Abstract
Folate deficiency in the periconceptional period contributes to neural tube defects; deficits in vitamin B12 (cobalamin) have negative consequences on the developing brain during infancy; and deficits of both vitamins are associated with a greater risk of depression during adulthood. This review examines two mechanisms linking folate and vitamin B12 deficiency to abnormal behavior and development in infants: disruptions to myelination and inflammatory processes. Future investigations should focus on the relationship between the timing of deficient and marginal vitamin B12 status and outcomes such as infant growth, cognition, social development, and depressive symptoms, along with prevention of folate and vitamin B12 deficiency.
Keywords: Child development, depression, folate, vitamin B12
Introduction
The link between diet and brain development and function has attracted global attention as evidence has emerged documenting the negative consequences of nutritional deficiencies on infant cognitive and motor functioning [1]. For example, the recognition that ensuring adequate folate intake during pregnancy reduces the risk of giving birth to an infant with a neural tube defect (NTD) [2] has led to folic acid fortification programs. Recent debates have centered on the optimal timing and amount of folate required, and fortification strategies [3].
Although the mechanisms underlying the effect of maternal folate status on neural tube development are not well understood, the shared metabolism between folate and vitamin B12 suggests that deficiencies in one vitamin may alter the metabolism of the other. This is perhaps related to the roles that vitamin B12 plays in myelination, or in the synthesis of methionine from homocysteine in combination with folic acid. This review was conducted to examine the evidence linking deficiencies in maternal and infant folate and/or vitamin B12 with infant cognitive and motor development.
Vitamin B12 deficiency
During pregnancy, vitamin B12 is concentrated in the fetus and stored in the liver [4, 5]. Infants born to vitamin B12-replete mothers have stores of vitamin B12 that are adequate to sustain them for the first several months postpartum. Vitamin B12 deficiency rarely occurs before about 4 months of age. Infants of vitamin B12-deficient breastfeeding mothers, or infants receiving low amounts of animal-source foods, may be vulnerable to vitamin B12 deficiency between 6 and 12 months of age. Neurological symptoms appear to affect the central nervous system [6] and, in severe cases, cause brain atrophy. In addition to neurological symptoms, infants may experience other physical symptoms, including abnormal pigmentation, hypotonia, enlarged liver and spleen, sparse hair, food refusal, anorexia, failure to thrive, and diarrhea.
Most of the initial data regarding vitamin B12 deficiency in infancy are from case studies of infants exclusively breastfed by mothers on vegan, vegetarian, or lacto-ovo vegetarian diets. Several authors have described developmental retardation and “infant tremor syndrome” in 4- to 11-month-old infants of vegetarian mothers from India [7, 8]. Four case studies from the United States described lethargy, irritability, and developmental delay among exclusively breastfed infants (ages 6 to 10 months) of vegan or vegetarian mothers [9–12]. Restoration of developmental skills after therapy was variable, with at least two cases reporting ongoing delays [9, 12], and one reporting developmental recovery [11]. Similar cases have been reported from Europe [13–17]. Again, the infants displayed delayed motor skills, along with lethargy, and were exclusively breastfed by mothers who were vegan or lacto-ovo vegetarian. After therapy, recovery was variable, with some children remaining moderately or severely retarded [13, 17–19].
Although infants who are deficient in vitamin B12 due to maternal and infant vegetarianism may also be deficient in other nutrients derived from animal-source foods, such as iron and zinc, it is unlikely that the developmental problems associated with vitamin B12 deficiency could be explained by iron or zinc deficiency. Recent reviews have suggested associations between iron deficiency and behavioral and developmental problems, mediated through changes in the developing brain [20, 21]. In addition, the role of iron in myelination, neurotransmitter function, and neuronal metabolism [22, 23] has been suggested as a possible explanation for the associations with behavioral and developmental functioning [24]. Associations between zinc deficiency and children’s behavior and development are less conclusive [1], but there is evidence in animals that zinc deficiency influences long-term growth and intellectual performance through changes in brain structure and function [25]. However, evidence from the treatment of children with vitamin B12 deficiency illustrates that administration of vitamin B12 for a short duration (e.g., even a few days) often leads to major improvements in functioning [26], suggesting that deficits in other micronutrients cannot explain the deficits associated with vitamin B12 deficiency.
Possible mechanisms of impaired development
Maternal pernicious anemia may serve as a model for the effects of vitamin B12 deficiency [27–29]. Pernicious anemia is a disorder caused by the absence of intrinsic factor, a substance from the gastrointestinal tract needed to absorb cobalamin. Symptoms among 9 infants of mothers with untreated pernicious anemia were remarkably similar to symptoms among 10 infants of vegetarian mothers [30]. In both cases, the most common symptom was anemia, which was present in 56% of the infants of mothers with pernicious anemia and 100% of the infants of vegetarian mothers. Lethargy and delayed developmental milestones were also common symptoms shared by both groups of infants.
There are at least two possible mechanisms linking vitamin B12 deficiency and abnormal behavior and development: 1) threats to early brain development, possibly through demyelination; and 2) inflammation, possibly simulating an autoimmune process that blocks intrinsic factor for cobalamin absorption, similar to pernicious anemia, an autoimmune disease that blocks intrinsic factor.
Brain development and myelination in infants
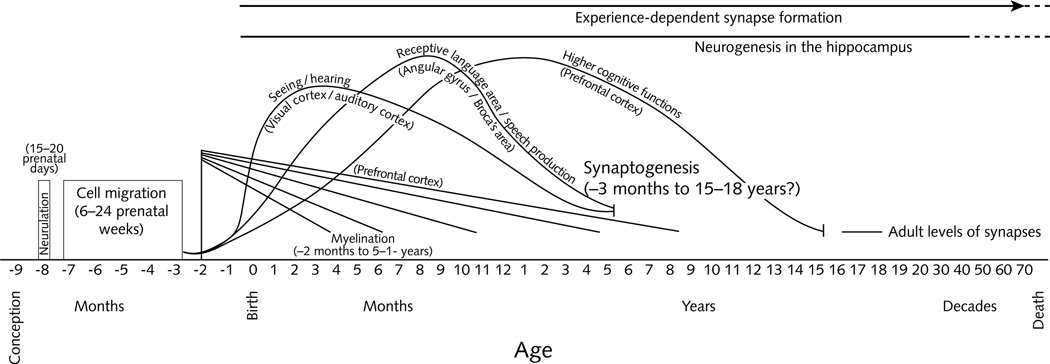
Recent advances in behavioral neuroscience have shown the important roles that nutrition plays in brain development [31, 32]. Brain development begins prenatally and continues through school age. It begins with the formation of brain cells, followed by cell migration and differentiation, and the development of synapses to enable cells to communicate with one another (fig. 1) [33]. Myelin is the supportive tissue that surrounds and protects the nerve cells and facilitates communication.
FIG. 1.
Human brain development. Source: adapted from Thompson and Nelson [29]
Nutrient deficiencies can interfere with early brain development and function, often by restricting the myelination, dendritic arborization, and synaptic connectivity that occur early in life [34]. The tissue levels of neurotransmitters (e.g., serotonin, dopamine, norepinephrine, acetylcholine) may be altered, resulting in neuroanatomical, neurochemical, or neurometabolic changes. The functional consequences of these alterations vary, depending on the specific nutritional deficiency and the timing of the deficiency relative to the developing neurological processes.
Brain growth is very rapid during the first 2 years of life, particularly in the cortex, which is associated with higher-order thinking. In addition, myelination of the brain, which is concentrated from mid-gestation through the second year of life, but continues through puberty, may be vulnerable to vitamin B12 deficiency. In infants, vitamin B12 deficiency has been associated with demyelination and brain atrophy [12, 34]. Although magnetic resonance imaging [35] using a circularly polarized head coil is an optimal way to study brain structure and function in infants, the requirement for sedation to ensure that infants do not move limits the procedure to infants undergoing clinical diagnosis. Thus, little is known about the consequences of mild vitamin B12 deficiency for early brain development and function.
Disruptions in myelination can have significant effects on central nervous system functioning by altering the speed of conduction in multiple systems. For example, slower conduction in the auditory and visual systems can interfere with learning and social interaction [32]. It is also likely that there are other intracerebral effects of vitamin B12 deficiency, given that so many brain systems are myelinating during the early developmental period. The acquisition of cognitive skills coincides with the pattern of central nervous system myelination. Therefore, retardation of myelination of the brain in infancy leads to delayed acquisition of cognitive skills, and brain atrophy leads to regression of these skills.
Inflammation
Vitamin B12 deficiency has been associated with gastric diseases such as atrophic gastritis (which sometimes progresses to pernicious anemia) and nonspecific gastritis (e.g., that caused by Helicobacter pylori). In the case of H. pylori infection, even patients with minimal or no gastric atrophy have presented with vitamin B12 deficiency [36]. Additionally, a case study from Poland described a 9-year-old girl who had undergone resection of the end of her small bowel early in life and presented with apathy, tiredness, and spastic paresis had inflammation of the esophagus and duodenum, low levels of vitamin B12 in her serum, impaired vitamin B12 absorption, and megaloblastic anemia [26]. Treatment with vitamin B12 and folic acid improved her neurological condition and hematologic status, illustrating a possible link with inflammation.
Prevalence of vitamin B12 deficiency
Studies in Guatemala among mothers and infants [37] and among school-age children [38] reported a high prevalence of deficient and marginal plasma vitamin B12 concentrations, possibly related to inadequate dietary intake, and for the mothers, the nutritional demands of pregnancy and lactation. These findings suggest that vitamin B12 deficiency is likely to be prevalent in other societies with low consumption of animal-source foods [39].
Vitamin B12 deficiency beyond infancy
Studies examining plasma vitamin B12 concentrations among preschool children and adolescents have suggested that vitamin B12 deficiency early in life compromises children’s subsequent growth and development. In a study in Boston, 42 preschoolers (median age, 3.9 years) who had been breastfed by vegetarian mothers and weaned to a macrobiotic diet were weighed and measured [40]. Approximately one-third (32%, 11/34) of the children were stunted (below the 5th percentile of height) and 15% (6/41) were wasted (below the 5th percentile of weight). Over half the children (55%) had high urinary methylmalonic acid concentrations, particularly those who had consumed a vegetarian diet throughout their lives. In a study conducted among adolescents in the Netherlands, cognitive assessments were conducted on 48 adolescents who had been raised on macrobiotic diets for the first 6 years of life followed by lacto-vegetarian or omnivorous diets, and 24 adolescents raised on omnivorous diets [41]. The children raised on omnivorous diets from birth obtained better scores on most cognitive assessments than children raised on macrobiotic diets, regardless of their cobalamin status during adolescence. Many of the adolescents raised on a macrobiotic diet early in life had poor cobalamin status during adolescence, even if they were consuming an omnivorous diet later in life, illustrating the difficulty in restoring cobalamin status following early vitamin B12 deficiency [42]. These studies are particularly important because they suggest that early cobalamin deficiency can have lasting consequences for children’s growth and cognitive development, and they raise questions about the possibility of mild cobalamin deficiency among the millions of children in developing countries breastfed by mothers who may be cobalamin-deficient due to low intake of animal-source foods and the nutritional demands of pregnancy and lactation.
Benefits from treatment
Cobalamin treatment has been effective in reducing the negative consequences for infants with vitamin B12-related neuropathy, but little is known about long-term effects of treatment. Several case studies among infants reported hematological improvement after vitamin B12 therapy, and rapid improvement of symptoms such as apathy and decreased activity. However, many infants continued to experience developmental delays several months after initiation of treatment [9, 12, 17]. There are few data on longer-term effects of treatment during infancy.
Environment
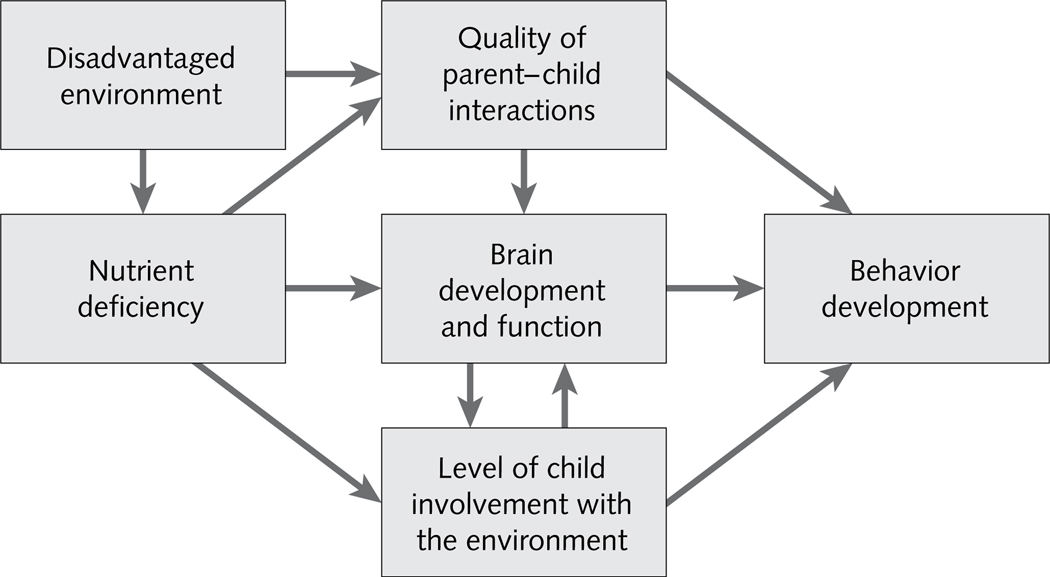
Nutrient deficiencies, such as vitamin B12 and folate deficiency, are more likely to occur in disadvantaged environments, which themselves have adverse effects on children [43]. In addition to direct effects on central nervous system development, through changes in neuroanatomy or neurotransmission, it is possible that behavior changes associated with micronutrient deficiencies alter the caregiving that the child receives, thereby compromising the child’s development even further [44]. For example, if a vitamin B12-deficient child is unable to elicit or to benefit from nurturant interactions from a caregiver, that child may be denied the enrichment that is known to promote early development. Animal studies have shown that alterations in maternal caregiving may also result in structural changes to the developing brain [45]. If these findings extend to humans, the result could be a child who experiences neurological changes together with limited environmental opportunities for enrichment. Over time, these combined influences may result in poor behavioral and developmental outcomes (fig. 2), suggesting that the impact of nutritional deficiencies on children’s behavior and development may be partially mediated through caregiving behavior [46]. Future research should consider how the caregiving system is related to child development and whether it mediates the effects of vitamin B12 deficiencies.
FIG. 2.
Association between nutritional deficiency and children’s behavior and development. Source: adapted from Levitsky and Barnes [46]
Depression among adults
Deficiencies in cobalamin and/or folic acid have also been related to symptoms of depression in adults. For example, investigators have found inverse relationships between symptoms of depression and plasma folate [47, 48] or plasma cobalamin [49]. One possible mechanism linking these vitamin deficiencies to depressive symptoms may be through elevated homocysteine and intracellular one-carbon metabolism. Plasma vitamin B12 and folate are required for the synthesis of methionine from homocysteine. Methionine is the precursor to S-adenosyl-L-methionine (SAM), a universal methyl donor active in metabolic pathways related to the synthesis of hormones, neurotransmitters, nucleic acids, and proteins, particularly in the brain. Elevated plasma homocysteine [50, 51] has been associated with low concentrations of SAM in plasma and cerebrospinal fluid [52], and with depressive symptoms. In addition, SAM has been used as an effective treatment for depression [52].
The evidence linking cobalamin status to the response to treatment of depression is also controversial. In a study among outpatients (mean age, 42 years) diagnosed with a major depressive disorder and treated with fluoxetine, treatment resistance was associated with low plasma folate, with no relationship to either cobalamin or homocysteine [53]. Yet, in a sample of depressed adults from Finland (mean age, 44 years), treatment outcome was positively associated with higher plasma cobalamin, but not folate [54]. In spite of the absence of clear findings related to cobalamin and folate, both vitamins have been recommended for the treatment of depression [55].
Summary
Although there is relatively clear evidence for the negative consequences of severe deficiencies in folate and vitamin B12 on the developing brain during infancy, and on depression during adulthood, neither the mechanisms, nor the impact of mild deficiencies, have been clearly specified. Both cobalamin and folic acid play important roles in the developing nervous system. Folic acid is necessary during early fetal development to prevent NTD, and cobalamin deficiency may interfere with early development through disruptions in myelination and dendritic formation or inflammation. Although treatment with cobalamin may remedy some of the negative effects of severe cobalamin deficiency on behavioral and developmental functioning, there is evidence suggesting that cobalamin deficiency early in life may compromise psychoeducational functioning through adolescence. The Institute of Medicine recommends continued research into the prevalence of cobalamin deficiency, methods to reduce the risk of deficiency resulting from malabsorption or a vegetarian diet, and the feasibility of fortification of grains [56]. Given the high prevalence of vitamin B12 deficiency in developing countries, future investigations should focus on the relationship between the timing of deficient and marginal vitamin B12 status and outcomes such as infant growth, cognition, social development, and depressive symptoms, along with strategies to prevent vitamin B12 deficiency.
References
- 1.Black MM. Nutrition and brain development. In: Walker WA, Duggan C, Watkins JB, editors. Nutrition in pediatrics. 3rd ed. New York: BC Decker; 2003. pp. 386–396. [Google Scholar]
- 2.Medical Research Council Vitamin Study. Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 3.Rosenberg IH. Science-based micronutrient fortification: which nutrients, how much, and how to know? Am J Clin Nutr. 2005;82:279–280. doi: 10.1093/ajcn.82.2.279. [DOI] [PubMed] [Google Scholar]
- 4.Hellegers A, Okuda K, Nesbitt RE, Jr, Smith DW, Chow BF. Vitamin B12 absorption in pregnancy and in the newborn. Am J Clin Nutr. 1957;5:327–331. doi: 10.1093/ajcn/5.3.327. [DOI] [PubMed] [Google Scholar]
- 5.Graber SE, Scheffel U, Hodkinson B, McIntyre PA. Placental transport of vitamin B12 in the pregnant rat. J Clin Invest. 1971;50:1000–1004. doi: 10.1172/JCI106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adhikari M, Coovadia HM, Hammond MG. Associations between HLA antigens and ephritic syndrome in African and Indian children in South Africa. Nephron. 1985;41:289–292. doi: 10.1159/000183599. [DOI] [PubMed] [Google Scholar]
- 7.Garewal G, Narang A, Das KC. Infantile tremor syndrome: A vitamin B12 deficiency syndrome in infants. J Trop Pediatr. 1988;34:174–178. doi: 10.1093/tropej/34.4.174. [DOI] [PubMed] [Google Scholar]
- 8.Srikantia SG, Redd V. Megaloblastic anaemia of infancy and vitamin B12. Br J Haematol. 1967;13:949–953. doi: 10.1111/j.1365-2141.1967.tb08865.x. [DOI] [PubMed] [Google Scholar]
- 9.Higginbottom MC, Sweetma L, Nyhan WL. A syndrome of methylmalonic aciduria, homocystinuria, megaloblastic anemia and neurologic abnormalities in a vitamin B12-deficient breast-fed infant of a strict vegetarian. N Engl J Med. 1978;299:317–323. doi: 10.1056/NEJM197808172990701. [DOI] [PubMed] [Google Scholar]
- 10.Lampkin BC, Saunders EF. Nutritional vitamin B12 deficiency in an infant. J Pediatr. 1969;75:1053–1055. doi: 10.1016/s0022-3476(69)80347-1. [DOI] [PubMed] [Google Scholar]
- 11.Sklar R. Nutritional vitamin B12 deficiency in a breast-fed infant of a vegan-diet mother. Clin Pediatr (Phila) 1986;25:219–221. doi: 10.1177/000992288602500409. [DOI] [PubMed] [Google Scholar]
- 12.Wighton MC, Manson JI, Speed I, Robertson E, Chapman E. Brain damage in infancy and dietary vitamin B12 deficiency. Med J Aust. 1979;2:1–3. doi: 10.5694/j.1326-5377.1979.tb112643.x. [DOI] [PubMed] [Google Scholar]
- 13.Gambon RC, Lentze MJ, Rossi E. Megaloblastic anaemia in one of monozygous twins breast fed by their vegetarian mother. Eur J Pediatr. 1986;145:570–571. doi: 10.1007/BF02429070. [DOI] [PubMed] [Google Scholar]
- 14.Hellebostad M, Markestad T, Seeger Halvorsen K. Vitamin D deficiency rickets and vitamin B12 deficiency in vegetarian children. Acta Paediatr Scand. 1985;74:191–195. doi: 10.1111/j.1651-2227.1985.tb10948.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuhne T, Bubl R, Baumgartner R. Maternal vegan diet causing a serious infantile neurological disorder due to vitamin B12 deficiency. Eur J Pediatr. 1991;150:205–208. doi: 10.1007/BF01963568. [DOI] [PubMed] [Google Scholar]
- 16.Renault F, Verstichel P, Ploussard JP, Costil J. Neuropathy in two cobalamin-deficient breast-fed infants of vegetarian mothers. Muscle Nerve. 1999;22:252–254. doi: 10.1002/(sici)1097-4598(199902)22:2<252::aid-mus13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 17.Stollhoff K, Schulte FJ. Vitamin B12 and brain development. Eur J Pediatr. 1987;146:201–205. doi: 10.1007/BF02343237. [DOI] [PubMed] [Google Scholar]
- 18.Graham SM, Arvela OM, Wise GA. Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J Pediatr. 1991;121:710–714. doi: 10.1016/s0022-3476(05)81897-9. [DOI] [PubMed] [Google Scholar]
- 19.von Schenck U, Bender-Gotze C, Koletzko B. Persistence of neurological damage induced by dietary vitamin B12 deficiency in infancy. Arch Dis Child. 1997;77:137–139. doi: 10.1136/adc.77.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Logan S, Martin S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev. 2001;2 doi: 10.1002/14651858.CD001444. CD001444. [DOI] [PubMed] [Google Scholar]
- 22.Beard J. Recent evidence from human and animal studies regarding iron status and infant development. J Nutr. 2007;134:524S–530S. doi: 10.1093/jn/137.2.524S. [DOI] [PubMed] [Google Scholar]
- 23.de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome coxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Chiron R, Dabadie A, Gandemer-Delignieres V, Balencon M, Legall E, Roussey M. Anemia and limping in a vegetarian adolescent. Arch Pediatr. 2001;8:62–65. doi: 10.1016/s0929-693x(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 25.Chowanadisai W, Kelleher SL, Lonnerdal B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J Neurochem. 2005;94:510–519. doi: 10.1111/j.1471-4159.2005.03246.x. [DOI] [PubMed] [Google Scholar]
- 26.Kopyta I, Jamroz E, Marszal E. Myelosis funicularis as a result of secondary vitamin B12 deficiency in a 9-year-old girl. Wiad Lek. 2002;55:228–234. [PubMed] [Google Scholar]
- 27.Hoey H, Linnell JC, Oberholzer VG, Laurance BM. Vitamin B12 deficiency in a breastfed infant of a mother with pernicious anaemia. J R Soc Med. 1982;75:656–658. doi: 10.1177/014107688207500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson PR, Jr, Roloff JS. Vitamin B12 deficiency in an infant strictly breast-fed by a mother with latent pernicious anemia. J Pediatr. 1982;100:917–919. doi: 10.1016/s0022-3476(82)80513-1. [DOI] [PubMed] [Google Scholar]
- 29.McPhee AJ, Davidson GP, Leahy M, Beare T. Vitamin B12 deficiency in a breast fed infant. Arch Dis Child. 1988;63:921–923. doi: 10.1136/adc.63.8.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen LH. Impact of vitamin B12 deficiency during lactation on maternal and infant health. Adv Exp Med Biol. 2002;503:57–67. doi: 10.1007/978-1-4615-0559-4_6. [DOI] [PubMed] [Google Scholar]
- 31.Fernstrom JD. Can nutrient supplements modify brain function? Am J Clin Nutr. 2000;71:1669S–1675S. doi: 10.1093/ajcn/71.6.1669S. [DOI] [PubMed] [Google Scholar]
- 32.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 33.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56:5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 34.Lovblad K, Ramelli G, Remonda L, Nirkko AC, Ozdoba C, Schroth G. Retardation of myelination due to dietary vitamin B12 deficiency: Cranial MRI findings. Pediatr Radiol. 1007;27:155–158. doi: 10.1007/s002470050090. [DOI] [PubMed] [Google Scholar]
- 35.Hall CA. Function of vitamin B12 in the central nervous system as revealed by congenital defects. Am J Hematol. 1990;34:121–127. doi: 10.1002/ajh.2830340208. [DOI] [PubMed] [Google Scholar]
- 36.Serin E, Gumurdulu Y, Ozer B, Kayaselcuk F, Yilmaz U, Kocak R. Impact of Helicobacter pylori on the development of vitamin B12 deficiency in the absence of gastric atrophy. Helicobacter. 2002;7:337–341. doi: 10.1046/j.1523-5378.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 37.Casterline JE, Allen LH, Ruel MT. Vitamin B12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J Nutr. 1997;127:1966–1972. doi: 10.1093/jn/127.10.1966. [DOI] [PubMed] [Google Scholar]
- 38.Rogers LM, Boy E, Miller JW, Green R, Sabel JC, Allen LH. High prevalence of cobalamin deficiency in Guatemalan schoolchildren: Associations with low plasma holotranscobalamin II and elevated serum methylmalonic acid and plasma homocysteine concentrations. Am J Clin Nutr. 2003;77:433–440. doi: 10.1093/ajcn/77.2.433. [DOI] [PubMed] [Google Scholar]
- 39.Allen LH. Folate and vitamin B12 status in the Americas. Nutr Rev. 2004;62:S29–S33. doi: 10.1111/j.1753-4887.2004.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 40.Dagnelie PC, Van Staveren WA, Hautvast JG. Health and nutritional status of ‘alternatively’ fed infants and young children, facts and uncertainties. II. Specific nutritional deficiencies; discussion. Tijdschr Kindergeneeskd. 1985;53:208–216. [PubMed] [Google Scholar]
- 41.Louwman MW, van Dusseldorp M, van de Vijver FJ, Thomas CM, Schneede J, Ueland PM, Refsum H, van Staveren WA. Signs of impaired cognitive function in adolescents with marginal cobalamin status. Am J Clin Nutr. 2000;72:762–769. doi: 10.1093/ajcn/72.3.762. [DOI] [PubMed] [Google Scholar]
- 42.van Dusseldorp M, Schneede J, Refsum H, Ueland PM, Thomas CM, de Boer E, van Staveren WA. Risk of persistent cobalamin deficiency in adolescents fed a macrobiotic diet in early life. Am J Clin Nutr. 1999;69:664–671. doi: 10.1093/ajcn/69.4.664. [DOI] [PubMed] [Google Scholar]
- 43.NICHD Network on Early Child Development. Duration and developmental timing of poverty and children’s cognitive and social development from birth through third grade. Child Dev. 2005;76:795–810. doi: 10.1111/j.1467-8624.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- 44.Shonkoff J, Phillips D. From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 45.Kuma H, Miki T, Matsumoto Y, Gu H, Li HP, Kusaka T, Satriotomo I, Okamoto H, Yokoyama T, Bedi KS, Onishi S, Suwaki H, Takeuchi Y. Early maternal deprivation induces alterations in brain-derived neurotrophic factor expression in the developing rat hippocampus. Neurosci Lett. 2004;372:68–73. doi: 10.1016/j.neulet.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Levitsky DA, Barnes RH. Nutritional and environmental interactions in the behavioral development of the rat: Long-term effects. Science. 1972;176:68–71. doi: 10.1126/science.176.4030.68. [DOI] [PubMed] [Google Scholar]
- 47.Hickie I, Naismith S, Ward PB, Scott E, Mitchell P, Wilhelm K, Parker G. Vascular risk and low serum B12 predict white matter lesions in patients with major depression. J Affect Disord. 2005;85:327–332. doi: 10.1016/j.jad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, Christensen H, Jorm AF. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–538. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- 49.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: The Rotterdam Study. Am J Psychiatry. 2002;159:2099–2101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 50.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tolmunen T, Hintikka J, Voutilainen S, Ruusunen A, Alfthan G, Nyyssonen K, Viinamaki H, Kaplan GA, Salonen JT. Association between depressive symptoms and serum concentrations of homocysteine in men: A population study. Am J Clin Nutr. 2004;80:1574–1578. doi: 10.1093/ajcn/80.6.1574. [DOI] [PubMed] [Google Scholar]
- 52.Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: A review of the evidence. Am J Clin Nutr. 2002;76:1158S–1161S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- 53.Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: Predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- 54.Hintikka J, Tolmunen T, Tanskanen A, Viinamaki H. High vitamin B12 level and good treatment outcome may be associated in major depressive disorder. BMC Psychiatry. 2003;3:17. doi: 10.1186/1471-244X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coppen A, Bolander-Gouaille C. Treatment of depression: Time to consider folic acid and vitamin B12. J Psychopharmacol. 2005;19:59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- 56.Institute of Medicine. Dietary reference intakes. Thiamin, riboflavin, niacin, vitamin B6, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]