer cell lines that express AR and are growth-responsive to the presence of androgens in their medium, expression of mRNA encoding hedgehogs was found to be markedly increased by a switch to an androgen-depleted medium [101,110]. For LNCaP cells, androgen depletion upregulated Shh by 30,000-fold, and the expression of Ihh and Dhh was also upregulated, although not to this extent. This response was not unique to LNCaP; other androgen-responsive prostate cancer cells demonstrated similar changes in hedgehog expression when treated in this manner. Moreover, the changes in Shh mRNA were accompanied by similar increases in the expression and release of the mature Shh polypeptide with intact paracrine function, shown by the finding that the conditioned growth medium from androgen-deprived, but not androgen-supplemented, LNCaP cells was able to elicit a hedgehog response from mouse fibroblasts [110]. The clinical relevance of these in vitro findings is supported by the previously mentioned survey of hedgehog expression in human prostate tumors [101], which included a group of tumors obtained from patients who had been adjuvantly treated with hormone therapy prior to surgery. Here, hormone treatment essentially doubled the percentage of tumors found to express Shh or Dhh in malignant epithelium compared with untreated tumors.
In addition to its effect on hedgehog expression, androgen deprivation was also shown to significantly increase the expression of Gli2 mRNA in LNCaP and other prostate cancer cell lines [110]. Considering the fact that this action was also accompanied by upregulated Ptch1 expression, one might reasonably suppose that the coincidental increases in Shh, Gli2 and Ptch expression represent the activities of an autocrine hedgehog cascade initiated by androgen deprivation. Indeed, since cyclopamine treatment conferred a small but significant decrease in Ptch expression under this condition [110], the outcome further supports the idea that androgen deprivation is associated with a reawakening of some autocrine-like activity in prostate cancer cells. Arguing against this is the fact that Gli1 mRNA expression was significantly decreased by this same condition and it is difficult to explain the striking discordance in the response of these two foremost Gli target genes (Gli1 and Ptch1), unless one invokes different regulatory mechanisms for each gene operating in the confines of the androgen-deprived cell. This remains an unresolved issue, which is further complicated by the evidence that active hedgehog/Gli affects androgen signaling in prostate cancer cells.
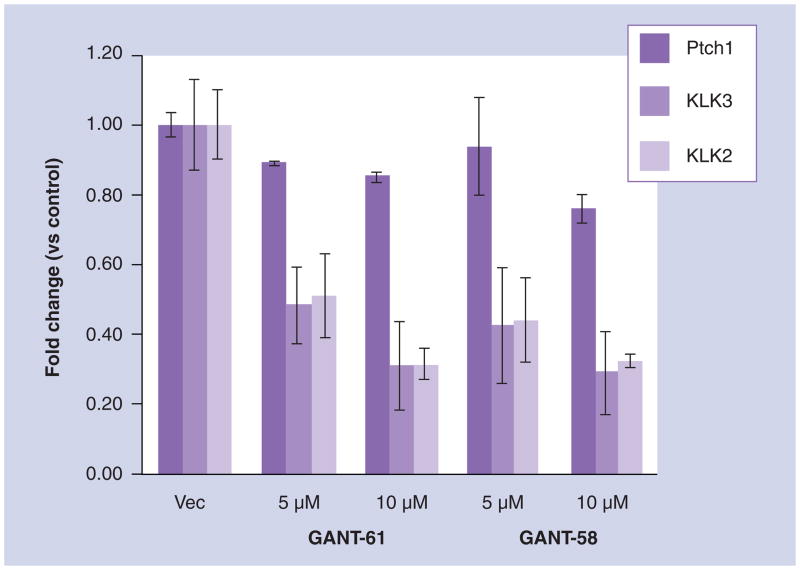
The notion that hedgehog/Gli also affects androgen signaling originated from observations of a dose-dependent effect of cyclopamine on the expression of androgen-regulated genes [111] in LNCaP and other prostate cancer cells. Here, cyclopamine treatment was shown to specifically suppress expression of kallikrein-related peptidase (KLK)2, KLK3 and PGC in androgen-deprived, but not androgen-supplemented, LNCaP cells, whereas it further induced expression of Shh, which represents an androgen-repressed gene. Cyclopamine had similar effects on expression of luciferase reporters from androgen-dependent promoter elements in these cells. These effects were most pronounced in androgen-deprived cells in which Gli2 levels were elevated. Whereas questions remain regarding cyclopamine’s specificity and its mechanism of action in prostate cancer cell lines, a similar outcome was observed after knockdown of Smo expression using siRNA. The fact that this effect also involves elements of hedgehog (Gli activity) downstream of Smo is indicated by the ability to suppress androgen-dependent gene expression by specific reduction of Gli2 expression or by treatments with the Gli inhibitor drugs, GANT-58 and -61 (Figure 2). Here, it is notable that the GANT drugs did not significantly affect expression of Ptch1. Finally, in the reverse paradigm, exogenous expression of Gli1 or Gli2 in androgen-deprived prostate cancer cells not only increased the expression of androgen-dependent genes but also enabled these cells to grow in an androgen-deficient medium [111]. Collectively, the outcomes of these studies support the presence of a Smo-dependent signaling process, at least in androgen-deprived prostate cancer cells, which cross-talks with the androgen signaling pathway through Gli to affect androgen-regulated gene expression. The involvement of Gli in the regulation of androgen-dependent genes suggests that the effect might be mediated by some form of Gli/AR interaction. Indeed, coimmunoprecipitation or two-hybrid analysis shows that Gli1 or Gli2 can directly bind to the AR protein [111,112]. Based on these reports, the Gli proteins may have AR coactivation functions that contribute to androgen signaling, especially in the androgen-deprived state.
Figure 2. Suppression of androgen-dependent gene expression in androgen-deprived prostate cancer cells by the Gli-suppressing drugs GANT-58 and -61.
LNCaP cells were seeded onto plates overnight in RPMI-1640 medium containing 10% fetal bovine serum, then switched to an androgen-depleted medium as was previously described [110] containing Vec or GANT-58 or GANT-61 dissolved in dimethyl sulfoxide at the indicated concentrations and was incubated for an additional 72 h. RNAs were then extracted from these cells and were assessed by quantitative real-time PCR for the expression of KLK2 or KLK3 (prostate-specific antigen), as described, and the results are normalized to the expression of GAPDH in the same samples. Each point indicates the means ± standard deviation from triplicate cultures.
GANT: Gli antagonist; KLK: Kallikrein-related peptidase; Ptch: Patched; Vec: Dimethyl sulfoxide vehicle only.
Expert commentary
Since its discovery in 1980, we have learned a great deal regarding the mechanistic aspects of hedgehog signaling and its role in vertebrate development. In addition, we have come to accept its causative role in some forms of human cancer. The association of hedgehog signaling abnormalities with human tumors has spurred the development and testing of clinically useful drugs that target hedgehog/Gli, some of which are already demonstrating efficacy as cancer therapeutics. However, our current knowledge regarding the role of hedgehog/Gli signaling in prostate cancer remains relatively limited to the notion that the disease, once acquired, benefits from a paracrine hedgehog signaling influence that is driven by the production of hedgehog ligands by prostate tumor cells that act on adjacent benign (stromal) cells and feeds back to the tumor, stimulating tumor cell growth and metastasis. With regards to prostate tumor cells themselves, there is little evidence for the types of mutations or defects in hedgehog signaling genes that are found in human skin and brain tumors, but this does not rule out the possibility that genetic anomalies in other hedgehog-regulating genes might be a factor in the disease. Furthermore, the indications that tumor Gli activity has a role in advanced/aggressive disease are relatively convincing, but there are many reasons to be skeptical as to whether the hyperactive Gli is a consequence of tumor cell-autonomous hedgehog signaling through an active autocrine-like signaling process. Recent findings that the hormone therapies used to treat advanced prostate cancers have the potential to augment the paracrine hedgehog signaling microenvironment of a prostate tumor, in conjunction with the findings that Gli proteins can interact with AR and confer androgen-independent growth behavior on human prostate cancer cells, support the consideration of hedgehog-blocking drug therapy used in conjunction with hormone therapy for patients with advanced/therapy-resistant disease. While drugs that target Smo are now clinically available and should be effective for suppression of hedgehog paracrine effects, the questions regarding the source of Gli activity in prostate cancers suggest that drugs that specifically target Gli may be more useful than Smo blockers alone as they might act on the paracrine hedgehog tumor microenvironment, as well as on tumor-autonomous Gli, allowing effective disease control when used as an adjunct to hormone therapy.
Five-year view
The availability of clinically tested drugs that target hedgehog/Gli suggests that clinical trials of hedgehog therapeutics for prostate cancer are likely to advance faster than the resolution of critical research issues that might guide the most effective application of these therapies. With this perspective, the field requires research advances in three focus areas to help resolve the hedgehog/Gli contribution to prostate cancer. The first involves further exploration of the hedgehog paracrine effect in prostate cancer. Here, the knowledge that hedgehog expression is induced by inflammation, as is common in the prostate, suggests that hyperactive paracrine hedgehog could explain the link between prostate inflammation and prostate carcinogenesis and identify a role for hedgehog in prostate cancer etiology. Development of this concept should encompass surveys of human prostate tissues to correlate the presence of prostate inflammation with hedgehog expression in adjacent epithelium and involve attempts to create a mouse model of prostate cancer by conditional targeted over-expression of Shh in the adult prostate epithelium. Further work is needed to identify the paracrine hedgehog-induced substances that are produced by hedgehog-stimulated tumor support cells that induce prostate tumor growth. The second area of focus involves addressing the source of Gli hyperactivity in prostate cancer cells and defining the extent to which increased tumor-autonomous Gli activity is associated with progression to aggressive (metastatic) disease. We have described the considerations leading many to questions about whether intermediary hedgehog signaling is even possible in prostate cancer cells and the evidence that Gli expression is not solely dependent upon an active hedgehog signaling process in prostate or other solid tumors. Can we then attribute Gli overexpression in prostate cancer to some specific alternate signaling process that increases with disease progression? The third area of research involves expanding our understanding of the cross-talk between hedgehog/Gli and its consequences for androgen signaling in prostate cancer cells. Research in this area should attempt to dissect the interaction sites of Gli with AR and define the extent to which the alternate Gli forms can coactivate or corepress AR transcription. More work is needed to resolve the question of the extent to which Gli is hijacked by the AR in prostate cancer cells and whether Gli activity is best measured in these cells by expression of androgen-regulated, rather than Gli-regulated, genes. Finally, the evidence that a reduction in Smo expression in prostate cancer cells affects the expression of androgen-regulated genes also suggests the need to better understand Smo function in the context of the prostate cancer cell.
Key issues.
Hedgehog signaling regulates the activities of Gli transcription factors.
Paracrine hedgehog signaling guides developmental growth of the prostate gland.
Gene anomalies that dysregulate hedgehog signaling are causative of some forms of human cancers.
These gene anomalies are rarely found in prostate tumor cells.
Aggressive prostate tumor behaviors correlate with high expression of hedgehog ligands and Gli2.
Overexpression of Sonic hedgehog increases the growth of human prostate cancer xenografts in mice, and treatment hedgehog/Gli inhibitors strongly inhibits tumor xenograft growth.
Knockdown of Gli1 or Gli2 expression reduces prostate cancer cell growth in vitro.
Gli proteins (1 and 2) bind to the androgen receptor and affect androgen signaling in prostate cancer cells.
Overexpression of Gli2 allows androgen-independent growth of prostate cancer cells in vitro and may be a factor in the development of castration-recurrent prostate cancers.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
Mengqian Chen is supported by a training grant from the United States Department of Defense Prostate Cancer Research Program (W81XH-10-1-0125). Ralph Buttyan is supported from grants from the NIH (RO1-CA11618) and from the United States Department of Defense Prostate Cancer Research Program (W81XH-10-1-0493 and W81XH-06-0061). The authors have no other relevant affiliations or financial involvements with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Kessler B, Albertsen P. The natural history of prostate cancer. Urol Clin North Am. 2003;30(2):219–226. doi: 10.1016/s0094-0143(02)00182-9. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW, Ankerst DP, Thompson IM. Screening for prostate cancer. CA Cancer J Clin. 2009;59(4):264–273. doi: 10.3322/caac.20026. [DOI] [PubMed] [Google Scholar]
- 3.Shteynshlyuger A, Andriole GL. Prostate cancer: to screen or not to screen? Urol Clin North Am. 2010;37(1):1–9. doi: 10.1016/j.ucl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60(2):70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 5.McConnell JD. Physiologic basis of endocrine therapy for prostatic cancer. Urol Clin North Am. 1991;18(1):1–13. [PubMed] [Google Scholar]
- 6.Culig Z, Bartsch G. Androgen axis in prostate cancer. J Cell Biochem. 2006;99(2):373–381. doi: 10.1002/jcb.20898. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL. Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol. 2008;617:223–234. doi: 10.1007/978-0-387-69080-3_21. [DOI] [PubMed] [Google Scholar]
- 8.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27(1):36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res. 2009;15(10):3251–3255. doi: 10.1158/1078-0432.CCR-08-1171. [DOI] [PubMed] [Google Scholar]
- 10.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton JP, Mongeau ME, Klimstra DS, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104(12):5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varnat F, Duquet A, Malerba M, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1(6–7):338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stecca B, Mas C, Clement V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci USA. 2007;104(14):5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper M, Jaks V, Fiaschi M, Toftgard R. Hedgehog signalling in breast cancer. Carcinogenesis. 2009;30(6):903–911. doi: 10.1093/carcin/bgp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15(23):3059–3087. doi: 10.1101/gad.938601. Outstanding early review of the hedgehog signaling process invertebrates. [DOI] [PubMed] [Google Scholar]
- 18••.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15(6):801–812. doi: 10.1016/j.devcel.2008.11.010. Updated review of the hedgehog signaling process that includes a focus on hedgehog in cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson CW, Chuang PT. Mechanism and evolution of cytosolic Hedgehog signal transduction. Development. 2010;137(13):2079–2094. doi: 10.1242/dev.045021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71(1):33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- 22.Marigo V, Roberts DJ, Lee SM, et al. Cloning, expression, and chromosomal location of SHH and IHH: two human homologues of the Drosophila segment polarity gene hedgehog. Genomics. 1995;28(1):44–51. doi: 10.1006/geno.1995.1104. [DOI] [PubMed] [Google Scholar]
- 23.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6(3):298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- 24.Breitling R. Greased hedgehogs: new links between hedgehog signaling and cholesterol metabolism. Bioessays. 2007;29(11):1085–1094. doi: 10.1002/bies.20663. [DOI] [PubMed] [Google Scholar]
- 25.Burke R, Nellen D, Bellotto M, et al. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99(7):803–815. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter D, Stone DM, Brush J, et al. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci USA. 1998;95(23):13630–13634. doi: 10.1073/pnas.95.23.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. An excellent review of primary cilium that focuses on its role in regulating hedgehog action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz i Altaba A, Sanchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2(5):361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 30.Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes Dev. 2008;22(18):2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 31.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 32.Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci USA. 2006;103(22):8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282(12):8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 34.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by Smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci USA. 2009;106(9):3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99(22):14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson CW, Chen MH, Chuang PT. Smoothened adopts multiple active and inactive conformations capable of trafficking to the primary cilium. PLoS ONE. 2009;4(4):e5182. doi: 10.1371/journal.pone.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallikas O, Palin K, Sinjushina N, et al. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell. 2006;124(1):47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Winklmayr M, Schmid C, Laner-Plamberger S, et al. Non-consensus GLI binding sites in Hedgehog target gene regulation. BMC Mol Biol. 2010;11:2. doi: 10.1186/1471-2199-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koebernick K, Pieler T. Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling. Differentiation. 2002;70(2–3):69–76. doi: 10.1046/j.1432-0436.2002.700201.x. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2009;326(1):177–189. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, Scales SJ. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol. 2010;30(8):1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smelkinson MG, Zhou Q, Kalderon D. Regulation of Ci-SCFSlimb binding, Ci proteolysis, and hedgehog pathway activity by Ci phosphorylation. Dev Cell. 2007;13(4):481–495. doi: 10.1016/j.devcel.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q, Shi Q, Chen Y, et al. Multiple Ser/Thr-rich degrons mediate the degradation of Ci/Gli by the Cul3-HIB/SPOP E3 ubiquitin ligase. Proc Natl Acad Sci USA. 2009;106(50):21191–21196. doi: 10.1073/pnas.0912008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C, Pan Y, Wang B. Suppressor of fused and Spop regulate the stability, processing and function of Gli2 and Gli3 full-length activators but not their repressors. Development. 2010;137(12):2001–2009. doi: 10.1242/dev.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42(4):437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 46.Ding Q, Motoyama J, Gasca S, et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125(14):2533–2543. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- 47.Park HL, Bai C, Platt KA, et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127(8):1593–1605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- 48.Chuang PT, McMahon AP. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 1999;397(6720):617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 49.McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455(7215):979–983. doi: 10.1038/nature07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1(4):e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci USA. 2009;106(51):21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canettieri G, Di Marcotullio L, Greco A, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12(2):132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 53.Cox B, Briscoe J, Ulloa F. SUMOylation by Pias1 regulates the activity of the Hedgehog dependent Gli transcription factors. PLoS ONE. 2010;5(8):e11996. doi: 10.1371/journal.pone.0011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen MH, Wilson CW, Li YJ, et al. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23(16):1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jia J, Kolterud A, Zeng H, et al. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol. 2009;330(2):452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2(1):29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 57.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125(18):3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 58.Roessler E, Du YZ, Mullor JL, et al. Loss-of-function mutations in the human GLI2 gene are associated with pituitary anomalies and holoprosencephaly-like features. Proc Natl Acad Sci USA. 2003;100(23):13424–13429. doi: 10.1073/pnas.2235734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 60.Mo R, Freer AM, Zinyk DL, et al. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124(1):113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- 61.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20(1):54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 62•.Podlasek CA, Barnett DH, Clemens JQ, Bak PM, Bushman W. Prostate development requires Sonic hedgehog expressed by the urogenital sinus epithelium. Dev Biol. 1999;209(1):28–39. doi: 10.1006/dbio.1999.9229. First and thorough description of an association between hedgehog signaling and prostate development. [DOI] [PubMed] [Google Scholar]
- 63.Berman DM, Desai N, Wang X, et al. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267(2):387–398. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Freestone SH, Marker P, Grace OC, et al. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264(2):352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Barsoum IB, Yao HH. Fetal Leydig cells: progenitor cell maintenance and differentiation. J Androl. 2010;31(1):11–15. doi: 10.2164/jandrol.109.008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–712. doi: 10.1038/nature02962. Highly publicized article citing evidence for hedgehog involvement in prostate cancer. [DOI] [PubMed] [Google Scholar]
- 67.Pu Y, Huang L, Prins GS. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273(2):257–275. doi: 10.1016/j.ydbio.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamm ML, Catbagan WS, Laciak RJ, et al. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249(2):349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- 69.Haraguchi R, Motoyama J, Sasaki H, et al. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134(3):525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- 70.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21(7):1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Hahn H, Wojnowski L, Miller G, Zimmer A. The patched signaling pathway in tumorigenesis and development: lessons from animal models. J Mol Med. 1999;77(6):459–468. doi: 10.1007/s001099900018. [DOI] [PubMed] [Google Scholar]
- 72.Zurawel RH, Allen C, Wechsler-Reya R, Scott MP, Raffel C. Evidence that haploinsufficiency of Ptch leads to medulloblastoma in mice. Genes Chromosomes Cancer. 2000;28(1):77–81. [PubMed] [Google Scholar]
- 73.Lee Y, Kawagoe R, Sasai K, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26(44):6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 74.Taylor MD, Liu L, Raffel C, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 75.Reifenberger J, Wolter M, Knobbe CB, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005;152(1):43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 76.Pan S, Dong Q, Sun LS, Li TJ. Mechanisms of inactivation of PTCH1 gene in nevoid basal cell carcinoma syndrome: modification of the two-hit hypothesis. Clin Cancer Res. 2010;16(2):442–450. doi: 10.1158/1078-0432.CCR-09-2574. [DOI] [PubMed] [Google Scholar]
- 77.Pastorino L, Ghiorzo P, Nasti S, et al. Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A. 2009;149A(7):1539–1543. doi: 10.1002/ajmg.a.32944. [DOI] [PubMed] [Google Scholar]
- 78.Lam CW, Xie J, To KF, et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18(3):833–836. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- 79.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 80.Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64(17):6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 81••.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. Succinct experimental platform that demonstrates primal relevance for paracrine hedgehog in several solid tumor systems as opposed to autocrine hedgehog. [DOI] [PubMed] [Google Scholar]
- 82•.Lauth M, Toftgard R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle. 2007;6(20):2458–2463. doi: 10.4161/cc.6.20.4808. Establishes the involvement of non-hedgehog signaling pathways in Gli activities that forms the basis for understanding Gli involvement in solid tumor systems that may not have autocrine hedgehog activities. [DOI] [PubMed] [Google Scholar]
- 83.Dennler S, Andre J, Verrecchia F, Mauviel A. Cloning of the human GLI2 promoter: transcriptional activation by transforming growth factor-β via SMAD3/β-catenin cooperation. J Biol Chem. 2009;284(46):31523–31531. doi: 10.1074/jbc.M109.059964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Lipinski RJ, Gipp JJ, Shaw AK, Bushman W. Hedgehog pathway responsiveness correlates with the presence of primary cilia on prostate stromal cells. BMC Dev Biol. 2009;9:50. doi: 10.1186/1471-213X-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang X, Harrington N, Moraes RC, Wu MF, Hilsenbeck SG, Lewis MT. Cyclopamine inhibition of human breast cancer cell growth independent of Smoothened (Smo) Breast Cancer Res Treat. 2009;115(3):505–521. doi: 10.1007/s10549-008-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tremblay MR, Lescarbeau A, Grogan MJ, et al. Discovery of a potent and orally active hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52(14):4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 87.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 88.Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor GDC-0449 in patients with refractory, locally-advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mimeault M, Johansson SL, Vankatraman G, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6(3):967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 90.Stanton BZ, Peng LF, Maloof N, et al. A small molecule that binds Hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5(3):154–156. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lauth M, Bergstrom A, Shimokawa T, Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104(20):8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyman JM, Firestone AJ, Heine VM, et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc Natl Acad Sci USA. 2009;106(33):14132–14137. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6(Suppl 2):22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 94.Kim J, Lee JJ, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci USA. 2010;107(30):13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beauchamp EM, Ringer L, Bulut G, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Invest. 2011;121(1):148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Sanchez P, Hernandez AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG–GLI1 signaling. Proc Natl Acad Sci USA. 2004;101(34):12561–12566. doi: 10.1073/pnas.0404956101. Seminal paper that first described evidence for hedgehog involvement in prostate cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao J, Ligon KL, Rakhlin EY, et al. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66(20):10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen BY, Lin DP, Liu JY, et al. A mouse prostate cancer model induced by Hedgehog overexpression. J Biomed Sci. 2006;13(3):373–384. doi: 10.1007/s11373-005-9050-x. [DOI] [PubMed] [Google Scholar]
- 99.Sheng T, Li C, Zhang X, et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan L, Pepicelli CV, Dibble CC, et al. Hedgehog signaling promotes prostate xenograft tumor growth. Endocrinology. 2004;145(8):3961–3970. doi: 10.1210/en.2004-0079. [DOI] [PubMed] [Google Scholar]
- 101.Azoulay S, Terry S, Chimingqi M, et al. Comparative expression of Hedgehog ligands at different stages of prostate carcinoma progression. J Pathol. 2008;216(4):460–470. doi: 10.1002/path.2427. [DOI] [PubMed] [Google Scholar]
- 102•.Narita S, So A, Ettinger S, et al. GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin Cancer Res. 2008;14(18):5769–5777. doi: 10.1158/1078-0432.CCR-07-4282. Finds clinical relevance for Gli2 expression in human prostate cancer progression. [DOI] [PubMed] [Google Scholar]
- 103•.Shaw A, Gipp J, Bushman W. The Sonic Hedgehog pathway stimulates prostate tumor growth by paracrine signaling and recapitulates embryonic gene expression in tumor myofibroblasts. Oncogene. 2009;28(50):4480–4490. doi: 10.1038/onc.2009.294. First paper to challenge the idea of autocrine hedgehog in prostate cancer cells that express hedgehog target genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Lipinski R, Shaw A, Gipp J, Bushman W. Lack of demonstrable autocrine hedgehog signaling in human prostate cancer cell lines. J Urol. 2007;177(3):1179–1185. doi: 10.1016/j.juro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 105.McCarthy FR, Brown AJ. Autonomous Hedgehog signalling is undetectable in PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2008;373(1):109–112. doi: 10.1016/j.bbrc.2008.05.169. [DOI] [PubMed] [Google Scholar]
- 106.Thiyagarajan S, Bhatia N, Reagan-Shaw S, et al. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res. 2007;67(22):10642–10646. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stecca B, Mas C, Ruiz i Altaba A. Interference with HH-GLI signaling inhibits prostate cancer. Trends Mol Med. 2005;11(5):199–203. doi: 10.1016/j.molmed.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Reddy GP, Barrack ER, Dou QP, et al. Regulatory processes affecting androgen receptor expression, stability, and function: potential targets to treat hormone-refractory prostate cancer. J Cell Biochem. 2006;98(6):1408–1423. doi: 10.1002/jcb.20927. [DOI] [PubMed] [Google Scholar]
- 109.Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99(2):402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- 110.Chen M, Tanner M, Levine AC, Levina E, Ohouo P, Buttyan R. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell Cycle. 2009;8(1):149–157. doi: 10.4161/cc.8.1.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111•.Chen M, Feuerstein MA, Levina E, et al. Hedgehog/Gli supports androgen signaling in androgen deprived and androgen independent prostate cancer cells. Mol Cancer. 2010;9:89. doi: 10.1186/1476-4598-9-89. First evidence for hedgehog/Gli interaction with androgen signaling pathway in prostate cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen G, Goto Y, Sakamoto R, et al. GLI1, a crucial mediator of sonic hedgehog signaling in prostate cancer, functions as a negative modulator for androgen receptor. Biochem Biophys Res Commun. 2011;404(3):809–815. doi: 10.1016/j.bbrc.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 113.Keys DN, Lewis DL, Selegue JE, et al. Recruitment of a hedgehog regulatory circuit in butterfly eyespot evolution. Science. 1999;283(5401):532–534. doi: 10.1126/science.283.5401.532. [DOI] [PubMed] [Google Scholar]
- 114.Agren M, Kogerman P, Kleman MI, Wessling M, Toftgard R. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. 2004;330:101–114. doi: 10.1016/j.gene.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 115.Bonifas JM, Pennypacker S, Chuang PT, et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J Invest Dermatol. 2001;116(5):739–742. doi: 10.1046/j.1523-1747.2001.01315.x. [DOI] [PubMed] [Google Scholar]
- 116.Bishop CL, Bergin AM, Fessart D, et al. Primary cilium-dependent and -independent Hedgehog signaling inhibits p16(INK4A) Mol Cell. 2010;40(4):533–547. doi: 10.1016/j.molcel.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 117.Yoon JW, Kita Y, Frank DJ, et al. Gene expression profiling leads to identification of GLI1-binding elements in target genes and a role for multiple downstream pathways in GLI1-induced cell transformation. J Biol Chem. 2002;277(7):5548–5555. doi: 10.1074/jbc.M105708200. [DOI] [PubMed] [Google Scholar]
- 118.Mill P, Mo R, Hu MC, Dagnino L, Rosenblum ND, Hui CC. Shh controls epithelial proliferation via independent pathways that converge on N-Myc. Dev Cell. 2005;9(2):293–303. doi: 10.1016/j.devcel.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Rizvi S, Demars CJ, Comba A, et al. Combinatorial chemoprevention reveals a novel smoothened-independent role of GLI1 in esophageal carcinogenesis. Cancer Res. 2010;70(17):6787–6796. doi: 10.1158/0008-5472.CAN-10-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124(7):1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 121.Teh MT, Wong ST, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62(16):4773–4780. [PubMed] [Google Scholar]
- 122.Eichberger T, Regl G, Ikram MS, et al. FOXE1, a new transcriptional target of GLI2 is expressed in human epidermis and basal cell carcinoma. J Invest Dermatol. 2004;122(5):1180–1187. doi: 10.1111/j.0022-202X.2004.22505.x. [DOI] [PubMed] [Google Scholar]
- 123.Laner-Plamberger S, Kaser A, Paulischta M, Hauser-Kronberger C, Eichberger T, Frischauf AM. Cooperation between GLI and JUN enhances transcription of JUN and selected GLI target genes. Oncogene. 2009;28(13):1639–1651. doi: 10.1038/onc.2009.10. [DOI] [PubMed] [Google Scholar]
- 124.Vokes SA, Ji H, McCuine S, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134(10):1977–1989. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- 125.Yoon JW, Gilbertson R, Iannaccone S, Iannaccone P, Walterhouse D. Defining a role for Sonic hedgehog pathway activation in desmoplastic medulloblastoma by identifying GLI1 target genes. Int J Cancer. 2009;124(1):109–119. doi: 10.1002/ijc.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22(19):2651–2663. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, Bushman W. Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem. 2009;284(9):5620–5629. doi: 10.1074/jbc.M809172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med. 2006;17(1):171–175. [PubMed] [Google Scholar]
- 129.Eichberger T, Kaser A, Pixner C, et al. GLI2-specific transcriptional activation of the bone morphogenetic protein/activin antagonist follistatin in human epidermal cells. J Biol Chem. 2008;283(18):12426–12437. doi: 10.1074/jbc.M707117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Regl G, Kasper M, Schnidar H, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64(21):7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 131.Tanese K, Fukuma M, Ishiko A, Sakamoto M. Endothelin-2 is upregulated in basal cell carcinoma under control of Hedgehog signaling pathway. Biochem Biophys Res Commun. 2010;391(1):486–491. doi: 10.1016/j.bbrc.2009.11.085. [DOI] [PubMed] [Google Scholar]
Website
- 201.American Cancer Society. Cancer facts and figures 2010. www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf.