Abstract
Mice with ablation of the Thra gene have cold intolerance due to an as yet undefined defect in the activation of brown adipose tissue (BAT) uncoupling protein (UCP). They develop an alternate form of facultative thermogenesis, activated at temperatures below thermoneutrality and associated with hypermetabolism and reduced sensitivity to diet-induced obesity. A consistent finding in Thra-0/0 mice is increased type-2 iodothyronine deiodinase (D2) mRNA in skeletal muscle and other tissues. With an improved assay to measure D2 activity, we show here that this enzyme activity is increased in proportion to the mRNA and as a function of the ambient cold. The activation is mediated by the sympathetic nervous system in Thra-0/0, as it is in wild-type genotype mice, but the sympathetic nervous system effect is greater in Thra-0/0 mice. Using D2-ablated mice (Dio2−/−), we reported elsewhere and show here that, in spite of sharing a severe deficiency in BAT thermogenesis with Thra-0/0 and UCP1-knockout mice, they do not have an increase in oxygen consumption, and they gain more weight than wild-type controls when fed a high-fat diet. UCP3 mRNA is highly responsive to thyroid hormone, and it is increased in Thra-0/0 mice, particularly when fed high-fat diets. We show here that muscle UCP3 mRNA in hypothyroid Thra-0/0 mice is responsive to small dose-short regimens of T4, indicating a role for locally, D2-generated T3. Lastly, we show that bile acids stimulate not only BAT but also muscle D2 activity, and this is associated with stimulation of muscle UCP3 mRNA expression provided T4 is present. These observations strongly support the concept that enhanced D2 activity in Thra-0/0 plays a critical role in their alternate form of facultative thermogenesis, stimulating increased fat oxidation by increasing local T3 generation in skeletal muscle.
Thyroid hormone is present in all vertebrates, where it controls the expression of genetic developmental or functional programs, the most illustrative example being the control of metamorphosis in amphibians (1). In homeothermic species, thyroid hormone acquires an additional physiological role, which is to support thermogenesis or heat production (reviewed in Ref. 2). Thyroid hormone stimulates the number and velocity of biochemical and physiological processes and, consequently, heat production. Such heat production is called obligatory thermogenesis, because it is the obligatory result of the vital energy transformations. In addition, in the last few decades, it has become evident that thyroid hormone is also a key player in the heat production for cold adaptation, called facultative thermogenesis. In mammals, this form of thermogenesis resides in the brown adipose tissue (BAT), which has the unique capacity of uncoupling ADP phosphorylation from respiration by expressing a protein called uncoupling protein (UCP), now UCP1, as new orthologs have been cloned (3). UCP1 can reduce the proton gradient through the inner membrane of the mitochondria in a regulated manner, so the energy accumulated in the gradient is dissipated as heat instead of being captured in ATP (4). In a cold-adapted rat, BAT can generate 400 W/kg in heat or 80 times that derived from basal metabolic rate of rat (5). Because of the reduced obligatory thermogenesis in hypothyroidism, BAT central sympathetic stimulation is increased, yet BAT fails to produce heat (6). Since this early observation, we have learned that thyroid hormone is also essential for the realization of the full thermogenic potential of BAT acting at different levels, from modulating the norepinephrine signal transduction to the expression of the UCP1 gene (Ucp1) and activation of the protein (2).
We have been studying a transgenic mouse lacking all known products of the thyroid hormone receptor α gene (Thra; Thra-0/0 mice) (7). Thra-0/0 mice, as other transgenic models lacking the bona fide receptor encoded by Thra, TRα1 have lower body temperature and bradycardia (7, 8). Our interest in this model was prompted by the belief that their lower body temperature reflected a thermogenic deficiency that could help us to understand the mechanisms whereby thyroid hormone increases obligatory thermogenesis. We found that the lower basal body temperature is not caused by a thermogenic deficiency, but it is a reflection of a reset of the hypothalamic thermostat to a lower temperature. However, Thra-0/0 mice are cold intolerant, i.e. they develop hypothermia at 4 C, a temperature mice normally endure in the wild, due to a failure of BAT to produce heat in response to adrenergic stimulation, even though all genes known to participate in cold-induced BAT thermogenesis respond normally (9).
Thermoneutrality (TN) temperature or zone is a narrow range of ambient temperature where a homeothermic animal is in thermal equilibrium with the environment, i.e. it can maintain its body temperature with just the heat resulting from basal metabolic rate, the obligatory thermogenesis, without the participation of any other body temperature regulatory mechanism. By definition, facultative thermogenesis is turned off at TN, which in the mouse is approximately 30 C (10). At room temperature, 8–10 C below TN, BAT contributes to 30% of heat production in rats (5) and about 40% in mice (9, 10). Remarkably, in spite the BAT deficiency, Thra-0/0 mice are under no apparent distress at room temperature, and moreover, they do not have reduced, but increased, metabolic rate (9). At room temperature, Thra-0/0 mice eat more than appropriate controls, yet are leaner and gain less weight when challenged with a high-fat diet (HFD), showing a preference to burn fat over carbohydrates (11). Such a phenotype is exaggerated at cooler temperatures, whereas it is attenuated as ambient temperature becomes warmer and disappears at TN (11). Thus, in the absence of BAT thermogenesis, Thra-0/0 mice develop an alternate form of facultative thermogenesis (AFT).
A consistent finding in Thra-0/0 mice has been an increase in type-2 iodothyronine deiodinase (D2) mRNA. D2 catalyzes the conversion of the main product of the follicular thyroidal secretion, T4, which has very weak metabolic activity (12), into T3, intrinsically 10 times more active. At the prevailing concentrations, little or no T4 is found bound to the thyroid hormone receptors, so it is considered that T3 is ultimately responsible for virtually all the thyromimetic potential of the thyroid secretion (13). D2 mRNA has been found elevated in muscle and BAT of Thra-0/0 mice (11), as well as in inguinal fat of another model of BAT deficiency, the UCP1 null mice (Ucp1−/−) (14). We have reported that there is measurable D2 activity in microsomes of skeletal muscle of C57Bl/6 mice (15) and have reported in abstract form that D2 activity is increased in Thra-0/0 mice (16, 17). In an accompanying paper (45), we demonstrated that D2 resides in sarcoplasmic reticulum and that its activity has been underestimated in skeletal muscle due to a number of factors that hamper its extraction and inhibit its activity. Thus, highly purified red muscle microsomes have D2-specific activities higher than crude microsomes of BAT of mice at room temperature and comparable with that of maximally cold-stimulated BAT. Here, we show that D2-specific activity is higher in red muscle of Thra-0/0 mice than in wild-type (WT) controls, that its activity is regulated by the sympathetic nervous system, and that it plays a key role in the AFT and metabolic phenotype of Thra-0/0.
Materials and Methods
Mice and treatments
All studies described here were approved by the Baystate Institutional Animal Care and Use Committee, protocol 05-003, version 2. All mice were C57Bl/6 mice from our colony. Thra-0/0 are devoid of all know products of the Thra gene and were created in the laboratory of Jacques Samarut (Lyon, France) (7). We have had these mice for over 6 yr, and they have been backcrossed in the C57Bl/6 mice innumerable times. Because they have no significant problem in fertility or survival, we keep the homozygous lines Thra+/+ and Thra-0/0 by inbreeding. They have basically maintained the original phenotype (9) with regard to temperature homeostasis and energy balance (11). In agreement with their BAT deficiency, Thra-0/0 isolated brown adipocytes have lower oxygen consumption, 70% reduced response to norepinephrine and oleate, whereas their isolated mitochondria show no detectable defect (Ramadan, W. et al., unpublished data) in agreement with our conclusion that the BAT deficiency is due to inability to activate UCP (11). D2 knockout (KO) mice were created by Schneider et al. (18) in a collaboration with Larsen, who gave the mice to us. They have been backcrossed 12 times into the C57Bl/6 background.
Mice were housed in a dedicated room, at four to five per cage when not being under study or pregnant, in plastic cages with ground corncob bedding. Temperature was kept at approximately 21 C with a 12-h light, 12-h dark cycle with 50–60% humidity. Unless otherwise indicated, they were fed a standard mouse diet from Harlan-Teklad, Rodent Teklad Global no. 2018 or a Teklad no. 7001 (Harlan Teklad, Indianapolis, IN). Both diets are very similar, containing approximately 3.3 Kcal/g, with about 12, 60, and 19% of the calories from fat, carbohydrate, and proteins, respectively. Where indicated, mice received a HFD TD 95121, also called Western diet, but without the supplement of cholesterol. The HFD has 5.24 Kcal/g and contains 42.7, 42, and 15.3% of the absorbable calories as carbohydrates, fat, and protein, respectively.
Hypothyroidism was induced by feeding a low iodine diet containing 0.15% propylthiouracil (PTU) by weight, TD 97061. Because of the resistance of mice to developing profound hypothyroidism, we added 0.5% NaClO4 to the drinking water. This regimen was maintained for a minimum of 6 wk, unless otherwise indicated. Typically, serum T4 becomes undetectable or barely so, whereas serum T3 is at most reduced to 50% of the euthyroid level.
Treatments
As indicated with the corresponding experiments, hypothyroid mice were given T4 or T3. T4 was given at a dose intended to replace normal secretion of 0.7 μg/100 g body weight (BW), sc, twice a day for 2 d. T3 was given at a supraphysiological dose of 3 μg/100 g BW, sc, twice a day for 2 d. The adrenergic system was blocked in several ways as indicated with the corresponding experiments. Propranolol was given at 10 μg/g ip, whereas prazosin was given also ip at a dose of 4 μg/g approximately 15 min before exposing mice to cold. For more prolonged sympathetic blockade, the same doses of propranolol or prazosin were given for 2 d every 12 h ip. α-Methyl-p-tyrosine (α-MPT), a blocker of tyrosine hydroxylase was given in 4 doses of 300 μg/g every 12 h to deplete the stores of catecholamines (19, 20). Cholic acid (CA) was given twice by gavage at 25 mg/100 g every 12 h before euthanizing the mice.
Blood and tissue sampling
Blood was rapidly obtained from the inferior vena cava or by cardiac puncture (usually 0.5–1 ml), under the isoflurane anesthesia, after which mice were killed by cervical dislocation to proceed to tissue harvesting. Blood was allowed to coagulate at room temperature, and cleanly collected serum was stored at −20 C until analyzed. Tissues were snap frozen in liquid nitrogen and kept at −80 ± 1 C until analyzed. Soleus, quadratus femoris, gastrocnemius (separating red fibers in the core of the muscle from fast-twitch peripheral fibers), vastus lateralis, and BAT were rapidly dissected, weighed, and either used fresh for subcellular fractionation and D2 assay or snap frozen in liquid nitrogen for other measurements, mainly mRNA indicated where appropriate.
Biochemical assays
These included D2 assay, total T4 and T3 levels, and mRNA assays by real-time quantitative RT-PCR.
D2 activities
As discussed in the accompanying paper, D2 activity in skeletal muscle has been underestimated. This has resulted from low extraction of inner cell membranes as well as factors inherent to muscle that reduce activity. We introduced a large number of changes in the methods to remedy these issues. Although the highest specific activities were obtained after washing microsomes obtained between 38 and 50% sucrose (gradient microsomes) with 0.1 m Na2CO3 (washed microsomes), the yield of protein after the Na2CO3 wash was too low and required large number of mice. Therefore, all the assays were performed with gradient microsomes, except at indicated time, when we used conventionally prepared microsomes (crude microsomes). The assay was performed using either 125I-T4 or 125I-rT3 as substrates and measurement 125I(−) release or 125I-T3 as products. 125I(−) was extracted and counted directly, and 125I-T3 was separated by paper chromatography and counted as described in the accompanying paper. The assay medium was 0.1 m Na phosphate buffer (pH7.0), containing EDTA-free protease inhibitors, 20 mm DTT, 25 nm unlabeled T3 to minimize the effect of small amounts of D3 (inner ring deiodinase) in some preparations, and 1 mm PTU, regardless of whether the substrate was rT3 or T4. The volume was 300 or 100 μl, depending on whether we measured 125I(−) or 125I-T3 as product.
Serum total T4 and T3 were measured by RIA using commercial kits (Diagnostic Products, Los Angeles, CA) with the modifications described elsewhere to optimize assay for mouse serum (11).
RNA isolation and PCR analysis
Tissue RNA was extracted and prepared for quantitative RT-PCR with reagents from QIAGEN (Valencia, CA). RT-PCR was done in a MX4000-v4.20 thermocycler (Stratagene, Cedar Creek, TX). Gene sequences were obtained from GenBank mouse genome (http://www.ncbi.nlm.nih.gov/entrez?db=gene). Primers were designed (PrimerQuest, http://www.idtdna.com/Scitools/Applications/Primerquest) to obtain products of 110–220 bp, spanning when possible at least one intron to control for eventual DNA contamination. Primers for mouse UCP3 were: forward, 5′-AGA AGT TGC TGG AGT CTC ACC TGT-3′ and reverse, 5′-GGA GCG TTC ATG TAT CGG GTC TTT-3′; and for liver mitochondrial glycerol 3-phosphate dehydrogenase (mGPD): forward, 5′-TTG ACA TCC TTG TTA TCG GAG GCG-3′ and reverse, 5′-GTG AAG GGC TTC TTT CAC CAT CCT-3′. Abundance of mRNA products was quantified by SYBR-green fluorescence (Stratagene), and mRNA were normalized by housekeeping genes cyclophilin D or Rp13L3 and expressed in arbitrary units.
In vivo measurements
Energy expenditure
Energy expenditure was measured basically as described (11), by indirect calorimetry in an open-circuit system (Qubits Systems, Kingston, Ontario, Canada). Mice were housed in individual, airtight plastic boxes, through which air was pumped at a constant flow rate, and where oxygen consumption (VO2) and carbon dioxide production were calculated from the corresponding concentrations in the air going in and out of the cage multiplied by the flow rate. Electrodes were calibrated before each experiment against commercial gas mixtures of accurate composition. Unless otherwise indicated, measurements were done for 24 h, six to seven mice at a time, three to four from each genotype. In experiments requiring repeated measurements on more than seven mice per day, measurements were made on consecutive days. For each mouse, readings were taken every 35 min, because this is what it takes to complete the gas readings on eight mouse chambers (one empty chamber is used as reference), so that each mouse VO2 was measured approximately 41–42 times in 24-h. Twenty-four-hour profiles were generated by the means obtained from three to six mice in successive 35-min cycles. VO2 is expressed in [ml × h−1 × 100 g−0.75] based on well-established principles (10). Percent of fat oxidation contribution to energy expenditure was calculated from well-established equations based on the respiratory quotient also called respiratory exchange ratio (see Ref. 21 and references therein). Gas exchange measurements were performed at “room temperature” (20–21 C) in a thermo-regulated walk-in chamber that maintains ambient temperature within 0.1 C of the set temperature (SureTemp, Raleigh, NC).
Food intake
Food intake, along with feces and urine collection, was measured in individual mouse metabolic cages (Mini Mitter; Respironics Co., Eugene, OR) placed at the desired ambient temperature. Food consumed was recorded daily for at least 3 d, averaged, and expressed on a per day and 100 g0.75 BW basis, except where indicated.
Body composition
This was measured by nuclear magnetic resonance using an ad hoc mouse device, ECHO MRI 1000, from Echo Medical Systems (Houston, TX).
Statistical analysis
Results are expressed as mean ± sem. Experiments were repeated at least once in experimental groups of three to six mice, unless indicated otherwise. Experiments involving several treatment or time groups were analyzed by ANOVA followed by post hoc tests for multiple comparisons. Two-way ANOVA was used to compare the effects of treatments on two experimental groups. Individual means were then compared by Bonferroni's test, whether to compare the differences in response to the experimental variable between genotypes (in a Two-way ANOVA) or to compare the effect of two levels of a variable, e.g. two different temperature within a genotype.
Results
We have reported (16, 17) that D2 activity is increased in Thra-0/0 mice. Figure 1 shows one of our earliest results obtained in mice maintained at room temperature. Indeed, there is significant D2 activity in conventionally prepared, crude microsomes of soleus muscles, and this is two to three times higher in Thra-0/0 than in WT soleus microsomes, as is the D2 mRNA. D2 activity is also increased in BAT homogenates of Thra-0/0 mice, where the activity is also greater by about the same factor. However, the level of activity in soleus microsomes was barely half that of BAT crude homogenates. Therefore, our earlier results show the difference between the two genotypes expected from the mRNA levels, but the level of D2 activity in muscle is low, as we reported in detail in crude microsomes from WT mice (15).
Fig. 1.
D2 activity in a BAT whole homogenate (Homg.) and soleus crude microsomes (Micrs.) from WT and Thra-0/0 mice. Activity was measured as 5′-iodine release from 2 nm 125I-rT3 by about 100 μg of homogenate protein or 30–40 μg of crude microsomal protein for 90 min. Note that in both tissues, the activity in Thra-0/0 preparations is two to three times that of the WT mice, as it occurs with D2 mRNA (9, 11). Data were analyzed by two-way ANOVA followed by Bonferroni's test. n = 8.
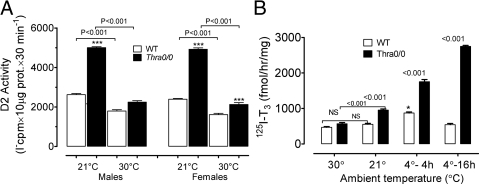
With the improvements in methodology, we find markedly higher levels of D2-specific activity in skeletal muscle and can clearly see the differences between the two genotypes. This allowed us to study aspects of the regulation, validate early experiments, and show the physiological relevance of the increased activity in skeletal muscle of Thra-0/0 mice. Thus, Fig. 2 shows the effect of ambient temperature on D2 activity in soleus gradient microsomes. Figure 2A shows the effect of keeping WT or Thra-0/0 mice at TN for about 24 h compared with those reared at room temperature. The figure also shows that there are no substantial gender differences. The sensitivity of D2 to this small temperature change is evident, because in both genotypes, D2 activity drops significantly when transferred from room temperature to TN. However, although the decrease is only 20–30% in WT mice, in Thra-0/0 mice, the reduction in D2 activity at TN is 5- to 6-fold greater, both in males and females. In males, at TN, there is usually no difference in D2 activity between the two genotypes, but in females, at least in this experiment, D2 activity was still modestly but significantly higher in Thra-0/0 than in WT females. Fig. 2B shows the effect of a wider range of ambient temperatures in males of both genotypes. To highlight the relevance of the changes, in this experiment, we measured 125I-T3 production. In WT mice, there was no effect between room temperature and TN, whereas there was a significant increase after 4 h of exposure to 4 C, but this increase did not persist if the cold was prolonged for 16 h. In Thra-0/0 mice, there was a significant doubling in D2 activity between TN and room temperature, similar to that observed in Fig. 2A. After 4 h at 4 C, there was a further increase, with D2 in Thra-0/0 doubling the response of WT mice. After 16 h of cold exposure, in contrast to WT, the D2 activity of which returned to baseline, that of Thra-0/0 mice, further increased more than 40% over the 4-h cold exposure value, becoming about 5-fold that of WT mice. These are very meaningful results as will be discussed later, supporting a role of D2 in AFT in Thra-0/0 mice.
Fig. 2.
Effect of ambient temperature on D2 activity of highly purified soleus (gradient) microsomes of WT and Thra-0/0 mice. A, Comparison of male and female gradient soleus microsomes at 30 and 21 C. Ten micrograms of microsomal protein were assayed for the production of 5′ 125I(−) from 1.5 nm 125I-T4 in the presence of 25 nm unlabeled T3 to avoid interference of D3. B, In a similar experiment, we tested the effect of cold, 4 C, for 4 and 16 h, this time measuring 125I-T3 as product from 125I-T4 but in otherwise identical assay conditions. There was no difference between WT and Thra-0/0 at 30 C, nor was there a significant stimulation of D2 by keeping WT mice at 21 C, but as in A, there was doubling of activity in Thra-0/0 mice with the move from TN to 21 C. In WT mice cold significantly stimulated D2 activity during 4 h but not during 16 h, whereas D2 activity increase substantially more in Thra-0/0 at 4 h, and the activity continued to increase as the cold exposure was prolonged to 16 h (5- to 6-fold the level of WT). NS, Statistically non-significant.
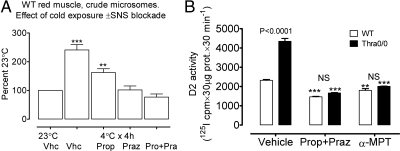
What drives the increase in D2 activity? This is illustrated in two experiments summarized in Fig. 3. In an early experiment, using crude microsomes from WT mice, we showed that a 4-h cold exposure induced an increase in D2 activity that was abolished by blocking the sympathetic nervous system. Propranolol alone significantly reduced D2 activity but did not abolish the increase, whereas prazosin totally abolished the stimulation, and a combination of propranolol and prazosin reduced D2 activity below the baseline. In Fig. 3B, we asked whether the higher activity seen at room temperature in Thra-0/0 was also dependent on sympathetic activity. It is evident that in WT and Thra-0/0 mice, skeletal muscle activity at room temperature requires the sympathetic nervous system. As previously shown (Fig. 1), D2 activity in Thra-0/0 mice is at least double that of WT mice at room temperature (P < 0.0001). However, in both WT and Thra-0/0, propranolol plus prazosin reduced D2 activity below that of controls and abolished the difference between WT and Thra-0/0. Depleting catecholamines with α-MPT (19, 20) also resulted in a decrease in D2 activity below the WT control value and the disappearance of the difference between the two genotypes. Thus, in both WT and Thra-0/0 mice, at room temperature, there is muscle D2 activity, which is in part sustained by sympathetic activity, with full adrenergic blockade bringing D2 activity below the baseline and causing the differences between the two genotypes to disappear. Therefore, it is likely that the increased D2 activity seen in Thra-0/0 mice at room temperature is the result of increased sympathetic tone, probably a response to the lack of BAT thermogenesis that in normal mice contributes to 40% of the heat production at 21 C (9).
Fig. 3.
Cold-dependent increase of D2 activity in muscle microsomes depends on sympathetic stimulation. A, Crude microsomes (conventional preparation) from a mix of red muscles (soleus, quadratus femoris, and red part of gastrocnemius) obtained from WT mice. Activity was measured as 5′ 125I(−) release from 2 nm 125I-rT3 by 100 μg of crude microsomal protein over 90 min in the presence 100 nm unlabeled T3. Four-hour cold exposure was associated with a 2.5-fold increase in D2 activity that was partially but significantly reduced by propranolol and totally abolished by prazosin ± propranolol. B, A similar experiment was performed in gradient microsomes of WT or Thra-0/0 mice to demonstrate the participation of sympathetic stimulation at room temperature. Effect of sympathetic blockade of D2 activity in 10 μg of gradient microsomal protein measuring 5′ 125I(−) release from 1.5 nm 125I-T4 in the presence of 25 nm unlabeled T3 for 30 min. Note the usual more than 2-fold higher D2 activity at 21 C and how this was reduced to the same low level by a combination of prazosin + propranolol or pretreatment with α-MPT, an inhibitor of catecholamine synthesis. Asterisks indicate statistically significant differences with vehicle-treated controls. ***, P < 0.001; **, P < 0.01. Vhc, Vehicle; Prop, propranolol; Rraz, prazosin; Pro, propranol; Pra, prazosin; NS, non-significant; SNS, sympathetic nervous system.
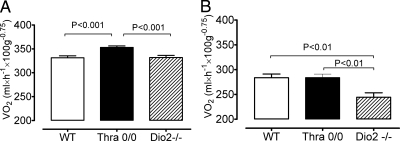
The most important question of these studies was whether the increased D2 activity contributed to the AFT, i.e. the increase in VO2 seen in Thra-0/0. Recall that this increase is cold dependent and disappears at TN, along with the resistance to diet-induced obesity (11). Both Thra-0/0 and Dio2−/− mice have a severe deficiency in BAT thermogenesis (9, 22), but Dio2−/− mice have not been reported to have increased energy expenditure (VO2). Representative results are shown in Fig. 4. Because the increase in VO2 of Thra-0/0 at room temperature is highly dependent on food availability, as is the capacity of these mice to defend body temperature, we examined VO2 for 24 h both in the presence (Fig. 4A) and in the absence (Fig. 4B) of food for 24 h. In the presence of food, the VO2 of all mice was higher, and as expected, Thra-0/0 showed a significant increase in VO2, whereas in D2KO mice, VO2 was not different from that of WT mice. When they were without food, the VO2 was reduced in the three genotypes. As expected, in Thra-0/0 mice, VO2 was not higher now than that of WT mice, but strikingly that of Dio2−/− mice was lower than in the other two genotypes. Mice did not lose significant weight when they had access to food, although Thra-0/0 mice had a clear trend to lose more, but when they were without food for 24 h, all three genotypes lost about the same amount of weight (data not shown). Thus, even though both Thra-0/0 and Dio2−/− mice have impaired BAT thermogenesis, Thra-0/0 mice, but not Dio2−/− mice, have an increase in overall thermogenesis at room temperature. This supports the idea that the increase in D2 activity seen in muscle (and possibly other tissues) of Thra-0/0 mice plays a role in AFT and the spendthrift phenotype of these mice.
Fig. 4.
Energy expenditure measured as VO2 in WT, Thra-0/0, and D2KO mice (Dio2−/−) with (A) and without (B) food. Water was provided ad libitum. Measurements were done for 24 h, at 22 C, with a total of 45–46 measurements per mouse with six mice per group. Note than in the presence of food Thra-0/0 mice have higher VO2 than the two other genotypes. In the absence of food, VO2 is expectedly lower and more so in Dio2−/−.
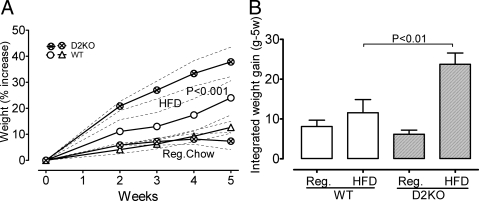
A relevant consequence of the more energy-demanding AFT is that the Thra-0/0 mice are less sensitive to diet-induced obesity, which is also a cold-dependent condition that disappears if these mice are placed at TN (11, 23). If the increase in D2 activity played a role, Dio2−/− mice should not be less, but perhaps more, sensitive to diet-induced obesity. As shown in Fig. 5, in marked contrast with Thra-0/0 mice kept at room temperature, Dio2−/− mice fed a HFD for 5 wk gain significantly more weight than do WT mice (P < 0.001). On standard rodent chow, these mice did not gain more weight, suggesting a role for D2 in oxidizing excess dietary fat and, consequently, Dio2−/− mice having a problem to deal with HFD. This confirms our previous findings reported elsewhere in detail (Marsili, A., C. Aguayo-Mazzucato, T. Chen, A. Kumar, M. Chung, E. P. Lunsford, J. W. Harney, T. Van-Tran, W. Ramadan, C. Chou, S. Bonner-Weir, P. Reed Larsen, J. E. Silva, and A. M. Zavacki, submitted for publication). Data just reported (24) are in apparent conflict with our findings and will be addressed under Discussion.
Fig. 5.
Weight gain by Dio2−/− mice on regular rodent chow or in a HFD. Study was carried out at room temperature (21 C). Data were analyzed by two-way ANOVA followed by Bonferroni's test. A, Weight gain is expressed as percent of the baseline, because this differed slightly among the experimental groups, even though they were of similar age. On HFD, the Dio2−/− mice gain significantly more weight than the WT (P < 0.001). B, The area under the curve of the weight gain (in grams) was calculated for each mouse to give an idea of the energy accumulated during 5 wk. The columns represent the mean ± sem of each group of six mice. On HFD, the Dio2−/− mice gained significantly more weight (accumulated more energy). Food intake was not different between the two groups. Reg, Regular diet.
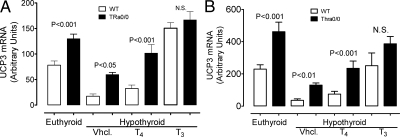
To obtain another perspective of the increase in muscle D2 in Thra-0/0 mice, we investigated a potential role for D2 activity in the UCP3 gene expression, usually stimulated in the skeletal muscle of Thra-0/0 mice, particularly when fed a HFD (11). UCP3 is known to be responsive to T3 (25). Figure 6 shows two independent experiments exploring the regulation of UCP3 mRNA by D2-generated T3. As expected (25), UCP3 in WT mice is markedly reduced in hypothyroidism. The hypothyroid mice were divided in three groups and were treated with vehicle, with a dose of T4 estimated to be close to the daily production rate for 2 d or, as positive control, a high dose of T3 of about 10 times the estimated daily production rate. Measured approximately 12 h after the last injections, serum concentration of T4 was slightly higher than normal, whereas serum T3 was over five times the euthyroid level (data not shown). The results of these two independent experiments were virtually identical. The T4 regimen significantly increased UCP3 mRNA in WT mice but did not normalize it, whereas T3 stimulated the gene over the euthyroid control level. In euthyroid Thra-0/0 mice, UCP3 mRNA was higher, and it decreased less than in euthyroid and hypothyroid WT mice, respectively. Most importantly, UCP3 mRNA was nearly normalized in hypothyroid Thra-0/0 mice by the low regimen of T4. As expected, the high dose of T3 brought UCP3 to a level slightly than in euthyroid WT mice. These results are reminiscent of the response of UCP1 in BAT of hypothyroid rats to such minitreatments with T4 and illustrate the power of D2 in generating T3 locally (26).
Fig. 6.
Possible role of D2 in regulating the expression of UCP3, a thyroid hormone-sensitive gene. A and B, Two independent but otherwise similar experiments. Mice were rendered hypothyroid with a PTU-low iodine diet (in B, we added NaClO4 to deepen the hypothyroidism) and treated for 2 d with an estimated replacement dose of T4 or a dose of T3 equivalent to 10 times the daily production rate (see text for foundation). Both experiments show how dramatically falls UCP3 mRNA in hypothyroidism. However, levels are higher in euthyroid Thra-0/0 than WT. The small dose and short treatment with T4 resulted in a significant increase in UCP3 mRNA in Thra-0/0 but not in WT. T3 treatment increased UCP3 in all mice, indicating that Thra is not necessary for this action of thyroid hormone and that the effect was mediated by Thrb. NS, Non-significant; Vhcl, vehicle.
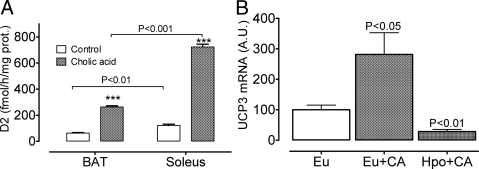
Bile acids have been shown to stimulate energy dissipation by stimulating D2 in BAT (27). Bile acids stimulate D2, and the thermogenic effect is not seen in D2KO mice, indicating a requirement of D2 for the effect and supporting the idea that such an effect is mediated by D2-generated T3 in BAT. These studies, however, did not look at the activity of D2 in other tissues, notably skeletal muscle, and therefore the contribution of this tissue to the global thermogenic response and reduced sensitivity to diet-induced obesity in the bile acid-treated mice remained unknown. To address the magnitude and tissue extent of bile acid stimulation of D2, we housed mice at TN for 2 d to avoid the confounding effect of adrenergic stimulation, and during this time, we gave them CA by gavage. Figure 7A shows that CA stimulates D2 activity in both BAT, as reported (27), but also stimulates D2 activity in soleus microsomes, and actually three times more than in BAT microsomes. To prove that the stimulation of UCP3 was not due to CA per se, we measured the response to CA in soleus muscle of euthyroid and hypothyroid mice. In hypothyroid mice, CA treatment had the opposite effect, decreasing UCP3 mRNA levels.
Fig. 7.
Effect of CA on D2 activity and the responsiveness of UCP3 mRNA. A, CA was given by gavage to WT mice. Activity was measured in crude microsomes with 2 nm 125I-rT3 in WT mice kept at 28 C. Baseline D2 activity was modestly but significantly higher in soleus than in BAT, and in both tissues, the biliary acid increased the activity by 5- to 6-fold, reaching significantly higher levels in soleus than in BAT. B, CA was given as in A to euthyroid and hypothyroid WT mice (PTU + low iodine diet) at room temperature. Note that in euthyroid but not in hypothyroid mice (undetectable serum T4) CA increased UCP3 mRNA in soleus muscle. Indeed, CA significantly decreased UCP3 mRNA levels vs. euthyroid controls. ***, P < 0.001; see text. Eu, Euthyroid; Hpo, hypothyroid.
Discussion
BAT is present only in mammals, and a large body of evidence indicates that it plays an essential role in cold adaptation by generating heat in a facultative manner (facultative or adaptive thermogenesis) (4). In addition, early observations showed that BAT was activated in various models of rodent overfeeding (28), leading to the idea that the tissue also played a role in dissipating calories ingested in excess as heat, a concept that received enthusiastic support from the selective ablation of the tissue by the transgenic expression of diphtheria toxin from the UCP1 BAT-specific promoter enhancer (29). Therefore, when the phenotype of UCP1-ablated (Ucp1−/−) mice was described, the cold intolerance was expected, but it was surprising that these mice were not obese despite eating more than WT controls (30). We made the same observation in another model of BAT deficiency, the Thra-0/0 mice (9). In both models, the reduced sensitivity to diet-induced obesity was lost as the environment became warmer and disappeared at TN (11, 23), indicating that in the absence of BAT, other, additional energy-demanding mechanisms were activated to defend body temperature. A corollary of these results is that BAT represents probably the culmination of an effective and fuel-efficient thermogenic tissue (reviewed in Ref. 31). A difference between the two models Thra-0/0 and Ucp1−/− is that the former has a readily demonstrable increase in metabolic rate (as shown once again here), whereas Ucp1−/− mice do not. The reason for this difference is not apparent but may be related to the neonatal protection of Ucp1−/− mice from cold stress (32), which may prevent a reprogramming in the metabolism to survive the cold stress of birth. Alternatively, it may be due to the lack of Thra in other tissues, because Thra is expressed in many other tissues, where its absence might lead to a hypermetabolic state (33), whereas Ucp1 is expressed only in BAT.
Regardless, a notable finding in Thra-0/0 mice was an increase in D2 mRNA (11) and, as demonstrated here, in D2 activity as well. In an accompanying paper, we demonstrated that there is a high D2 activity in red muscle microsomes, similar to that of cold-stimulated BAT microsomes. We estimate that the soleus muscles alone may produce several nanograms of T3 per day.
Here, we show that as it occurs with metabolic rate and food consumption in Thra-0/0 mice, muscle D2 activity is highly sensitive to cold. Although the activity is two to three times higher in mice kept at room temperature, 8–10 C below TN, the difference disappears at TN (Fig. 2). Furthermore, we reported (17) and show here (Fig. 3A) that 4 h at 4 C clearly stimulated D2 activity and that this stimulation was abolished by prazosin alone or in combination with propranolol. However, we have also reported elsewhere that in WT mice, overnight exposure (16 h) to cold was not associated with stimulation of D2 activity in crude microsomes from red muscle (15). Here, with improvements to increase the D2 assay sensitivity, we explain these apparently conflicting results. As shown in Fig. 2B, WT mice soleus microsomes double the production of T3 after a 4-h exposure to 4 C, as reported previously. In contrast, even though D2 was stimulated by 4 h of cold exposure in WT mice, it returned to baseline after 16 h of cold exposure, whereas in Thra-0/0 mice, D2 response was more vigorous at 4 h, about double that of WT, and after 16 h of cold exposure increased further instead of returning to normal as in WT mice. Indeed, by 16 h of cold exposure, D2 activity increased in Thra-0/0 microsomes to more than 5-fold the level of WT mice. This is because in mice living below TN, BAT is active and primed, contributing 30–40% to heat production (5, 9), UCP1 gene transcription is activated within seconds of cold exposure, and by a few hours of cold exposure, BAT thermogenesis is activated and sufficient to maintain body temperature (34). This does not occur in Thra-0/0 mice, which have disabled BAT, unresponsive to norepinephrine (9), and therefore, they need not only to maintain but to stimulate further AFT. These findings are not only consistent with D2 playing a role in AFT, but the time course of events adds support to the concept that AFT is a compensation to the lack of BAT thermogenesis.
The activation of D2 is mediated by the sympathetic nervous system, and this sustains the increased levels of activity seen at room temperature in Thra-0/0 mice, because the differences with WT disappear by blocking adrenergic receptors or depleting the mice of catecholamines. Furthermore, in both WT and Thra-0/0 mice, the adrenergic blockade reduced the level of D2 below baseline, indicating that sympathetic stimulation is not unique to Thra-0/0; thus, even in normal mice, D2 plays a role that is regulated by the sympathetic nervous system.
Further support for a role of increased D2 activity in Thra-0/0 mice to maintain body temperature and causing or contributing to the hypermetabolic state, leanness, and reduced sensitivity to diet-induced obesity comes from the comparison of Thra-0/0 mice with D2-ablated mice (Dio2−/−) and WT mice at room temperature (Fig. 4). Note that Dio2−/− mice also have a BAT thermogenic deficiency, with impaired activation of the norepinephrine signaling pathway, lipolysis, and UCP synthesis and activation (22). If such deficiency in Thra-0/0 and Ucp1−/− mice is partially compensated by an AFT, then Dio2−/− mice should also show a similar phenotype unless D2 itself plays a role. Our data support the concept that the increased D2 activity plays an important role. We show that the 24-h mean VO2 of Thra-0/0 is significantly higher than in WT mice and Dio2−/−. When VO2 is measured for 24 h in the absence of food, it is reduced in all three genotypes but much more in Dio2−/− mice.
Moreover, Thra-0/0 and Ucp1−/− mice eat more and gain less weight than controls when reared at room temperature, they are less sensitive to HFD than controls, and such difference disappears when ambient temperature approaches TN (22, 23). We show here (as reported elsewhere, Marsili, A., C. Aguayo-Mazzucato, T. Chen, A. Kumar, M. Chung, E. P. Lunsford, J. W. Harney, T. Van-Tran, W. Ramadan, C. Chou, S. Bonner-Weir, P. Reed Larsen, J. E. Silva, and A. M. Zavacki, submitted for publication) that at room temperature Dio2−/− mice gain significantly more weight when exposed to a HFD than WT controls. This is in marked contrast with the other two models of BAT-deficient thermogenesis. This furthers suggests that D2 may be necessary for using fat as fuel. In this regard, we find that the respiratory exchange ratio of D2KO mice on the HFD used here, containing 43 and 42% of the calories as fat and carbohydrates, is significantly higher than in WT mice (Silva, J.E., unpublished data). These findings, however, are in conflict with those just reported by Castillo et al. (24), in that D2KO mice fed a HFD gain more weight than WT controls at TN but not at room temperature (22 C). The explanation for the discrepancy is not readily evident. In both studies, D2KO mice are from the same lineage, are in the same genetic background (C57Bl/6), and were fed the same diet. A possible explanation is that the initial weight of the D2KO mice was much lower than of WT control in Castillo et al. (24), approximately 19 vs. 26–27 g, as calculated from the reported weight gain and final weight (they only report the weight change in grams).
Although the role of UCP3 in metabolism has not yet been clearly defined, UCP3 levels increased in animals fed HFD and in starvation, a condition when the muscle turns to fat as fuel (35–37). UCP3 is thyroid hormone responsive (25), and it is increased in Thra-0/0 mice (11). This made it an ideal model gene to probe the role of D2 in stimulating a relevant thyroid hormone-responsive gene. As shown in Fig. 6, two independent experiments show a marked reduction of UCP3 mRNA with hypothyroidism. To test the role of D2, we used the same paradigm that we used to demonstrate the role D2 as a local source of T3 in BAT, namely to make animals hypothyroid and compare the effect of a small dose-short treatment with T4 on a target gene [UCP1 in BAT (26) and UCP3 here] with that of a higher dose of T3 as positive control (26). Such treatment with T4 would not cause a substantial elevation of circulating T3 levels, whereas the T3 injection would. As happened with UCP1 in BAT, this small-short regimen of T4 increased UCP3 in WT but nearly normalized it in Thra-0/0 mice, whereas in the two genotypes, the high dose of T3 elevated UCP3 mRNA at or over the euthyroid level. Because mGPD is highly sensitive to thyroid hormone (38) and liver has no D2, liver thyroid status and mGPD depend largely on plasma borne T3 (39). Therefore, using the same strategy to show UCP1 is stimulated by locally D2-generated T3 in BAT (26), we used liver mGPD to test whether the short low-dose regimen has stimulated muscle UCP3 via elevating circulating T3. As it occurred in BAT with UCP1, in the present experiments the low-dose short regimen of T4 stimulated UCP3 in the soleus but barely stimulated liver mGPD, whereas injected T3 stimulated liver mGPD to the same high level in both genotypes (data not shown). Thus, D2 in skeletal muscle appears to play the same physiological role as in BAT (26). These results suggest that skeletal muscle D2 generates locally T3 to stimulate T3-responsive genes and that the increase activity of D2 in Thra-0/0 mice may play a role in increasing muscle thermogenesis to compensate for the lack of BAT thermogenesis. Keep in mind that the choice of measurable thyroid hormone-dependent genes in muscle of Thra-0/0 mice is difficult, because they lack TRα1, very highly expressed in muscle (8). Thus, for example sarco/endoplasmic reticulum Ca2+-ATPase 1, which is normally very sensitive to T3, is less responsive in TRα1-null mice (40), and other genes, such as myogenin, myosin, troponin, are either thyroid hormone-dependent during development or are affected by the lack of Thra gene products (41, 42).
Although we do not know what role bile acids may play in the phenotype of Thra-0/0 mice, it is likely that their production is increased, because these mice eat more than controls. To test the potential of bile acids, we showed here two novel findings. First, bile acids do not only stimulate BAT D2 but also skeletal muscle D2. Secondly, CA alone stimulates UCP3 mRNA expression in soleus of euthyroid mice, whereas it has no effect in soleus from hypothyroid mice, showing that this is not a nonspecific effect of CA but likely mediated by producing more T3 locally. Actually, CA in hypothyroid mice reduced UCP3 mRNA below the euthyroid control. This is possibly because mice are quite resistant to hypothyroidism and when treated with PTU-low iodine diet serum T3 is hardly reduced to 50–60% of the euthyroid level, and because bile acids also stimulate D3 to some extent, although in the presence of T4, the effect is offset by the generation of T3 (Zavacki et al: unpublished observations).
In summary, we have demonstrated that there are physiologically relevant amounts of D2 in skeletal muscle. With an improved assay, we demonstrate the presence of D2 activity in muscle, we show that it is sensitive to sympathetic stimulation, and that it plays a major role in the phenotype of the Thra-0/0 mice and probably is a sine qua non for the AFT of these mice. The dependence on the sympathetic activity correlates closely with the cold-dependent spendthrift phenotype of Thra-0/0 mice, strongly supporting a role in AFT. Furthermore, D2 activity is also present in WT mice, where its activity is also dependent on adrenergic stimulation, suggesting that it may be an important source of T3 for red, aerobic muscle for thermogenesis. These are exciting results that open several avenues to explore regarding the role of thyroid hormone in regulating intermediary metabolism. Polymorphisms of D2 have been related to insulin resistance (43) and changes in the sensitivity of the feedback mechanism to T4 levels (44), adding further support to the concept that D2 plays a major role modulating the action of thyroid hormone in a tissue selective manner.
Acknowledgments
We thank the valuable technical contributions of Paula Pelletier and Cyril Chou.
This work was supported by the Department of Medicine and the Academic Affairs Office of Baystate Medical Center, by internal grants from the Combined Biomedical Research Grants of Baystate Health and University of Massachusetts, and partially by National Institutes of Health Grants DK 44128 (to P.R.L.) and DK076117 (A.M.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AFT
- Alternate form of facultative thermogenesis
- BAT
- brown adipose tissue
- BW
- body weight
- CA
- cholic acid
- D2
- type-2 iodothyronine deiodinase
- HFD
- high-fat diet
- KO
- knockout
- mGPD
- mitochondrial glycerol 3-phosphate dehydrogenase
- α-MPT
- α-methyl-p-tyrosine
- PTU
- propylthiouracil
- TN
- thermoneutrality
- TRα1
- thyroid hormone receptor α-1
- UCP
- uncoupling protein
- VO2
- oxygen consumption
- WT
- wild type.
References
- 1. Galton VA. 1992. The role of thyroid hormone in amphibian metamorphosis. Trends Endocrinol Metab 3:96–100 [DOI] [PubMed] [Google Scholar]
- 2. Silva JE. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86:435–464 [DOI] [PubMed] [Google Scholar]
- 3. Borecký J, Maia IG, Arruda P. 2001. Mitochondrial uncoupling proteins in mammals and plants. Biosci Rep 21:201–212 [DOI] [PubMed] [Google Scholar]
- 4. Cannon B, Nedergaard J. 2004. Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359 [DOI] [PubMed] [Google Scholar]
- 5. Foster DO. 1984. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can J Biochem Cell Biol 62:618–622 [DOI] [PubMed] [Google Scholar]
- 6. Mory G, Ricquier D, Pesquiés P, Hémon P. 1981. Effects of hypothyroidism on the brown adipose tissue of adult rats: comparison with the effects of adaptation to the cold. J Endocrinol 91:515–524 [DOI] [PubMed] [Google Scholar]
- 7. Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. 2001. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol 21:4748–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forrest D, Vennström B. 2000. Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 [DOI] [PubMed] [Google Scholar]
- 9. Marrif H, Schifman A, Stepanyan Z, Gillis MA, Calderone A, Weiss RE, Samarut J, Silva JE. 2005. Temperature homeostasis in transgenic mice lacking thyroid hormone receptor α gene products. Endocrinology 146:2872–2884 [DOI] [PubMed] [Google Scholar]
- 10. Gordon CJ. 1993. Temperature regulation in laboratory rodents. New York: Cambridge University Press [Google Scholar]
- 11. Pelletier P, Gauthier K, Sideleva O, Samarut J, Silva JE. 2008. Mice lacking the thyroid hormone receptor-α gene spend more energy in thermogenesis, burn more fat, and are less sensitive to high-fat diet-induced obesity. Endocrinology 149:6471–6486 [DOI] [PubMed] [Google Scholar]
- 12. Oppenheimer JH, Schwartz HL, Surks MI. 1972. Propylthiouracil inhibits the conversion of L-thyroxine to L-triiodothyronine. J Clin Invest 51:2493–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oppenheimer JH, Schwartz HL, Surks MI, Koerner D, Dillmann WH. 1976. Nuclear receptors and the initiation of thyroid hormone action. Rec Prog Horm Res 32:529–565 [DOI] [PubMed] [Google Scholar]
- 14. Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. 2006. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem 281:31894–31908 [DOI] [PubMed] [Google Scholar]
- 15. Marsili A, Ramadan W, Harney JW, Mulcahey M, Castroneves LA, Goemann IM, Wajner SM, Huang SA, Zavacki AM, Maia AL, Dentice M, Salvatore D, Silva JE, Larsen PR. 2010. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology 151:5952–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Silva JE, Ramadan W, Marsili A, Larsen PR. 2010. Type-2 iodoythyronine deiodinase (D2) in skeletal muscle of C57Bl mice: authenticity and increased responses to adrenergic stimulation in thyroid hormone receptor-α null mice (Thra0/0). Proceedings 14th International Thyroid Congress, Paris, Sep 11–16, OC-139, Abstract [Google Scholar]
- 17. Ramadan W, Huang S, Marsili A, Larsen PR, Silva JE. 2009. Mouse skeletal muscle microsomes have type-2 iodothyronine deiodinase (D2) mRNA and activity. Possible role in the hypermetabolism of TRα-deficient mice. Program and Meeting Abstracts, 80th Annual Meeting of the American Thyroid Association, Palm Beach, Fl, Sep 23–27 [Google Scholar]
- 18. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. 2001. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- 19. Silva JE, Larsen PR. 1983. Adrenergic activation of triiodothyronine production in brown adipose tissue. Nature 305:712–713 [DOI] [PubMed] [Google Scholar]
- 20. Silva JE, Larsen PR. 1985. Potential of brown adipose tissue type II thyroxine 5′-deiodinase as a local and systemic source of triiodothyronine in rats. J Clin Invest 76:2296–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lusk G. 1924. Animal calorimetry. Analysis of the oxidation of mixtures of carbohydrate and fat. J Biol Chem 59:41–42 [Google Scholar]
- 22. de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. 2003. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castillo M, Hall JA, Correa-Medina M, Ueta C, Won Kang H, Cohen DE, Bianco AC. 2011. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60:1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gong DW, He Y, Karas M, Reitman M. 1997. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, β3-adrenergic agonists, and leptin. J Biol Chem 272:24129–24132 [DOI] [PubMed] [Google Scholar]
- 26. Bianco AC, Silva JE. 1987. Intracellular conversion of thyroxine to triiodothyronine is required for the optimal thermogenic function of brown adipose tissue. J Clin Invest 79:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. 2006. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- 28. Rothwell NJ, Stock MJ. 1979. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281:31–35 [DOI] [PubMed] [Google Scholar]
- 29. Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. 1993. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366:740–742 [DOI] [PubMed] [Google Scholar]
- 30. Enerbäck S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. 1997. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387:90–94 [DOI] [PubMed] [Google Scholar]
- 31. Silva JE. 2011. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci 3:352–371 [DOI] [PubMed] [Google Scholar]
- 32. Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. 2006. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology 147:2468–2480 [DOI] [PubMed] [Google Scholar]
- 33. Sjögren M, Alkemade A, Mittag J, Nordström K, Katz A, Rozell B, Westerblad H, Arner A, Vennström B. 2007. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J 26:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ricquier D, Mory G, Bouillaud F, Thibault J, Weissenbach J. 1984. Rapid increase of mitochondrial uncoupling protein and its mRNA in stimulated brown adipose tissue. FEBS Lett 178:240–244 [DOI] [PubMed] [Google Scholar]
- 35. Schrauwen P, Hesselink MK. 2004. The role of uncoupling protein 3 in fatty acid metabolism: protection against lipotoxicity? Proc Nutr Soc 63:287–292 [DOI] [PubMed] [Google Scholar]
- 36. Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, Laville M, Langin D. 1997. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. J Clin Invest 100:2665–2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weigle DS, Selfridge LE, Schwartz MW, Seeley RJ, Cummings DE, Havel PJ, Kuijper JL, BeltrandelRio H. 1998. Elevated free fatty acids induce uncoupling protein 3 expression in muscle: a potential explanation for the effect of fasting. Diabetes 47:298–302 [DOI] [PubMed] [Google Scholar]
- 38. Lee YP, Lardy HA. 1965. Influence of thyroid hormones on l-α-glycerophosphate dehydrogenases and other dehydrogenases in various organs of the rat. J Biol Chem 240:1427–1436 [PubMed] [Google Scholar]
- 39. Silva JE, Leonard JL, Crantz FR, Larsen PR. 1982. Evidence for two tissue-specific pathways for in vivo thyroxine 5′-deiodination in the rat. J Clin Invest 69:1176–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johansson C, Lännergren J, Lunde PK, Vennström B, Thorén P, Westerblad H. 2000. Isometric force and endurance in soleus muscle of thyroid hormone receptor-α(1)- or -β-deficient mice. Am J Physiol Regul Integr Comp Physiol 278:R598–R603 [DOI] [PubMed] [Google Scholar]
- 41. Anderson JE, McIntosh LM, Moor AN, Yablonka-Reuveni Z. 1998. Levels of MyoD protein expression following injury of mdx and normal limb muscle are modified by thyroid hormone. J Histochem Cytochem 46:59–67 [DOI] [PubMed] [Google Scholar]
- 42. Mansén A, Yu F, Forrest D, Larsson L, Vennström B. 2001. TRs have common and isoform-specific functions in regulation of the cardiac myosin heavy chain genes. Mol Endocrinol 15:2106–2114 [DOI] [PubMed] [Google Scholar]
- 43. Canani LH, Capp C, Dora JM, Meyer EL, Wagner MS, Harney JW, Larsen PR, Gross JL, Bianco AC, Maia AL. 2005. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 90:3472–3478 [DOI] [PubMed] [Google Scholar]
- 44. Butler PW, Smith SM, Linderman JD, Brychta RJ, Alberobello AT, Dubaz OM, Luzon JA, Skarulis MC, Cochran CS, Wesley RA, Pucino F, Celi FS. 2010. The Thr92Ala 5′ type 2 deiodinase gene polymorphism is associated with a delayed triiodothyronine secretion in response to the thyrotropin-releasing hormone-stimulation test: a pharmacogenomic study. Thyroid 20:1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramadan W, Marsili A, Huang S, Larsen PR, Silva JE. 2011. Type-2 iodothyronine 5'deiodinase in skeletal muscle of C57Bl/6 mice. I. Identity, subcellular localization, and characterization. Endocrinology 152:3082–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]